Abstract
Initial clinical trials and surveillance data have shown that the most commonly administered BNT162b2 COVID-19 mRNA vaccine is effective and safe. However, several cases of mRNA vaccine-induced mild to moderate adverse events were recently reported. Here, we report a rare case of myositis after injection of the first dose of BNT162b2 COVID-19 mRNA vaccine into the left deltoid muscle of a 34-year-old, previously healthy woman who presented progressive proximal muscle weakness, progressive dysphagia, and dyspnea with respiratory failure. One month after vaccination, BNT162b2 vaccine mRNA expression was detected in a tissue biopsy of the right deltoid and quadriceps muscles. We propose this case as a rare example of COVID-19 mRNA vaccine-induced myositis. This study comprehensively characterizes the clinical and molecular features of BNT162b2 mRNA vaccine-associated myositis in which the patient was severely affected.
1. Introduction
The Food and Drug Administration (FDA) granted emergency approval for the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine to combat the COVID-19 pandemic [1] on 11 December 2020. In Israel, a nationwide COVID-19 vaccination campaign began in late December 2020, in which more than five million people were successfully vaccinated, leading to a rapid decline in COVID-19 cases throughout the country [2]. In phase III clinical trials, the BNT162b2 vaccine was found to be 95% effective against COVID-19 [3]. However, the safety and risk of adverse reactions to the BNT162b2 vaccine are now the major concerns. In the clinical trials, participants reported short-term mild local adverse reactions, including injection site pain, swelling, fever, fatigue, myalgia, and lymphadenopathy [4]. The occurrence of adverse events was found significantly to be higher in the younger populations than in older people and in females than in males [4,5,6]. The majority of adverse events in the younger population is one of the important causes behind COVID-19 vaccine hesitancy among youth [7].
Several cases of mild autoimmune reactions followed by BNT162b2 vaccine administration were reported where the requirement for hospital care was rare [8,9]. However, recent studies showed that in autoimmune inflammatory rheumatic diseases (AIIRD), the BNTb262 vaccine could generate immunogenic response in the majority of patients, which raised safety concerns for patients with AIIRDs [10,11,12]. Some evidence demonstrated that BNT162b2 could induce the development of Guillain-Barre syndrome (GBS), which is a rare neurological autoimmune disorder of the peripheral nervous system [13,14,15,16,17]. Furthermore, a recent case study reported that the BNT162b2 vaccine induced the development of acute transverse myelitis followed by fatal neurological complications [18]. A few cases have been reported for BNT162b2 vaccine-induced myasthenia gravis (MG) development, a chronic autoimmune condition affecting the neuromuscular junction [19,20,21]. However, some studies demonstrated that BNT162b2 vaccination showed overall good short-term safety in MG patients [22,23].
Recently, several mild to moderate rare cases of anaphylaxis [24,25,26,27], thrombocytopenia [28,29,30,31], myocarditis [32,33,34,35,36], myositis [37,38,39,40,41], and rhabdomyolysis [42,43,44] associated with the BNT162b2 vaccine have been reported. Here, we report a rare case of BNT162b2 vaccine-associated myositis in which the patient was severely affected. To understand the molecular mechanism behind the severe conditions of myositis, we analyzed the vaccine mRNA expression in the DNA and RNA samples from patient blood and muscle tissue biopsy.
2. Materials and Methods
2.1. Ethical Considerations
Written informed consent for publication was obtained from the patient. The authors assure the accuracy and comprehensiveness of the data in this report.
2.2. Immunoassays and Serological Testing
Nasopharyngeal swabs were taken and examined for SARS-CoV-2 by real-time RT-PCR performed with internal positive and negative controls, according to World Health Organization (WHO) guidelines. The COBAS SARS-CoV-2 6800/8800 assay (Roche Pharmaceuticals, Basel, Switzerland) was employed. SARS-CoV-2 IgG II quantitative testing was performed on the Abbott Alinity i platform.
2.3. DNA and RNA Extraction and Sequencing
A blood sample was collected from the patient 7 days after administration of the first vaccine dose. As a control, a blood sample was taken 7 days after the second vaccine dose from a second individual. Total RNA was extracted from peripheral blood mononuclear cells (PBMCs) using a RNeasy Mini Kit (Qiagen, Germany, catalogue no. 74104) according to the manufacturer’s protocol. FFPE (formalin-fixed paraffin-embedded) tissue sections were processed for total RNA and genomic DNA using a RNeasy FFPE kit (Qiagen, Germany, catalogue no. 73504) and a DNeasy Blood and Tissue kit (Qiagen, Germany, catalogue no. 69504), according to the manufacturer’s protocols. All samples were sent for RNA and DNA sequencing on an Illumina NextSeq 550 machine.
2.4. Nested PCR Assay for BNT162b2 Vaccine
A PCR was performed for 20 cycles with the outside primers, after which the primers were removed with a MN Nucleospin PCR and Gel Clean-up kit. A nested PCR was performed for 30 cycles, when the products were separated on a 3% agarose gel.
3. Results
3.1. Case Representation
A previously healthy 34-year-old woman with no evidence of prior SARS-CoV-2 infection presented with complaints of severe muscle weakness, pain, and tenderness. The patient denied vigorous exercise, seizures, or heavy physical labor before the onset of symptoms. Her symptoms began on day four after the first dose of BNT162b2 mRNA vaccine, which was administered into her left deltoid muscle. On admission, she had no fever, chills, or malaise. A COVID-19 swab (PCR) test was performed twice and was negative both times. She did not complain of cough or shortness of breath. Upon physical examination, the patient was afebrile and had a blood pressure of 118/72 mmHg, a pulse rate of 84 b.p.m., and oxygen saturation of 97% on room air. There was remarkable swelling, severe tenderness, and proximal muscle weakness in the flexor muscles of the neck, pelvic region, thigh and shoulders, with symmetrical distribution. A neurological examination showed intact higher mental function. Sensory and cranial nerve examination results were within normal limits. There was significant muscle weakness, with a strength grade of 3/5 for the shoulders and hips, and 2/5 for the elbows, ankles and wrists. All nerve reflexes were normal. A chest radiograph was normal, and an echocardiogram showed no pericardial effusion and good biventricular function.
Upon admission, a blood workup revealed a creatine kinase (CK) level of 15750 IU/L (n < 195 IU/L), an aspartate aminotransaminase (AST) level of 351 U/L, an alanine aminotransaminase (ALT) level of 138 U/L, and a C-reactive protein (CRP) level of 134 mg/L (n < 5 mg/L), as well as a normal hemoglobin level, leukocyte count, and platelet count. The patient showed lymphopenia at 0.43 × 109 cells/L (normal 1.5–4.5 × 109 cells/L), and her high-sensitivity troponin T (hsTnT) level was 12 ng/L (normal < 14 ng/L). Antinuclear antibody tested using indirect immunofluorescence on human type 2 epithelial cells (Hep2) was strongly positive with a speckled pattern. Testing for anti-double-stranded DNA, anti-SSA/SSB, anti-Sm, anti-melanoma differentiation-associated gene 5 (anti-MDA5), anti-Jo1, anti-Scl70, anti-Ro52, and anti-neutrophil cytoplasmic antibodies (ANCA), were all negative. Anti-acetylcholine receptor (AChR) and anti-muscle-specific tyrosine kinase (MuSK) autoantibodies were negative. Anti-TIF-1g, NXP-2/MJ and Mi-2 antibodies were not available in our clinical laboratory.
High-resolution computed tomography (CT) of the lungs showed bilateral mild pleural effusion. Influenza PCR and HIV, EBV, CMV, HBV, HCV, and parvovirus serological results were all negative. Urinalysis excluded myoglobinuria. Eight weeks after one dose of BNT162b2 vaccine, the SARS-CoV-2 IgG level was 37 AU/L (Alinity i System, Abbott Laboratories, IL; a value < 50 AU/mL is considered negative, a value > 150 AU/mL is considered protective, and a value between 50 and 150 AU/mL reflects the uncertainty of protection).
Electromyography and nerve conduction studies performed three weeks after disease onset showed evidence of myositis with muscle fiber denervation confined to the proximal upper limbs (specifically to the deltoid muscle). There was no electrodiagnostic evidence for large fiber polyneuropathy or lumbosacral radiculopathy. Magnetic resonance imaging (MRI) of the deltoid and thigh muscles was suggestive of myositis with bilateral symmetric involvement (Figure 1). No other polymyositis-associated features, including specific heliotrope or Gottron’s signs or papules, other skin or cuticular changes, Raynaud’s phenomenon, lymphadenopathy, arthritis, or cardiac involvement were noted. A diagnosis of myositis was made on the basis of characteristic proximal muscle weakness, elevated creatine phosphokinase (CPK) levels, a strongly positive ANA HEp2, and electrodiagnostic evidence for active myositis with precise MRI findings.
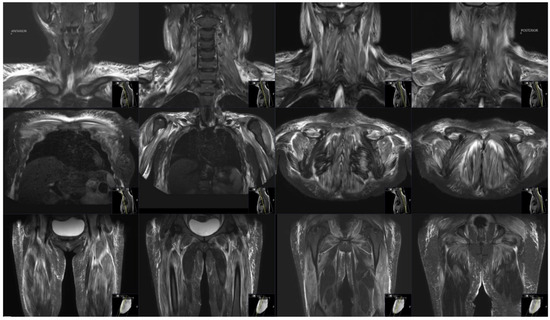
Figure 1.
MRI of deltoid and thigh muscles. Bilateral patchy and diffuse areas of high T2/ STIR signal intensity in muscles are seen, consistent with active myositis.
Open muscle biopsies from the right deltoid and quadriceps performed one month after vaccination showed similar lesions in both muscles that included perivascular inflammation, perifascicular fiber atrophy with vacuoles, major histocompatibility complex class I (MHC-I) staining, and C5b9 complement deposition in the capillaries. Other regions showed diffuse fiber necrosis suggestive of muscle infarction. These findings were most consistent with a diagnosis of myositis (Figure 2).
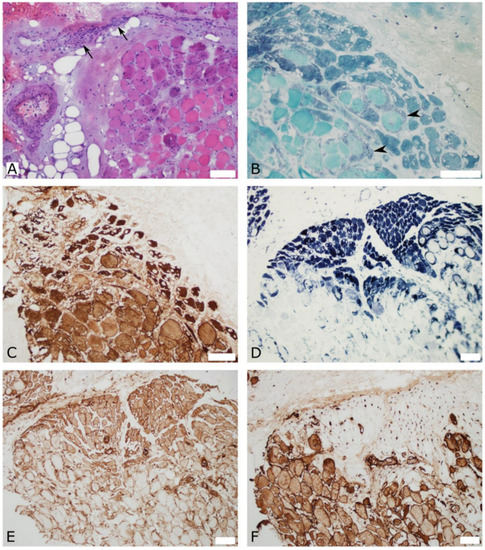
Figure 2.
Quadriceps muscle biopsy with perifascicular findings that are suggestive of myositis. (A) Hematoxylin and eosin stain shows perivascular mononuclear cellularity (arrows) in the perimysium, and perimysial and endomysial fibrosis. (B) Gomori trichome stain shows perifascicular atrophic fibers and small vacuoles, as well as signs of regeneration (arrowheads). (C) Slow myosin immunostaining highlights perifascicular atrophy and small vacuoles. (D) Dense staining with reduced nicotinamide adenine dinucleotide (NADH) dehydrogenase in perifascicular atrophic fibers, and no staining of necrotic fibers. (E) Major histocompatibility complex class I (MHC-I) is up-regulated in perifascicular myofibers. (F) Membranolytic attack complex (C5b-9) immunostaining shows significant capillary deposition in the perifascicular region and highlights necrotic fibers. Scale bars = 100 μm.
The patient was treated with intravenous methylprednisolone (125 mg b.i.d. as a bolus) for ten days, followed by oral prednisone (1 mg/kg qd). Subsequently, there was a transient ~50% improvement in proximal muscle weakness, with the patient’s serum CPK being monitored daily as it continued to rise. On day 15, the patient developed progressive dysphagia, dyspnea with hypoxemia, and hypercapnia requiring invasive mechanical ventilation and nasogastric feeding. She was resuscitated and transferred to the intensive care unit. Pulmonary embolism was ruled out by CT angiography. The patient was treated with methylprednisolone pulse therapy (1000 mg daily) and intravenous immune globulin (IVIG), which resulted in mild improvement. Although partial bulbar weakness persisted, the patient was able to resume and continue oral prednisone and azathioprine. After multiple failures to wean the patient from mechanical ventilation, a tracheostomy was performed. She is now undergoing respiratory and neurological rehabilitation, albeit without further improvement.
3.2. Evaluation of Vaccine mRNA Expression in Blood and Muscle Tissue Biopsy Samples
To understand the association of BNT162b2 mRNA expression with the development of myositis, we sequenced the patient’s blood and muscle tissue biopsy samples. After generating raw sequence reads from control and patient blood samples, quality control was performed, followed by the removal of index and adapter sequences. Next, trimmed reads were further used for mapping to the human reference sequences + BNT162b2 vaccine spike mRNA using Bowtie2 [45]. Then, total mapped reads to the vaccine spike mRNA sequence region were calculated. A total of 17,626 and 639 reads were mapped to the vaccine spike protein mRNA sequence in the RNA-seq data of the control and the patient, respectively. Upon visualizing the spike mRNA reads mapping region using the Integrative Genomics Viewer (IGV) [46], we found that in the control sample, reads covered and were equally mapped across 98% region of the vaccine spike protein mRNA sequence. In the patient sample, reads were mapped to just a few regions covering only 36% of the vaccine spike protein mRNA sequence (Figure 3I). Partial mapping of the vaccine spike protein mRNA sequence in the patient’s sample indicated an unusual pattern of vaccine mRNA expression in blood cells, namely, “chopped” parts of the mRNA vaccine molecules from the Pfizer vaccine. This was supported by the low level of anti-SARS-CoV-2 IgGs detected, suggesting that the mRNA vaccine was not translated into the spike protein in this patient, resulting in no immune response to SARS-CoV-2.
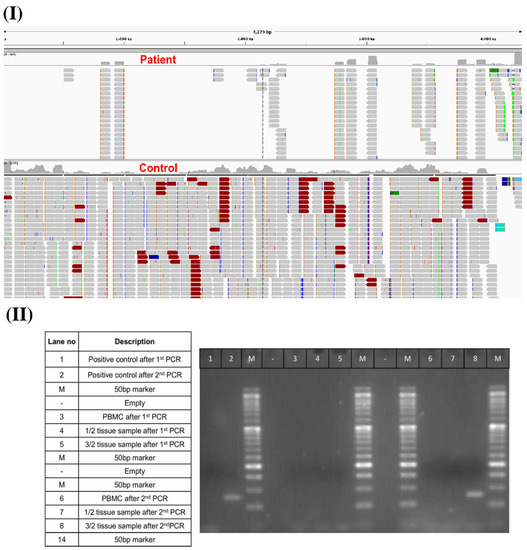
Figure 3.
(I) Distribution of mapped RNA-seq reads across spike mRNA. (II) PCR validation of vaccine spike mRNA expression in the patient’s muscle tissue biopsy samples one-month post-vaccination.
Next, to understand the development of vaccine-induced myositis, we performed a DNA sequencing analysis of a right quadriceps muscle biopsy sample from the patient one-month post-vaccination. We did not find any mapped reads of the vaccine spike mRNA sequence in the genomic DNA sequencing data. It can thus be inferred that the vaccine mRNA sequences did not integrate into the patient’s genome. Next, to check whether vaccine mRNA expression could be detected in the RNA of the tissue biopsy sample, we performed a nested PCR using two sets of primers against the 3′UTR (untranslated region) of the vaccine mRNA. A synthetic construct containing the 3′UTR of the BNT162b2 mRNA vaccine served as a control for PCR validation of the mRNA vaccine. To achieve maximum sensitivity and specificity in PCR in efforts to detect the presence of vaccine mRNA in the quadriceps muscle tissue, a nested PCR was developed in which the forward primers annealed to the TLE5 3′UTR sequence and the reverse primers annealed to the mito-nc sequence of the BNT162b2 vaccine sequence. The expected size of the vaccine mRNA sequence was thus 75 bp. We observed a band of this size in the total tissue RNA, confirming the expression of the BNT162b2 vaccine mRNA in the biopsy samples of the right quadriceps muscle one month after BNT162b2 vaccination (Figure 3II). This result highlights that although the BNT162b2 vaccine mRNA was not properly expressed in blood cells seven days after receipt of the first vaccine dose, it was still expressed in muscle tissue distant from the vaccination site one month after receipt of the first vaccine dose. This suggests that the unusual BNT162b2 mRNA expression pattern observed in muscle cells may be related to the development of myositis.
4. Discussion
In this study, we represent a rare example of BNT162b2 vaccine-induced myositis. According to mRNA vaccine technology, most endogenous mRNA transcripts are rapidly degraded, usually within 10–15 min after inoculation [47]. The rate of degradation of synthetic mRNA vaccines in tissues is inversely proportional to the kinetics of translation initiation and duration. Therefore, to increase the stability of vaccine mRNA, it is necessary to optimize codon usage and UTR sequences [48]. The BNT162b2 mRNA vaccine is a lipid nanoparticle-encapsulated vaccine, with the nanoparticles protecting the mRNA from degradation by mediating endocytosis and endosomal escape [49]. After injection into the muscle, the synthetically produced BNT162b2 mRNA vaccine should be degraded by both extracellular and intracellular RNases, thus remaining in human tissues for only a few days [50]. However, in our patient, we observed the expression of vaccine mRNA in muscle tissues as late as one month after vaccination, which may indicate that the exogenously expressed mRNA was stable enough to persist over a long period of time. One of the known autoimmune manifestations of COVID-19-induced muscle disease is myositis with severe bulbar weakness [51]. Most commonly, SARS-CoV-2-associated muscle inflammation is triggered by clonal expansion of T cells and production of pro-inflammatory cytokines, leading to muscle damage [52,53].
Although BNT162b2 COVID-19 vaccination is safe and most of the adverse effects are mild [54], some moderate to serious adverse events such as anaphylaxis [24,25,26,27], thrombocytopenia [28,29,30,31], myocarditis [32,33,34,35,36], myositis [37,38,39,40,41] and rhabdomyolysis [42,43,44] were recently reported. Therefore, it is crucial to identify the factors associated with adverse effects after BNT162b2 vaccination. A direct relationship between the reduced BNT162b2-induced immunogenicity and the risk of autoimmunity has yet to be demonstrated. Thus, the case presented here raises many difficult questions and unanswered concerns, such as the efficacy and safety of mRNA vaccines in subgroups previously excluded from BNT162b2 vaccine trials and underscores the need for further studies in order to understand autoimmunity induced by modified-mRNA vaccines.
As the new COVID-19 strains are continuously emerging [55,56] and, in several countries, the COVID-19 surge shows a seasonal pattern [57,58], the vaccination strategy should be planned accordingly. The new clinical trials should be designed to understand the effect of BNT162b2 vaccination in groups of different autoimmune disorder patients, which could help to design an effective strategy for COVID-19 vaccination with minimal risk of adverse reactions. At present, considering the global scenario for effectiveness and risk of the COVID-19 vaccination, it could be suggested that the development of different strategies [59,60,61,62,63,64] with proper clinical trials is needed to design such new COVID-19 vaccines which could be effective against all SARS-CoV-2 variants with a minimal risk of adverse effects.
Author Contributions
Conceptualization, E.M. (Eli Magen), S.M., E.M. (Eugene Merzon), I.B., A.L., M.S., R.T., E.C., A.D. and M.F.-M.; methodology, E.M. (Eli Magen), S.M., M.B., R.D., E.M. (Eugene Merzon), I.B., A.L., M.S., G.B., R.T., E.C., A.D. and M.F-M.; software, S.M., R.D. and M.F.-M.; validation, M.B., G.B. and M.F.-M.; formal analysis, S.M., R.D. and M.F.-M.; investigation, E.M. (Eli Magen), S.M., M.B., R.D., E.M. (Eugene Merzon), I.B., A.L., M.S., G.B., R.T., E.C., A.D. and M.F.-M.; resources, E.M. (Eli Magen) and M.F.-M.; writing—original draft preparation, E.M. (Eli Magen), S.M., R.D., E.M. (Eugene Merzon), G.B. and M.F.-M.; writing—review and editing, E.M. (Eli Magen), S.M. and M.F.-M.; supervision, E.M. (Eli Magen) and M.F.-M.; project administration, E.M. (Eli Magen) and M.F.-M.; funding acquisition, E.M. (Eli Magen) and M.F.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Since the normal clinical practice is described in this case report, formal ethical approval by the Independent Review Board was not required in accordance with the policy of our institution.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank members of the Cancer Genomics and Biocomputing of Complex Diseases Lab for multiple discussions at different stages of this project. S.M. was supported by the Israeli Council of Higher Education and Research through the PBC fellowship program for outstanding postdoctoral researchers from China and India (2019–2021). M.F-M. was supported by the COVID-19 Data Science Institute (DSI) grant, Bar-Ilan University (#247017, 2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tanne, J.H. COVID-19: FDA panel votes to approve Pfizer BioNTech vaccine. BMJ 2020, 371, m4799. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Nationwide Vaccination Campaign with BNT162b2 in Israel Demonstrates High Vaccine Effectiveness and Marked Declines in Incidence of SARS-CoV-2 Infections and COVID-19 Cases, Hospitalizations, and Deaths. SSRN. 2021. Available online: https://ssrn.com/abstract=3811387 (accessed on 17 June 2022).
- Chagla, Z. The BNT162b2 (BioNTech/Pfizer) vaccine had 95% efficacy against COVID-19 ≥ 7 days after the 2nd dose. Ann. Intern. Med. 2021, 174, JC15. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Kaiki, Y.; Sugiyama, A.; Nagashima, S.; Kurisu, A.; Nomura, T.; Omori, K.; Akita, T.; Shigemoto, N.; Tanaka, J.; et al. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan. J. Infect. Chemother. 2022, 28, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Willis, D.E.; Presley, J.; Williams, M.; Zaller, N.; McElfish, P.A. COVID-19 vaccine hesitancy among youth. Hum. Vaccines Immunother. 2021, 17, 5013–5015. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, L.; Tong, X.; Chan, V.K.Y.; Chui, C.S.L.; Lai, F.T.T.; Wong, C.K.H.; Wan, E.Y.F.; Chan, E.W.Y.; Lau, K.K.; et al. Autoimmune conditions following mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: A descriptive cohort study among 1.1 million vaccinated people in Hong Kong. J. Autoimmun. 2022, 130, 102830. [Google Scholar] [CrossRef] [PubMed]
- Ishay, Y.; Kenig, A.; Tsemach-Toren, T.; Amer, R.; Rubin, L.; Hershkovitz, Y.; Kharouf, F. Autoimmune phenomena following SARS-CoV-2 vaccination. Int. Immunopharmacol. 2021, 99, 107970. [Google Scholar] [CrossRef]
- Velikova, T.; Georgiev, T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol. Int. 2021, 41, 509–518. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Braun-Moscovici, Y.; Balbir-Gurman, A.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; et al. Predictors of Immunogenic Response to the BNT162b2 mRNA COVID-19 Vaccination in Patients with Autoimmune Inflammatory Rheumatic Diseases Treated with Rituximab. Vaccines 2022, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Ahmad, U.; Ualiyeva, D.; Amissah, O.B.; Khan, A.; Noor, Z.; Zaman, N. Guillain-Barre syndrome: An autoimmune disorder post-COVID-19 vaccination? Clin. Immunol. Commun. 2022, 2, 1–5. [Google Scholar] [CrossRef]
- Trimboli, M.; Zoleo, P.; Arabia, G.; Gambardella, A. Guillain-Barré syndrome following BNT162b2 COVID-19 vaccine. Neurol. Sci. 2021, 42, 4401–4402. [Google Scholar] [CrossRef]
- Takahashi, K.; Tomoda, Y.; Kadena, S.; Kanbayashi, T.; Kobayashi, S.; Kato, R. Guillain–Barré syndrome after BNT162b2 (Pfizer-BioNTec) vaccination. QJM Int. J. Med. 2022, 115, 331–333. [Google Scholar] [CrossRef]
- Shapiro Ben David, S.; Potasman, I.; Rahamim-Cohen, D. Rate of Recurrent Guillain-Barré Syndrome After mRNA COVID-19 Vaccine BNT162b2. JAMA Neurol. 2021, 78, 1409–1411. [Google Scholar] [CrossRef]
- Kim, N.; Kim, J.H.; Park, J.S. Guillain–Barré syndrome associated with BNT162b2 COVID vaccination: A first case report from South Korea. Neurol. Sci. 2022, 43, 1491–1493. [Google Scholar] [CrossRef]
- Nakano, H.; Yamaguchi, K.; Kawabata, K.; Asakawa, M.; Matsumoto, Y. Acute transverse myelitis after BNT162b2 vaccination against COVID-19: Report of a fatal case and review of the literature. J. Neurol. Sci. 2022, 434, 120102. [Google Scholar] [CrossRef]
- Chavez, A.; Pougnier, C. A Case of COVID-19 Vaccine Associated New Diagnosis Myasthenia Gravis. J. Prim. Care Community Health 2021, 12, 215013272110519. [Google Scholar] [CrossRef]
- Ishizuchi, K.; Takizawa, T.; Sekiguchi, K.; Motegi, H.; Oyama, M.; Nakahara, J.; Suzuki, S. Flare of myasthenia gravis induced by COVID-19 vaccines. J. Neurol. Sci. 2022, 436, 120225. [Google Scholar] [CrossRef]
- Sansone, G.; Bonifati, D.M. Vaccines and myasthenia gravis: A comprehensive review and retrospective study of SARS-CoV-2 vaccination in a large cohort of myasthenic patients. J. Neurol. 2022, 269, 3965–3981. [Google Scholar] [CrossRef]
- Lupica, A.; Di Stefano, V.; Iacono, S.; Pignolo, A.; Quartana, M.; Gagliardo, A.; Fierro, B.; Brighina, F. Impact of COVID-19 in AChR Myasthenia Gravis and the Safety of Vaccines: Data from an Italian Cohort. Neurol. Int. 2022, 14, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Lotan, I.; Hellmann, M.A.; Friedman, Y.; Stiebel-Kalish, H.; Steiner, I.; Wilf-Yarkoni, A. Early safety and tolerability profile of the BNT162b2 COVID-19 vaccine in myasthenia gravis. Neuromuscul. Disord. 2022, 32, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Novak, N.; Hamelmann, E.; Werfel, T.; Wagenmann, M.; Taube, C.; Bauer, A.; Merk, H.; Rabe, U.; Jung, K.; et al. Severe allergic reactions after COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA. Allergo J. Int. 2021, 30, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Lim, X.R.; Leung, B.P.; Ng, C.Y.L.; Tan, J.W.L.; Chan, G.Y.L.; Loh, C.M.; Tan, G.L.X.; Goh, V.H.H.; Wong, L.T.; Chua, C.R.; et al. Pseudo-anaphylactic reactions to pfizer bnt162b2 vaccine: Report of 3 cases of anaphylaxis post pfizer bnt162b2 vaccination. Vaccines 2021, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, A.; Bhandari, D.; Sawano, T.; Sah, R.; Tanimoto, T. High anaphylaxis rates following vaccination with the Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese healthcare workers: A secondary analysis of initial post-approval safety data. J. Travel Med. 2021, 28, taab090. [Google Scholar] [CrossRef]
- Shavit, R.; Maoz-Segal, R.; Iancovici-Kidon, M.; Offengenden, I.; Haj Yahia, S.; Machnes Maayan, D.; Lifshitz-Tunitsky, Y.; Niznik, S.; Frizinsky, S.; Deutch, M.; et al. Prevalence of Allergic Reactions after Pfizer-BioNTech COVID-19 Vaccination among Adults with High Allergy Risk. JAMA Netw. Open 2021, 4, e2122255. [Google Scholar] [CrossRef]
- Lee, E.J.; Cines, D.B.; Gernsheimer, T.; Kessler, C.; Michel, M.; Tarantino, M.D.; Semple, J.W.; Arnold, D.M.; Godeau, B.; Lambert, M.P.; et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021, 96, 534–537. [Google Scholar] [CrossRef]
- Akiyama, H.; Kakiuchi, S.; Rikitake, J.; Matsuba, H.; Sekinada, D.; Kozuki, Y.; Iwata, N. Immune thrombocytopenia associated with Pfizer-BioNTech’s BNT162b2 mRNA COVID-19 vaccine. IDCases 2021, 25, e01245. [Google Scholar] [CrossRef]
- Malayala, S.V.; Papudesi, B.N.; Sharma, R.; Vusqa, U.T.; Raza, A. A Case of Idiopathic Thrombocytopenic Purpura After Booster Dose of BNT162b2 (Pfizer-Biontech) COVID-19 Vaccine. Cureus 2021, 13, e18985. [Google Scholar] [CrossRef]
- Okada, Y.; Sakai, R.; Sato-Fitoussi, M.; Nodera, M.; Yoshinaga, S.; Shibata, A.; Kurasawa, T.; Kondo, T.; Amano, K. Potential Triggers for Thrombocytopenia and/or Hemorrhage by the BNT162b2 Vaccine, Pfizer-BioNTech. Front. Med. 2021, 8, 751598. [Google Scholar] [CrossRef]
- Abu Mouch, S.; Roguin, A.; Hellou, E.; Ishai, A.; Shoshan, U.; Mahamid, L.; Zoabi, M.; Aisman, M.; Goldschmid, N.; Berar Yanay, N. Myocarditis following COVID-19 mRNA vaccination. Vaccine 2021, 39, 3790–3793. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Friedensohn, L.; Levin, D.; Fadlon-Derai, M.; Gershovitz, L.; Fink, N.; Glassberg, E.; Gordon, B. Myocarditis Following a Third BNT162b2 Vaccination Dose in Military Recruits in Israel. JAMA J. Am. Med. Assoc. 2022, 327, 1611–1612. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Volpe, M. Myocarditis after BNT162b2 mRNA SARS-CoV-2 vaccine: Low incidence and mild severity. Eur. Heart J. 2022. [Google Scholar] [CrossRef]
- Fosch, X.; Serra, J.; Torres, P.L.; Preda, L.; González, R.; Mojer, F. Acute myocarditis after a third dose of the BNT162b2 COVID-19 vaccine. Rev. Esp. Cardiol. 2022, 75, 614–616. [Google Scholar] [CrossRef]
- Theodorou, D.J.; Theodorou, S.J.; Axiotis, A.; Gianniki, M.; Tsifetaki, N. COVID-19 vaccine-related myositis. QJM Int. J. Med. 2021, 114, 424–425. [Google Scholar] [CrossRef]
- Vutipongsatorn, K.; Isaacs, A.; Farah, Z. Inflammatory myopathy occurring shortly after severe acute respiratory syndrome coronavirus 2 vaccination: Two case reports. J. Med. Case Rep. 2022, 16, 57. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Woo, C.G. Clinicopathological Characteristics of Inflammatory Myositis Induced by COVID-19 Vaccine (Pfizer-BioNTech BNT162b2): A Case Report. J. Korean Med. Sci. 2022, 37, e91. [Google Scholar] [CrossRef]
- Ramalingam, S.; Arora, H.; Lewis, S.; Gunasekaran, K.; Muruganandam, M.; Nagaraju, S.; Padmanabhan, P. COVID-19 vaccine-induced cellulitis and myositis. Cleve. Clin. J. Med. 2021, 88, 648–650. [Google Scholar] [CrossRef]
- Gouda, W.; Albasri, A.; Alsaqabi, F.; Al Sabah, H.Y.; Alkandari, M.; Abdelnaby, H. Dermatomyositis Following BNT162b2 mRNA COVID-19 Vaccination. J. Korean Med. Sci. 2022, 37, e32. [Google Scholar] [CrossRef]
- Hakroush, S.; Tampe, B. Case Report: ANCA-Associated Vasculitis Presenting with Rhabdomyolysis and Pauci-Immune Crescentic Glomerulonephritis After Pfizer-BioNTech COVID-19 mRNA Vaccination. Front. Immunol. 2021, 12, 762006. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Chung, H.; Dhayaparan, Y.; Nyein, A.; Acevedo, B.J.; Chicos, C.; Zheng, D.; Barras, M.; Mohamed, M.; Alfishawy, M.; et al. COVID-19 vaccine induced rhabdomyolysis: Case report with literature review. Diabetes Metab. Syndr. 2021, 15, 102170. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Niwa, J.-I.; Doyu, M. Recurring Weakness in Rhabdomyolysis Following Pfizer–BioNTech Coronavirus Disease 2019 mRNA Vaccination. Vaccines 2022, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S. Fast gapped-read alignment with Bowtie2. Nat. Methods 2013, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Neymotin, B.; Athanasiadou, R.; Gresham, D. Determination of in vivo RNA kinetics using RATE-seq. RNA 2014, 20, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Xia, X. Detailed dissection and critical evaluation of the pfizer/biontech and moderna mrna vaccines. Vaccines 2021, 9, 734. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Chan, L.Y.; Mugler, C.F.; Heinrich, S.; Vallotton, P.; Weis, K. Non-invasive measurement of mRNA decay reveals translation initiation as the major determinant of mRNA stability. eLife 2018, 7, e32536. [Google Scholar] [CrossRef]
- Zhang, H.; Charmchi, Z.; Seidman, R.J.; Anziska, Y.; Velayudhan, V.; Perk, J. COVID-19–associated myositis with severe proximal and bulbar weakness. Muscle Nerve 2020, 62, E57–E60. [Google Scholar] [CrossRef]
- Saud, A.; Naveen, R.; Aggarwal, R.; Gupta, L. COVID-19 and Myositis: What We Know So Far. Curr. Rheumatol. Rep. 2021, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- El-Shabasy, R.M.; Nayel, M.A.; Taher, M.M.; Abdelmonem, R.; Shoueir, K.R.; Kenawy, E.R. Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int. J. Biol. Macromol. 2022, 204, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Fast-evolving COVID variants complicate vaccine updates. Nature 2022, 607, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Merow, C.; Urban, M.C. Seasonality and uncertainty in global COVID-19 growth rates. Proc. Natl. Acad. Sci. USA 2020, 117, 27456–27464. [Google Scholar] [CrossRef]
- Mukherjee, S.B.; Gorohovski, A.; Merzon, E.; Levy, E.; Mukherjee, S.; Frenkel-Morgenstern, M. Seasonal UV exposure and vitamin D: Association with the dynamics of COVID-19 transmission in Europe. FEBS Open Bio 2022, 12, 106–117. [Google Scholar] [CrossRef]
- Papi, M.; Pozzi, D.; Palmieri, V.; Caracciolo, G. Principles for optimization and validation of mRNA lipid nanoparticle vaccines against COVID-19 using 3D bioprinting. Nano Today 2022, 43, 101403. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Mukherjee, S.; Tworowski, D.; Detroja, R.; Mukherjee, S.B.; Frenkel-Morgenstern, M. Immunoinformatics and structural analysis for identification of immunodominant epitopes in SARS-CoV-2 as potential vaccine targets. Vaccines 2020, 8, 290. [Google Scholar] [CrossRef]
- Tworowski, D.; Gorohovski, A.; Mukherjee, S.; Carmi, G.; Levy, E.; Detroja, R.; Mukherjee, S.B.; Frenkel-Morgenstern, M. COVID19 Drug Repository: Text-mining the literature in search of putative COVID19 therapeutics. Nucleic Acids Res. 2021, 49, D1113–D1121. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, J.S.; Bilich, T.; Tandler, C.; Nelde, A.; Maringer, Y.; Marconato, M.; Reusch, J.; Jäger, S.; Denk, M.; Richter, M.; et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature 2022, 601, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.A.; van Doremalen, N.; Greaney, A.J.; Andersen, H.; Sharma, A.; Starr, T.N.; Keeffe, J.R.; Fan, C.; Schulz, J.E.; Gnanapragasam, P.N.P.; et al. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).