Persistent Health Issues, Adverse Events, and Effectiveness of Vaccines during the Second Wave of COVID-19: A Cohort Study from a Tertiary Hospital in North India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
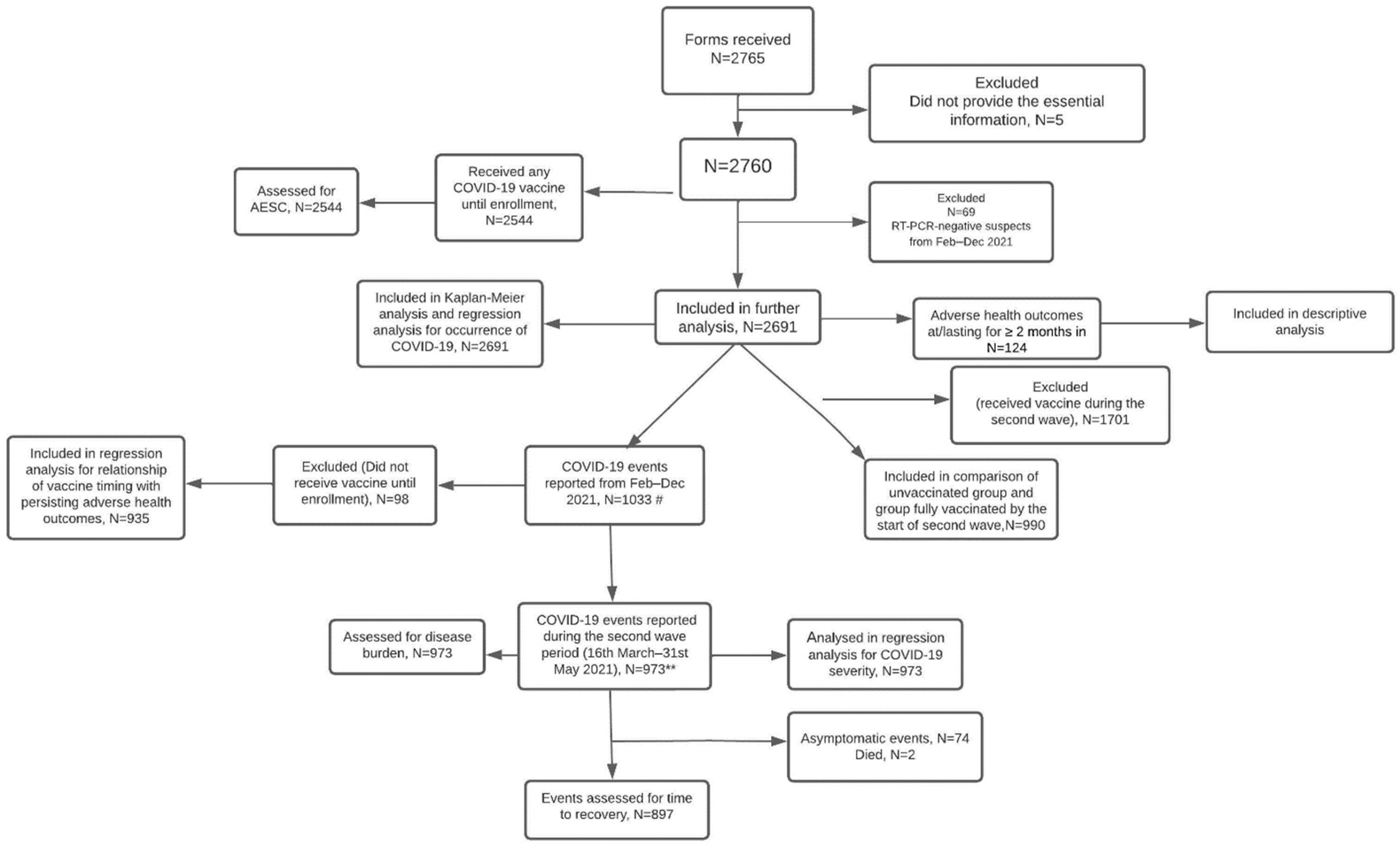
2.2. Study Participants
2.3. Ethical Permission
2.4. Data Sources/Measurement
2.5. Outcome Measures
2.5.1. Primary Outcomes
2.5.2. Secondary Outcomes
- Any serious AEFI;
- Any severe AEFI (FDA grade 3);
- Any moderate–severe AEFI (FDA grade 2–3) which persisted for ≥7 days;
- Any moderate AEFI (FDA grade 2) which persisted for ≥4 weeks;
- Any mild–moderate AEFI (FDA grade 1–2) that persisted for ≥12 weeks.
- Vaccine (post-COVID-19)-associated: If health events were reported in those who received COVID-19 vaccine after recovering from natural SARS-CoV-2 infection in the past;
- COVID-19 (post-vaccine)-associated: If events were reported in those who developed COVID-19 after receiving COVID-19 vaccine;
- COVID-19-associated: If events were reported in unvaccinated individuals who developed COVID-19;
- Vaccine-associated: If events were reported in vaccinated individuals with no history of COVID-19 until date of enrollment.
2.6. Sample Size
2.7. Statistical Analysis
2.8. Role of Funding Source
3. Results
3.1. Descriptive Data
3.2. Main Results
3.2.1. Occurrence of COVID-19
3.2.2. Severity of COVID-19
3.2.3. Persistent Health Issues
3.2.4. Descriptive Data of Persistent Health Issues
3.2.5. Adverse Events of Significant Concern (AESCs)
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Blackwelder, W.; Potdar, V.; Yadav, P.; Sarangi, V.; Aileni, V.K.; Kanungo, S.; Rai, S.; Reddy, P.; et al. Efficacy, Safety, and Lot-to-Lot Immunogenicity of an Inactivated SARS-CoV-2 Vaccine (BBV152): Interim Results of a Randomised, Double-Blind, Controlled, Phase 3 Trial. Lancet 2021, 398, 2173–2184. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca Vaccines on Covid-19 Related Symptoms, Hospital Admissions, and Mortality in Older Adults in England: Test Negative Case-Control Study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Desai, D.; Khan, A.R.; Soneja, M.; Mittal, A.; Naik, S.; Kodan, P.; Mandal, A.; Maher, G.T.; Kumar, R.; Agarwal, A.; et al. Effectiveness of an Inactivated Virus-Based SARS-CoV-2 Vaccine, BBV152, in India: A Test-Negative, Case-Control Study. Lancet. Infect. Dis. 2022, 22, 349–356. [Google Scholar] [CrossRef]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, Risk of Hospital Admission, and Vaccine Effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Dean, N.E.; Hogan, J.W.; Schnitzer, M.E. Covid-19 Vaccine Effectiveness and the Test-Negative Design. N. Engl. J. Med. 2021, 385, 1431–1433. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; Pearce, N. Test-Negative Designs. Epidemiology 2019, 30, 838–844. [Google Scholar] [CrossRef]
- Pilishvili, T.; Gierke, R.; Fleming-Dutra, K.E.; Farrar, J.L.; Mohr, N.M.; Talan, D.A.; Krishnadasan, A.; Harland, K.K.; Smithline, H.A.; Hou, P.C.; et al. Effectiveness of MRNA Covid-19 Vaccine among U.S. Health Care Personnel. N. Engl. J. Med. 2021, 385, e90. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Evaluation of COVID-19 Vaccine Effectiveness; Interim Guidance; WHO: Geneva, Switzerland, 2021; 70p. [Google Scholar]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization with MRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chakrabarti, S.S.; Gambhir, I.S.; Verma, A.; Kumar, I.; Ghosh, S.; Tiwari, A.; Chandan, G.; Chakrabarti, S.; Kaur, U. Acute Cardiac Events after ChAdOx1 NCoV-19 Corona Virus Vaccine. Am. J. Ther. 2022. [Google Scholar] [CrossRef] [PubMed]
- Tagini, F.; Carrel, L.; Fallet, B.; Gachoud, D.; Ribi, C.; Monti, M. Behçet’s-like Adverse Event or Inaugural Behçet’s Disease after SARS-CoV-2 MRNA-1273 Vaccination? Rheumatology 2022, 61, e112–e113. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Ojha, B.; Pathak, B.K.; Singh, A.; Giri, K.R.; Singh, A.; Das, A.; Misra, A.; Yadav, A.K.; Kansal, S.; et al. A Prospective Observational Safety Study on ChAdOx1 NCoV-19 Corona Virus Vaccine (Recombinant) Use in Healthcare Workers- First Results from India. eClinicalMedicine 2021, 38, 101038. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Bala, S.; Ojha, B.; Jaiswal, S.; Kansal, S.; Chakrabarti, S.S. Occurrence of COVID-19 in Priority Groups Receiving ChAdOx1 NCoV-19 Coronavirus Vaccine (Recombinant): A Preliminary Analysis from North India. J. Med. Virol. 2022, 94, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Xu, J.; Xia, H.; Wang, Y.; Zhang, C.; Chen, W.; Zhang, H.; Liu, Q.; Zhu, R.; et al. Comprehensive Investigations Revealed Consistent Pathophysiological Alterations after Vaccination with COVID-19 Vaccines. Cell Discov. 2021, 7, 99. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-Dose Administration and the Influence of the Timing of the Booster Dose on Immunogenicity and Efficacy of ChAdOx1 NCoV-19 (AZD1222) Vaccine: A Pooled Analysis of Four Randomised Trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Victor, P.J.; Mathews, K.P.; Paul, H.; Murugesan, M.; Mammen, J.J. Protective Effect of COVID-19 Vaccine Among Health Care Workers During the Second Wave of the Pandemic in India. Mayo Clin. Proc. 2021, 96, 2493–2494. [Google Scholar] [CrossRef]
- Satwik, R.; Satwik, A.; Katoch, S.; Saluja, S. ChAdOx1 NCoV-19 Effectiveness during an Unprecedented Surge in SARS COV-2 Infections. Eur. J. Intern. Med. 2021, 93, 112–113. [Google Scholar] [CrossRef]
- Kaur, U.; Bala, S.; Ojha, B.; Pathak, B.K.; Joshi, A.; Yadav, A.K.; Singh, A.; Kansal, S. Patterns and Predictors of COVID-19 Occurrence and Severity in Vaccinated Priority Groups. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, X.; Pilia, F.; Ojanguren, I.; Romero-Mesones, C.; Cruz, M.-J. Is Asthma a Risk Factor for COVID-19? Are Phenotypes Important? ERJ Open Res. 2021, 7, 00216–02020. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, X.; Wang, Y.; Zeng, X.; Luo, T.; Liu, Q. Clinical Determinants of the Severity of COVID-19: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0250602. [Google Scholar] [CrossRef]
- Gallè, F.; Sabella, E.A.; Roma, P.; Ferracuti, S.; Da Molin, G.; Diella, G.; Montagna, M.T.; Orsi, G.B.; Liguori, G.; Napoli, C. Knowledge and Lifestyle Behaviors Related to COVID-19 Pandemic in People over 65 Years Old from Southern Italy. Int. J. Environ. Res. Public Health 2021, 18, 10872. [Google Scholar] [CrossRef]


| 1a | 1b | |||||
|---|---|---|---|---|---|---|
| Participants (N = 2691) | COVID-19 Cases, n (%) | p-Value (Effect Size) | COVID-19 Events (N = 973) * | Events of Moderate– Severe Grade | p-Value (Effect Size) | |
| Age (years) | <0.001 (OR 1.5) | |||||
| <40 | 2009 | 773 (38.5) | 777 | 185 (23.8) | 0.35 | |
| ≥40 (reference) | 682 | 196 (28.7) | 196 | 53 (27) | ||
| Sex | <0.001 (OR 1.4) | |||||
| Male (reference) | 1699 | 567 (33.4) | 569 | 134 (23.6) | 0.43 | |
| Female | 992 | 402 (40.5) | 404 | 104 (25.7) | ||
| Body mass index (kg/m2) ** | ||||||
| ≥25 | 1056 | 397 (37.6) | 0.23 | 398 | 106 (26.6) | 0.19 |
| <25 | 1633 | 572 (35) | 575 | 132 (23) | ||
| Diabetes mellitus | ||||||
| Yes | 154 | 45 (29.2) | 0.07 | 45 | 11 (24.4) | 0.99 |
| No | 2537 | 924 (36.4) | 928 | 227 (24.5) | ||
| Hypertension | ||||||
| Yes | 201 | 74 (36.8) | 0.80 | 74 | 18 (24.3) | 0.97 |
| No | 2490 | 895 (35.9) | 899 | 220 (24.5) | ||
| Heart disease | ||||||
| Yes | 28 | 7 (25) | 0.22 | 7 | 3 (42.9) | 0.26 |
| No | 2663 | 962 (36.1) | 966 | 235 (24.3) | ||
| Lung disease | 0.03 (OR 2.3) | |||||
| Yes | 80 | 26 (32.5) | 0.51 | 26 | 11 (42.3) | |
| No (reference) | 2611 | 943 (36.1) | 947 | 227 (24) | ||
| Hypothyroidism | 0.03 (OR 1.5) | |||||
| Yes | 107 | 49 (45.8) | 49 | 15 (30.6) | 0.30 | |
| No (reference) | 2584 | 920 (35.6) | 924 | 223 (24.1) | ||
| Inflammatory arthritis | ||||||
| Yes | 14 | 6 (43) | 0.59 | 6 | 3 (50) | 0.14 |
| No | 2677 | 963 (36) | 967 | 235 (24.3) | ||
| History of allergy | ||||||
| Yes | 342 | 137 (40.1) | 0.09 | 137 | 34 (24.8) | 0.92 |
| No | 2349 | 832 (35.4) | 836 | 204 (24.4) | ||
| Use of RAAS blockers | ||||||
| Yes | 111 | 43 (38.7) | 0.54 | 43 | 10 (23.3) | 0.85 |
| No | 2580 | 926 (36) | 930 | 228 (24.5) | ||
| Prior history of COVID-19 Yes No (reference) | 387 2304 | 104 (26.8) 865 (37.5) | <0.001 (OR 1.6) | 105 868 | 32 (30.5) 206 (23.7) | 0.13 |
| Type of Vaccine (N = 2476) COVISHIELD COVAXIN COVISHIELD/COVAXIN COVAXIN/COVISHIELD Pfizer | 2412 61 1 1 1 | 859 (35.6) 16 (26.2) 1 1 0 | 0.16 | 863 16 - - - | 195 (22.6) 4 (25) - - - | 0.07 |
| Vaccination status (Definition A) 0 dose 1 dose 2 doses | 935 394 1362 | 346 (37) 148 (37.6) 475 (34.9) | 0.45 | 348 149 476 | 113 (32.5) 42 (28.2) 83 (17.4) | <0.001(Cramer’s V 0.16) |
| Vaccine effectiveness (Definition A) 2 versus 0 1 versus 0 | 5.7% −1.6% | 46.4% 13.2% | ||||
| Vaccination status (Definition B) 0 dose 1 dose 2 doses | 759 570 1362 | 262 (34.5) 232 (40.7) 475 (34.9) | 0.03(Cramer’s V 0.05) | 263 234 476 | 90 (34.2) 65 (27.8) 83 (17.4) | <0.001(Cramer’s V 0.17) |
| Vaccine effectiveness (Definition B) 2 versus 0 1 versus 0 | −1.1% −18% | 49.1% 18.7% | ||||
| Pure analysis 0 dose 2 doses (reference) | 500 490 | 227 (45.4) 184 (37.6) | 0.01 (OR 1.4) | 227 184 | 81 (35.7) 32 (17.4) | <0.001 (OR 2.6) |
| Vaccine effectiveness (Pure analysis) 2 versus 0 | 17.2% | 51.2% | ||||
| 2a | 2b | ||||
|---|---|---|---|---|---|
| Tentative Risk Factors (n = 2691) | aHR | p-Value | Tentative Risk Factors (n = 973 COVID-19 Events) | aOR | p-Value |
| Age (years) <40 ≥40 (Reference) | 1.45 (1.23–1.69) | <0.001 | Pre-existing lung disease Yes No (Reference) | 2.54 (1.13–5.7) | 0.02 |
Sex Female Male (Reference) | 1.22 (1.07–1.39) | 0.004 | |||
| Prior history of COVID-19 Yes No (Reference) | 0.66 (0.54–0.81) | <0.001 | Vaccination status ** 2 doses 1 dose 0 dose (Reference) | 0.43 (0.31–0.60) 0.83 (0.54–1.26) | <0.001 0.38 |
Hypothyroidism Yes No (Reference) | 1.34 (0.99–1.8) | 0.055 | |||
| Vaccination status * 2 doses 1 dose 0 dose (Reference) | 1.1 (0.92–1.26) 1.27 (1.10–1.52) | 0.34 0.007 | |||
| Risk Factor | COVID-19 Events | Persistent Adverse Health Outcomes, N (%) | p-Value (Effect Size) |
|---|---|---|---|
| Age (years) | 0.04 (OR 1.6) | ||
| <40 (reference) | 740 | 69 (9.3) | |
| ≥40 | 195 | 28 (14.4) | |
| Sex | 0.12 | ||
| Male | 560 | 51 (9.1) | |
| Female | 375 | 46 (12.3) | |
| Body mass index (kg/m2) | 0.56 | ||
| ≥25 | 382 | 37 (9.7) | |
| <25 | 553 | 60 (10.8) | |
| Diabetes mellitus | 0.04 (OR 2.2) | ||
| Yes | 46 | 9 (19.6) | |
| No (reference) | 889 | 88 (9.9) | |
| Hypertension | 0.69 | ||
| Yes | 68 | 8 (11.8) | |
| No | 867 | 89 (10.3) | |
| Heart disease | 0.40 | ||
| Yes | 6 | 0 (0) | |
| No | 929 | 97 (10.4) | |
| Lung disease | 0.22 | ||
| Yes | 29 | 5 (17.2) | |
| No | 906 | 92 (10.2) | |
| Hypothyroidism | <0.001 (OR 4.8) | ||
| Yes | 49 | 16 (32.7) | |
| No (reference) | 886 | 81 (9.1) | |
| Inflammatory arthritis | 0.004 (OR 26.7) | ||
| Yes | 4 | 3 (75) | |
| No (reference) | 931 | 94 (10.1) | |
| History of allergy | 0.008 (OR 2) | ||
| Yes | 137 | 23 (16.8) | |
| No (reference) | 798 | 74 (9.3) | |
| Vaccine received after COVID-19 recovery | <0.001 (OR 2.5) | ||
| Yes | 331 | 54 (16.3) | |
| No (reference) | 604 | 43 (7.1) | |
| Type of Vaccine | 0.21 | ||
| COVISHIELD | 916 | 94 (10.3) | |
| COVAXIN | 17 | 2 (11.8) | |
| COVISHIELD/COVAXIN | 2 | 1 (50) |
| Tentative Risk Factor | Adjusted Odds Ratio | p-Value |
|---|---|---|
| Vaccination after COVID-19 Yes No (reference) | 2.8 (1.8–4.4) | <0.001 |
| Age (years) <40 ≥40 (reference) | 0.7 (0.4–1.1) | 0.14 |
| Diabetes mellitus Yes No (reference) | 1.1 (0.4–2.7) | 0.83 |
| Inflammatory arthritis Yes No (reference) | 30 (3–304) | 0.004 |
| History of allergy Yes No (reference) | 2 (1.2–3.4) | 0.01 |
| Hypothyroidism Yes No (reference) | 5.3 (2.6–10.5) | <0.001 |
| Age/Sex | Comorbidity | Type of Vaccine | Time of AEFI since COVID-19 Vaccine | Description of AEFI | Outcome | Causality |
|---|---|---|---|---|---|---|
| 29 years/Female | History of allergy, Polycystic ovarian disease | COVAXIN | Within 24 h of first dose | Fever, severe vomiting, and diarrhoea within 24 h of first dose, requiring hospitalization | Recovered in 5 days | Probable |
| 37 years/Female | Hypothyroidism | COVISHIELD | Within 24 h of first dose | Tingling, dizziness, palpitations, heaviness in chest, tachycardia, and fluctuating blood pressure. On admission, blood pressure 150/80 mm Hg, heart rate 130/min, remaining vitals stable and routine blood investigations including cardiac enzymes were normal. | Recovered in 4 days | Possible |
| 38 years/Female | Diabetes mellitus | COVISHIELD | Within three months of second dose | Miscarriage | NA | Possible |
| 32 years/Female | -- | COVISHIELD | Within 24 h of first dose | Abdominal distress and severe diarrhoea requiring emergency room visit | Recovered in 5 days | Possible |
| 45 years/Male | Diabetes mellitus, hypertension, obesity | COVISHIELD | Within 8–10 weeks of first dose | Cardiac arrest | Died, NA | Unlikely |
| 39 years/Female | -- | COVISHIELD | Within 24 h of second dose | Rashes, breathlessness, drowsiness, hypophonia, tachycardia, mild headache. Rash also followed first vaccine dose. | Recovered fully in 3–4 days | Probable |
| 47 years/Female | Hypothyroidism, hypertension, old lung cyst, RT-PCR positive for SARS-CoV-2 three and a half months before vaccination | COVISHIELD | Within seven days of first dose | Fever, nausea, chest pain, dyspnoea, palpitation, difficulty in talking, increased erythrocyte sedimentation rate, eosinophilia (6.2%). Routine kidney and liver function tests were normal. Cardiac enzymes done after 1 week of symptom onset normal, 2D Echocardiography normal. Cardiac magnetic resonance imaging was suggestive of myocarditis. | Recovered in 30 days | Probable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, U.; Bala, S.; Joshi, A.; Reddy, N.T.S.; Japur, C.; Chauhan, M.; Pedapanga, N.; Kumar, S.; Mukherjee, A.; Mishra, V.; et al. Persistent Health Issues, Adverse Events, and Effectiveness of Vaccines during the Second Wave of COVID-19: A Cohort Study from a Tertiary Hospital in North India. Vaccines 2022, 10, 1153. https://doi.org/10.3390/vaccines10071153
Kaur U, Bala S, Joshi A, Reddy NTS, Japur C, Chauhan M, Pedapanga N, Kumar S, Mukherjee A, Mishra V, et al. Persistent Health Issues, Adverse Events, and Effectiveness of Vaccines during the Second Wave of COVID-19: A Cohort Study from a Tertiary Hospital in North India. Vaccines. 2022; 10(7):1153. https://doi.org/10.3390/vaccines10071153
Chicago/Turabian StyleKaur, Upinder, Sapna Bala, Aditi Joshi, Noti Taruni Srija Reddy, Chetan Japur, Mayank Chauhan, Nikitha Pedapanga, Shubham Kumar, Anurup Mukherjee, Vaibhav Mishra, and et al. 2022. "Persistent Health Issues, Adverse Events, and Effectiveness of Vaccines during the Second Wave of COVID-19: A Cohort Study from a Tertiary Hospital in North India" Vaccines 10, no. 7: 1153. https://doi.org/10.3390/vaccines10071153
APA StyleKaur, U., Bala, S., Joshi, A., Reddy, N. T. S., Japur, C., Chauhan, M., Pedapanga, N., Kumar, S., Mukherjee, A., Mishra, V., Talda, D., Singh, R., Gupta, R. K., Yadav, A. K., Rana, P. J., Srivastava, J., Bhat K., S., Singh, A., P.G., N. K., ... Chakrabarti, S. S. (2022). Persistent Health Issues, Adverse Events, and Effectiveness of Vaccines during the Second Wave of COVID-19: A Cohort Study from a Tertiary Hospital in North India. Vaccines, 10(7), 1153. https://doi.org/10.3390/vaccines10071153






