Abstract
COVID-19, caused by SARS-CoV-2, is one of the longest viral pandemics in the history of mankind, which have caused millions of deaths globally and induced severe deformities in the survivals. For instance, fibrosis and cavities in the infected lungs of COVID-19 are some of the complications observed in infected patients post COVID-19 recovery. These health abnormalities, including is multiple organ failure—the most striking pathological features of COVID-19—have been linked with diverse distribution of ACE2 receptor. Additionally, several health complications reports were reported after administration of COVID-19 vaccines in healthy individuals, but clinical or molecular pathways causing such complications are not yet studied in detail. Thus, the present systematic review established the comparison of health complication noted in vaccinated and non-vaccinated individuals (COVID-19 infected patients) to identify the association between vaccination and the multiorgan failure based on the data obtained from case studies, research articles, clinical trials/Cohort based studies and review articles published between 2020–2022. This review also includes the biological rationale behind the COVID-19 infection and its subsequent symptoms and effects including multiorgan failure. In addition, multisystem inflammatory syndrome (MIS) has been informed in individuals post vaccination that resulted in multiorgan failure but, no direct correlation of vaccination with MIS has been established. Similarly, hemophagocytic lymphohistiocytosis (HLH) also noted to cause multiorgan failure in some individuals following full vaccination. Furthermore, severe complications were recorded in elderly patients (+40 years of age), indicates that older age individuals are higher risk by COVID-19 and post vaccination, but available literature is not sufficient to comply with any conclusive statements on relationship between vaccination and multiorgan failure.
1. Introduction
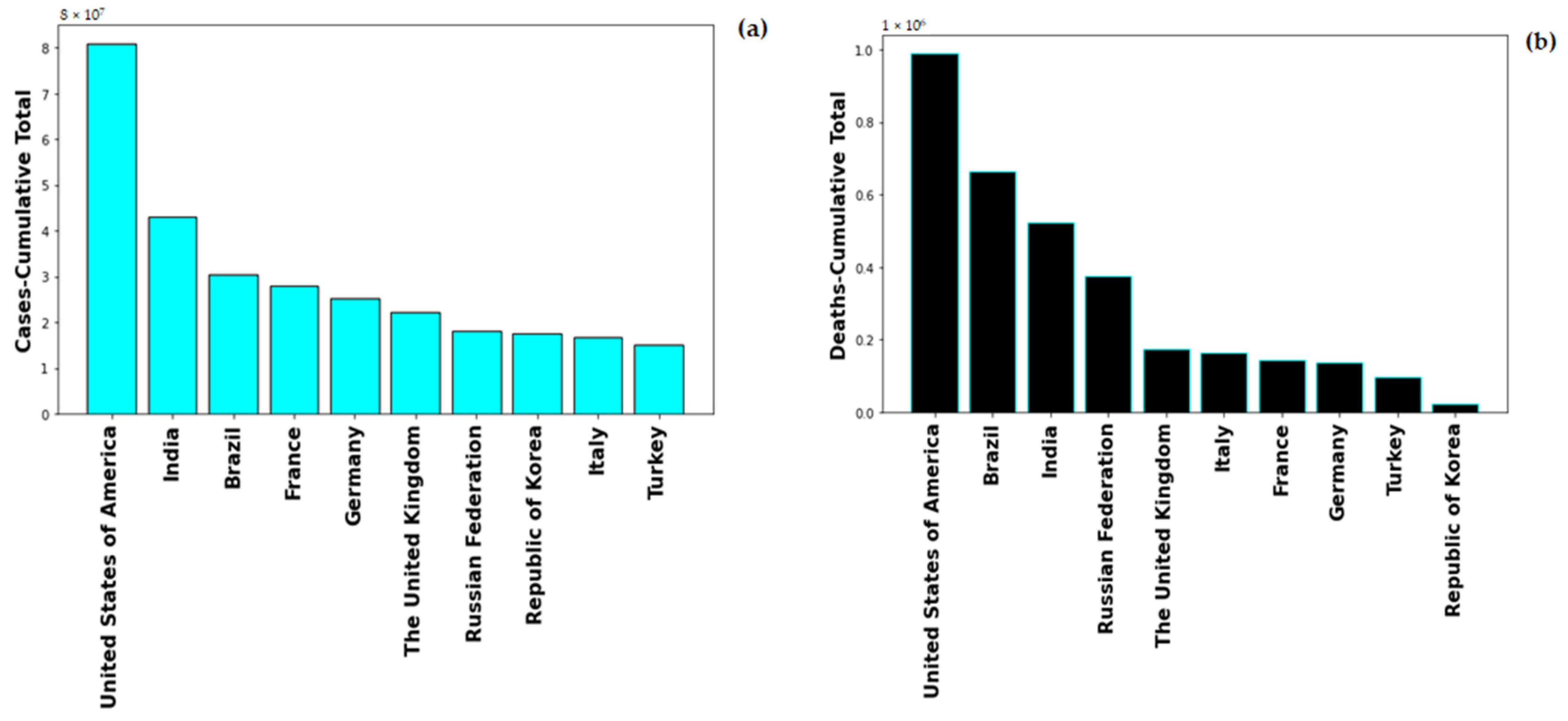
Global healthcare system was severely alarmed by the rate of Coronavirus-19 infection, on 1st May 2022, the global number of the novel Coronavirus-19 (nCOVID-19) infected patients stands at 513,348,944 with number of deaths at 6,260,762. The highest number of COVID-19 cases are in USA with 83,066,907 and also maximum number of COVID-19 induced deaths, i.e., 1,030,833. Figure 1 shows the cumulative cases and deaths for top 10 countries globally. United States of America has maximum number reported both in number of cases and number of deaths. The pandemic spread in multiple were lethal for numerous people and rendering others with severe deformities. The severity of COVID-19 enhances with the presence of co-morbidity factors such as diabetes, hypertension, immunocompromised conditions.
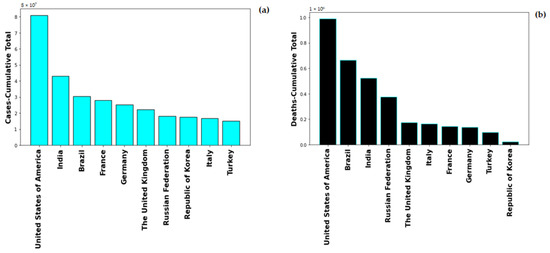
Figure 1.
Total Number of (a) cases and (b) deaths for top 10 countries globally.
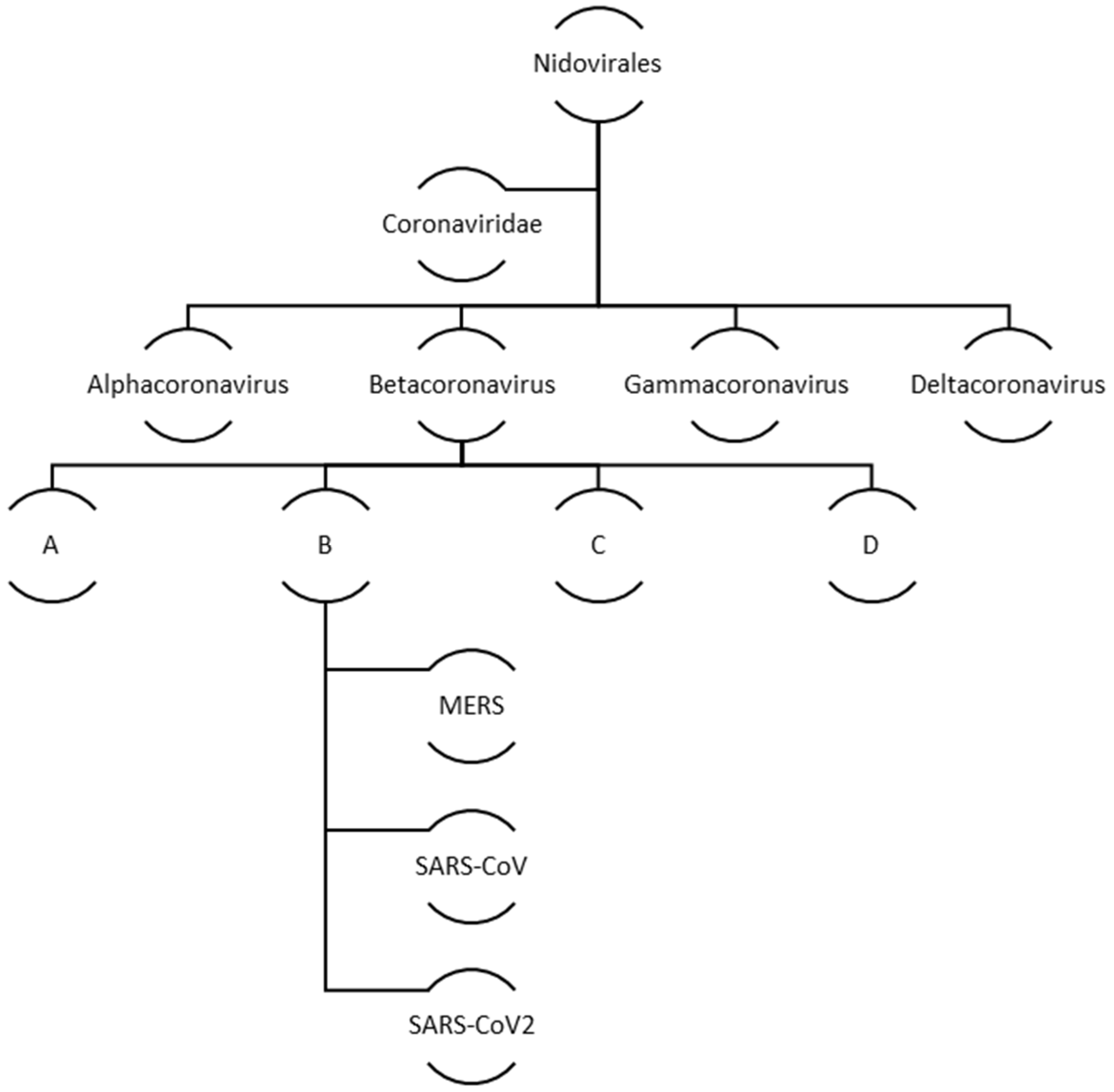
Coronaviruses belong to a big family of viruses, Coronaviridae that comprises of enveloped single stranded RNA viruses. They can infect human, bats, pangolin and other mammals, birds, livestock and even pets. There are four genera within the Coronaviridae family: α-Coronaviruses, β-Coronaviruses, γ-Coronaviruses, and δ-Coronaviruses. While the first two genera exclusively infect mammals the latter two have a diverse range of host species that also include birds. In case of humans and other animals, coronaviruses infections mainly manifest as the respiratory and enteric diseases. Figure 2 illustrates the family and evolution of corona virus.
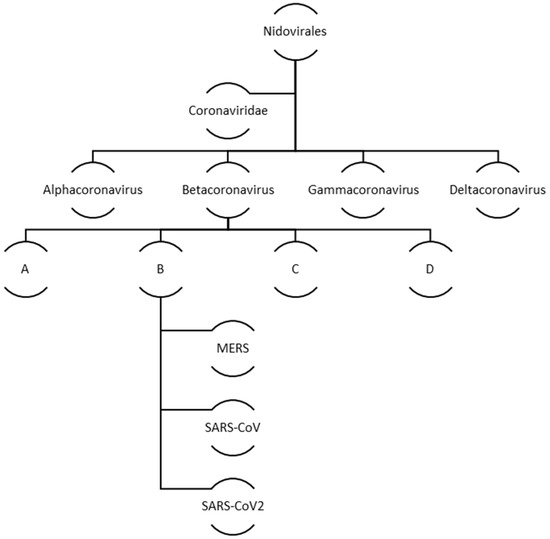
Figure 2.
Evolution and classification of human coronavirus.
Most of the COVID-19 patients are mainly asymptomatic or display mild symptoms. The older age group and especially those suffering from other forms of illnesses, such as lung or heart diseases, are at greater possibility of developing the acute form of COVID-19 infections. The severe forms of the disease can also be observed in healthy younger individuals lacking any health disorder. In case of children, most of the COVID-19 patients either does not display any symptoms or show symptoms of milder magnitude. However, recent studies suggest the development of multisystem inflammatory syndrome thus significantly increasing the mortality rates [1,2,3]. The major symptoms displayed by the patients infected by SARS-CoV-2 are fever, dry cough, fatigue, dyspnea [4]. The cells of many organs possess the ACE2 receptor beyond which SARS-CoV-2 may spread within the host body causing multiple organ failure [5].
Although, various vaccine candidates have been approved by WHO and used in COVID-19 patients, the occurrence of multi-organ failure have still been reported by several case studies. This review discusses extensively, the co-relation between vaccination status of COVID-19 patients and occurrences of multi organ failure, which has not been covered elsewhere. This co-relation study shall help the researchers to study the long-term effects of COVID-19 vaccines on the virus infected and non-infected individuals.
2. Methods
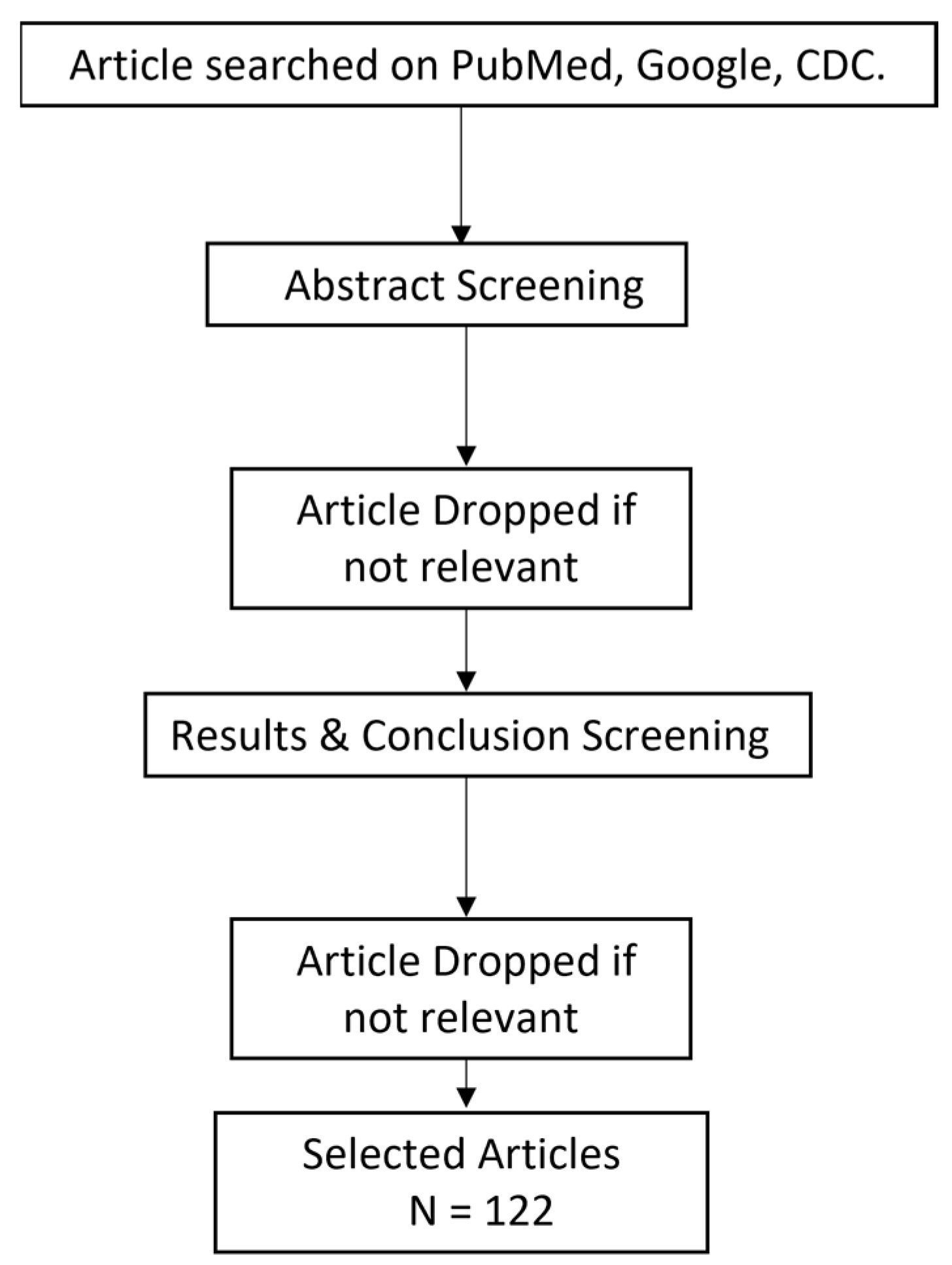
This metareview includes the data obtained from 38 research articles, 16 review articles, 32 case studies and 46 clinical trials/cohort-based studies conducted between 2020–2022 in various countries. Flowchart of articles selected is shown in Figure 3.
Figure 3.
Flowchart for the selection of articles.
The number of patients from cohort-based studies which displayed multi organ complications from vaccinated and non-vaccinated groups were used for further evaluations to draw the correlations. Official WHO web site https://covid19.who.int/ (accessed on 25 April 2022) was used for data collection on number of cases and vaccination. ‘R’ statistical package was used for the data analysis and plotting bar and stem plots. Images were drawn using power point Microsoft application and license free images web sources (https://www.pngwing.com/ (accessed on 25 April 2022)). Central disease control and prevention database is used to search for the case studies at https://wwwnc.cdc.gov/ (accessed on 25 April 2022) URL. Data collection in this study used following inclusion and exclusion criteria.
Inclusion Criteria: Original research work, review on post COVID and post vaccination, Case series, Case studies, cross-sectional studies
Exclusion Criteria: News articles, un-authorized reports, and web articles.
3. Viral Pathogenesis
3.1. Genomic Architecture
Coronaviruses are named so due to their crownlike exterior owing to the occurrence of spike shaped glycoproteins on the viral envelope and belongs to the family of B—Coronaviruses [6]. The genome of COVID-19 is made up of single strand RNA, nearly 30 kb in length (sense strand) [7]. Two dominant open reading frames (ORFs) namely 1a and 1b constitutes approximately 70% of the viral genome. These ORFs codes for numerous conserved protein sequences with distinct functions such as replication of virus and evasion of host innate immune responses [8,9]. In the midst of all the coronavirus genome sequences, the genome of COVID-19 shares maximum similarity with two other coronaviruses, the bat coronavirus (96%) and the pangolin coronavirus (91%) [10,11].
3.2. Structural Elements
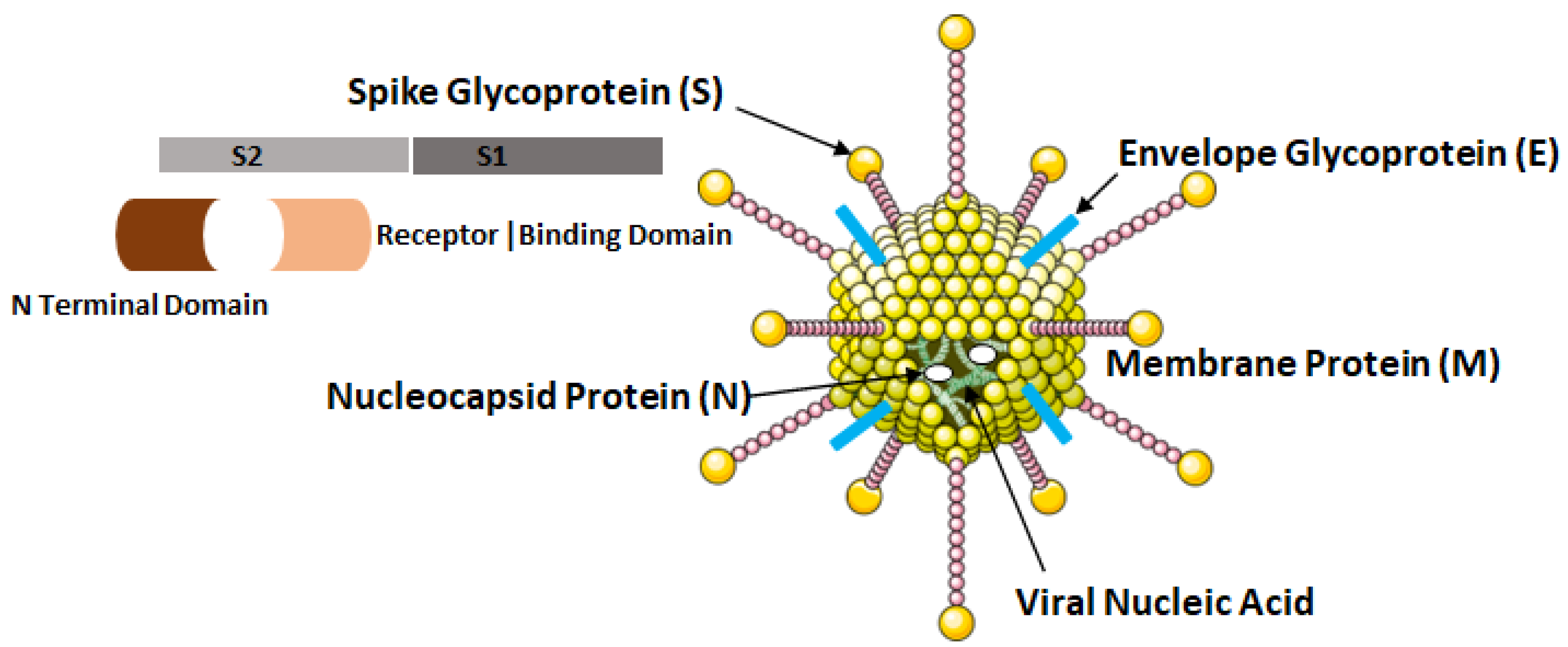
The COVID-19 virus possesses four major structural proteins, namely the spike glycoproteins, matrix glycoproteins, envelope glycoproteins and the nucleocapsid proteins as shown in Figure 4. All of these proteins can induce the immune response inside the hosts [12].
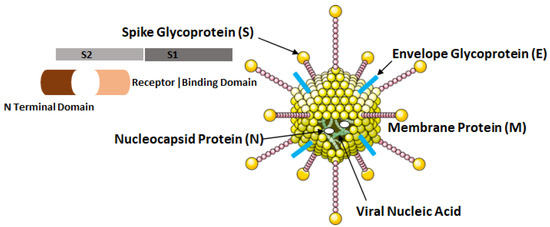
Figure 4.
Structural elements of COVID-19 virus, i.e., SARS-CoV-2.
The nucleocapsid proteins are phosphorylated that enables them to bind to viral nucleic acid and carry out essential functions during the life cycle of the virus. The glycoprotein on viral envelope is a membrane integral protein which makes up the significant part of the viral structure. These proteins play essential role during assembly of the viral particles by cross linking with other structural components of the virus [13]. The viral spike glycoprotein spans the host membrane and is present in the outermost layer of the virus. The two subunits, S1 and S2 which makes up these proteins are processed by the host proteases. The S1 subunit consisting of two domains: (a) N terminal and (b) Receptor Binding Domain (RBD) influences the cellular tropism of the virus. After the fusion of viral envelope with host membrane, the S1 subunit is dissociated that induces a change in the conformation of S2 subunit which then act as an anchor for the viral membrane [8].
3.3. Host Cell Entry
The SARS-CoV-2 virus enter the host cells using ACE2 (Angiotensin Converting Enzyme-2) which is a carboxypeptidase and maintains the balance of fluid and electrolytes essential for the proper functioning of multiple organs in the body. The binding affinity of ACE2 and the spike protein influences the severity and the outcome of COVID-19 infection. ACE-2 converts angiotensin-I (ang-I) to angiotensin-II (ang-II) which in turn can interact with the Angiotensin Type-1 Receptor to induce pro-inflammatory and fibrotic response in the host body [14,15]. The same ACE-2 is also involved in the conversion of ang-II to ang-I and further to ang. The Ang-I interacts with Mas receptors and reverses the downstream effects induced by ang-II. However, the affinity of ACE2 for ang-I is the highest, thus, favoring its conversion to ang-II [16]. In addition to ACE2, other receptors involved in the cellular entry of coronaviruses are Human Aminopeptidase N (APN) for Human Coronavirus 229E and Dipeptidyl Peptidase 4 in case of Middle East Respiratory Syndrome Related Coronaviruses [17].
3.4. Mechanism of Viral Replication
The RBD domain of COVID-19 binds to ACE2 to enter into the host cells. This process is aided by the host cell surface TMPRSS2 (Transmembrane Serine Protease 2), cathepsin L and cathepsin B [18,19,20]. These interactions then induce the uptake of ACE2 receptor along with COVID-19 virus through the process of endocytosis that further led to the combination of the viral membrane with host cell. The intracellular COVID-19 then reduces the levels of ACE2 both at the mRNA and protein levels [21]. Inside the endosomes, the spike protein of the virus becomes cleaved by the cysteine proteases causing its cleavage to dissociate S1 subunit initially and then to expose the fusion peptide within the S2 subunit [21]. This event releases the packaged virus into cell cytoplasm to produce more progeny virions using the host cellular machinery. The viral genome consisting of a single stranded copy of RNA also act as a messenger RNA to direct the host ribosomes towards the synthesis of viral proteins. The naked RNA genome of the virus codes for polypeptides 1a and 1b which are then cleaved to form the non-structural proteins. These polypeptides then interact with viral genome to form the sub genomic viral particles that translate structural proteins [22]. Gordon et al., detected 332 protein–protein interactions (high confidence) between the COVID-19 and humans which were involved in virus life cycle [23]. Upon the production of all the viral proteins and assembled in the ER-Golgi compartments, the progeny virions are released through the process of exocytosis outside the host cell [22,24].
4. COVID-19 Induced Pathophysiological Complications
Pathophysiological changes or complications refer to altered physiological changes occurring in diseased state and its impact on the mortality and disease outcome. COVID-19 has been shown to affect multiple organs and systems in human body such as respiratory, cardiovascular, hematological, gastrointestinal, urogenital and central nervous system. This multi organ involvement and damage is due to abundance of ACE2 surface protein, a major route of virus entry on the cells of these organs/systems. These pathophysiological alterations involving multiple organs and systems are enlisted below.
4.1. Multiple Organ Injury
Even though many aspects of SARS-CoV-2 virus-host interactions are unclear, some of them are shown to mimic the pathogenesis of other human coronaviruses. Five primary pathogenic processes underpin COVID-19 genesis and progression are: cytotoxicity induced by the virus directly into ACE2-expressing cells, RAAS dysregulation as a byproduct of virus-facilitated ACE2 downregulation, immunological dysregulation, endothelial cell damage and thrombosis, and tissue fibrosis [25]. The lungs have various characteristics that make them ideal for serving as a preliminary reservoir for viral transmission and replication from person to person. Due to high surface area of lung, it is more vulnerable to viruses absorbed through the air. It’s also well-vascularized, allowing virus particles to spread quickly to nearby organs. Additionally, ACE2 is expressed in a variety of lung cell types with alveolar epithelial type II cells displaying the highest abundance [26,27]. Various viral functional genes, such as those involved in the genome replication are expressed by alveolar epithelial type II cells, according to gene ontology enrichment study [27]. The generation and exocytosis releasing new viral progeny can cause pyroptosis. The host cell responds to the viral infection through the recognition of damage-associated molecular pattern (DAMP) signals. DAMP are also identified by nearby epithelial as well as endothelial cells in lung tissue, which further activates the transcription factors, interferon regulatory factor 3 (IRF3) and nuclear factor-B (NF-B), causing production and secretion of pro-inflammatory mediators [6]. Concurrently, type I interferons are secreted, which have antiviral effects through a variety of pathways. Antigen-presenting cells (APCs), such as resident tissue macrophages and dendritic cells, identify these first inflammatory signals and deliver the antigen to TH cells, which subsequently prime other TH cells, TC cells, and B-cells in an immunocompetent reaction [28]. B-cells generate neutralizing antibodies against the spike protein and nucleocapsid, whereas TC are cytotoxic and destroy host cells infected with virus immediately. Phagocytes eliminate apoptotic cells and viruses that have been neutralized, results in a well-coordinated infection elimination with little lung tissue harm [6,28]. A defective immune response, on the other hand, leads to increased inflammation, ineffective viral removal, hyperactivation of innate immune system and increased production of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, tumor necrosis factor (TNF), etc. The alveolar-capillary membrane is disrupted by this unmanaged inflammatory process, and viruses with the subsequent cytokine storm, can subsequently move to other body sites, leading to multi-organ failure [29]. In patients with acute COVID-19, histological investigation of lung cells revealed neutrophil migration into the alveolar space as well as acute capillaritis [30,31,32]. The initiation of the coagulation system, neutrophil extracellular traps (NETs), and leakage of tissue fluid in the sub-endothelial region may all be linked to the inflammatory reaction caused by the host-virus interaction in severe COVID-19 patients [30,33,34,35]. Individual heterogeneity in expression of ACE2, TMPRSS2 receptors, as well as genetic elements linked to inflammatory and immunological responses, might explain COVID-19’s diverse clinical symptoms [36,37,38,39]. As a result, patients with severe COVID-19 may experience increasing acute inflammatory reactions, which can lead to MODS and mortality [40,41]. The acute inflammatory responses leads to cytokine storm with significantly elevated levels of proinflammatory mediators, such as IL-1β, IL-2, IL-6, IL-7, IL8, TNF-α, interferon induced protein 10, monocyte chemoattractant protein 1, granulocyte colony-stimulating factor, macrophage inflammatory protein-1, platelet-derived growth factor, vascular endothelial growth factor [29,42,43,44,45]. This storm is pronounced in patients hospitalized to the intensive care unit, revealed by higher blood concentrations of various pro-inflammatory mediators, suggesting a link between illness severity and serum concentrations [29,43,44,45]. In acute COVID-19, abnormal CD4+ TH cells co-expressing IFN- and G-CFS are also observed [46]. A higher proportion of neutrophils over lymphocytes ratio is linked to death in the hospitalized COVID-19 patients [47]. Patients with severe infection, however, may develop lymphopenia, which is defined by a large decrease in peripheral CD4+ as well as CD8+ T-cells. These counterbalancing responses could hinder SARS-CoV-2 from being completely cleared and may lead to the development of secondary infections likely to result in a sepsis-like condition [43,45,48].
Recently, a cohort-based study was conducted to assess the multi organ health in 443 non hospitalized German individuals with a previous mild to moderate COVID-19 infection compared to healthy uninfected controls. The assessment of pulmonary, cardiovascular, urogenital, and neurological systems was carried out, and the outcome was examined carefully. The assessment of cardiac system revealed enhanced levels of cardiac biomarkers along with lowered ventricular function. Ultrasonography revealed the presence of deep vein thrombosis indicated by the less compressible nature of femur veins. In addition, lower glomerular filtration rate and the increased mean thickness of brain cortex was observed. Other parameters such as brain volume and cognitive functions were non-impaired [49].
4.2. Gastrointestinal System
COVID-19 infected women are reported to illicit a stronger T-cell response than males, which may lead to more effective viral clearance [50]. The presence of stomach discomfort, nausea, diarrhea, and vomiting in a significant number of COVID-19 patients indicates virus induced gastrointestinal dysfunction [41,43,48,51,52]. ACE2 is abundant on the luminal surface of intestinal epithelial cells and functions as a co-receptor towards the amino acid absorption [26,53]. The nucleocapsid protein of COVID-19 is detected in various gastrointestinal regions [54,55] as well as in stool samples [56,57,58], suggesting that oral-fecal transmission is possible. Histopathological study of COVID-19 subjects revealed inflammation of the intestinal endothelial lining and mesenteric ischemia [34]. Whereas gastrointestinal symptoms have not been linked to a higher risk of death, they do appear to be linked to a longer disease duration [59,60].
Furthermore, increased levels of transaminases, overall bilirubin [41,43,51], and glutamyl transferase were found in multiple people with COVID-19 [56,61]. A series of COVID-19 patients showed modest lymphocyte infiltration, sinusoidal dilatation, uneven liver necrosis at autopsy [62,63]. Additional case series revealed Kupffer cell growth and persistent hepatic obstruction [64]. Cholangiocytes are vulnerable to direct virus-induced cytotoxicity because they express ACE2 [65]. However, it’s unclear if COVID-19’s liver impairment is caused by SARS-CoV-2 infection, inflammation, medication, or a combination of factors. The liver’s function should be examined on a regular basis since most medications under consideration as COVID-19 treatments are metabolized there.
4.3. Circulatory System
Increased D-dimer levels and extended prothrombin duration, and overt thrombotic symptoms, have been reported in a large percentage of COVID-19 patients [33,35,41,43,51]. The viral RNA is found in blood samples obtained from COVID-19 patients [43,58]. ACE2 is expressed in smooth muscles and endothelium lining the blood arteries and veins in many organs, SARS-CoV-2 may specifically attack these tissues, causing endothelial dysfunction [26,34,42]. Additionally, high synthesis of pro-inflammatory mediators results in an imbalance among pro- and anti-coagulant factors, as well as platelet aggregation [35,66]. Alongside NET generation, a rise in tissue factor V and VIII, thrombin, and fibrinogen levels resulting in a hypercoagulation state and an elevated risk of systemic macro- and micro-thrombosis [30,35,66,67]. Fibrous exudates and thrombi are observed in tissue specimens obtained from COVID-19 patients [5,42,62]. Severe COVID-19 has been linked to a higher risk of cardiovascular complications, including a higher rate of clinical heart disease symptoms (increased heart rate), increased levels of cardiac biomarker levels, and irregularities in EEG [41,43,68]. The majority of COVID-19 patients had cardiomegaly, right ventricular dilatation [31], and moderate fibrosis [62], according to postmortem findings. Since ACE2 is abundantly expressed in cardiomyocytes, the heart is vulnerable to SARS-CoV-2–mediated organ dysfunction [26,34]. Pericytes, however, produce even larger amounts of ACE2 compared to cardiomyocytes, and hence can become attacked by the virus, resulting in capillary endothelial dysfunction [9,52]. Heterozygosis with respect to ACE2 activity reduction is proved to be enough to enhance the chances of heart disease [69]. Although the significance of circulating soluble ACE2 is unknown, it is considerably elevated in case of cardiovascular dysfunction due to COVID-19 infection, suggesting that it might be employed as a biomarker [63,69,70]. Nevertheless, cytokine storm may cause direct myocardial damage when combined with a hypoxic condition caused by pulmonary failure. In COVID-19, initial acute myocardial damage is linked to a greater risk of deaths. COVID-19 may increase cardiac tissue damage and dysfunction, therefore already existing cardiovascular disorders are linked to a poorer prognosis [44,48,71]. It is advised that heart function and standard biomarkers be monitored.
4.4. Respiratory System
Since, ACE2 is present in numerous cell types along the respiratory tract, lung is without a doubt is the most sensitive and impacted by COVID-19 infection [58,72]. In chronic cases, acute pneumonia is the most prevalent and significant clinical symptom [43,46]. The virus may infiltrate through mucosal membranes of upper respiratory tract or immediately infect bronchial as well as alveolar epithelial cells in the lower respiratory tract since ACE2 is present in numerous cell types along the respiratory tract [58,72]. Fascinatingly, nasal ACE2 expression is decreased in children compared to adults which explains age-related changes of COVID-19 infection [73,74]. The viral RNA is found in the sputum of infected patients even before the onset of clinical symptoms [75], and even 2 weeks after remission [58,76]. In comparison to non-smokers and healthy patients, people with chronic obstructive pulmonary disease, current and former smokers, overexpress ACE2 in airway cells, which might underlie at least part of the elevated chances of severe infection in these people [74,77,78]. In both the SARS-CoV and SARS-CoV-2 contagion, ACE2 serves similar to a double-edged sword: it works as a receptor for virus entry into host cells and protects lung from damage by counterbalancing the vasoconstrictive and pro-fibrotic effects of Ang II on pulmonary vascular and epithelial cells [79,80]. Virus-ACE2 internalization [81] or cleavage and shedding of ACE2 [63,82] reduces its expression while enhancing Ang II buildup, synthesis of the receptors for TNF-α and IL-6, and stimulation of M1 macrophages (pro-inflammatory state) [16,82,83,84]. Moreover, the viral capsid proteins may combine with Smad3 to inhibit the death of infected host cells, inducing tissue fibrosis mediated by transforming growth factor (TGF)-β [85]. Hyperinflammation and fibrosis were reported in critical COVID19 patients where galectin-3 was proposed as potential biomarker [86]. Similarly, suppression of INFß while higher level of IL-1α and TGF-ß were also proposed as biomarker for COVID patients with acute lung fibrosis [87]. The self-renewing alveolar epithelial type II cells respond to high ACE2 amounts which can be repeatedly targeted for viral entry, leading to a cycle of tissue injury and repair resulting in the replacement of gas-exchange areas with fibrotic lesions. ACE2 is expressed in lung progenitor cells. Therefore, viral replication in them can perturb lung tissue healing [88]. A genome-wide associated study discovered novel host gene variables that may lead to the improvement of respiratory dysfunction induced by COVID-19. SNPs detected in six genes (SLC6A20, ZTFL1, CCR9, FYCO1, CXCR6, and XCR1) are thought discovered to be potential significance [39]. Additionally, the ABO blood type has been linked to SARS and COVID-19 vulnerability [27,39,89]. ARDS, SARS, and COVID-19 all exhibit lung histopathology findings that are very similar [5,62,63]. Elevated counts of neutrophils and mononuclear cells scattered alveolar damage with proteinaceous exudates, and epithelial cell hyperplasia are among the symptoms. In other situations, giant cells with multiple nuclei have been discovered in alveoli [5,62,63]. Despite of the usage of anticoagulation agents, an increase of deep venous thrombosis was observed in COVID-19 cases [33,90]; this condition may worsen further leading to the respiratory failure. Impairment of lungs caused by COVID-19 can be classified into three phenotypes based on chest computed tomography results: (1) focal, multiple, potently over perfused opacities; (2) non-homogeneously diffused atelectasis; and (3) moderate/severe ARDS, alveolar edema and reduced complain [52]. Since these traits are linked to various pathogenic processes and illness development, individualized mechanical ventilation systems should be used to facilitate an effective recovery for every individual.
4.5. Urogenital Tract
To maintain normal kidney function, fine balance between the activities of ACE2/Ang (1–7) as well as Ang II must be maintained. Since ACE2 is found in kidney cells, especially at the border of proximal cells, they are vulnerable towards SARS-CoV-2 invasion [26,91]. Proteinuria, hematuria, elevated serum creatinine along with blood urea nitrogen levels have all been seen in people with COVID-19 in laboratory testing [92,93,94]. On computed tomography, anomalies in the kidneys indicative of inflammation and edema were also seen [92]. The severe kidney damage in COVID-19 patients is linked to a much greater danger (approximately 5 times higher) of in-hospital death than those without acute kidney injury [41,44,51,92,94]. Dialysis is required in a significant proportion of COVID-19 patients with acute renal damage [93]. During autopsy, viral antigens are discovered in urine as well as tissue specimens from kidney, mainly the tubular epithelial cells and podocytes [60,95,96]; histopathological study showed tubular damage in addition to reduced brush border integrity, vacuolar degeneration as well as necrosis [96]. There has also been evidence of erythrocyte aggregation in capillary lumen without the involvement of fibrous debris [96]. Despite the possibility of direct viral invasion of kidney, inflammatory response and antiviral treatment might cause nephrotoxicity; hence, biomarkers for renal health as well as the balance of fluid and electrolyte should be continuously managed for the reduction in the extent of kidney damage. It is also observed that males with COVID-19 have a greater case fatality rate, regardless of age [97]. This may be due to the observation that testicular and vas deferens cells express substantially more ACE2 than ovaries [91,98,99], which might help explain disparities in disease severity and, as a result, mortality. Additionally, genes located on the sex-chromosome along with the sex hormones may decide the gender-specific control of immune responses in COVID-19 patients [100,101]. The X chromosome possess a large repertoire of immune system related genes, including ACE2. This functional mosaic may have an impact on innate and adaptive immune systems, as well as the severity of illness [101].
4.6. Hematological Disorders
The impacts of COVID-19 on blood were first reported from patients in China. However, the exact molecular pathway remains to be elucidated. One of the clinical manifestations of the COVID-19 thought to enhance the rate of mortalities is Venous thromboembolism (VTE). Autopsy samples from COVID-19 patients reveals the widespread occurrences of microthrombi not only in blood vessels associated with lungs but also with many other organs. These findings highlight the essentiality of thromboprophylaxis towards the reduction in the COVID-19 severity especially in ICU patients. In the patients who died of COVID-19, the levels of lymphopenia, coagulation abnormalities and thrombocytopenia are highly enhanced.
The pulmonary VTE causes sudden increase in the arterial CO2 pressure causing the increase in dead space ventilation. A study conducted in the New York involving 3334 COVID-19 patients correlates the direct association between VTE and the increased mortality rate [102]. The COVID-19 patients also show hypercoagulation however bleeding was observed to be less common which makes it different from the Disseminated Intravascular Coagulation (DIC). In case of acute DIC cases, bleeding is predominant along with low levels of fibrinogen and platelets while in chronic cases, levels of fibrinogen are elevated. In case of COVID-19, a mildly decreased levels of platelets induces the state of hypercoagulation [103].
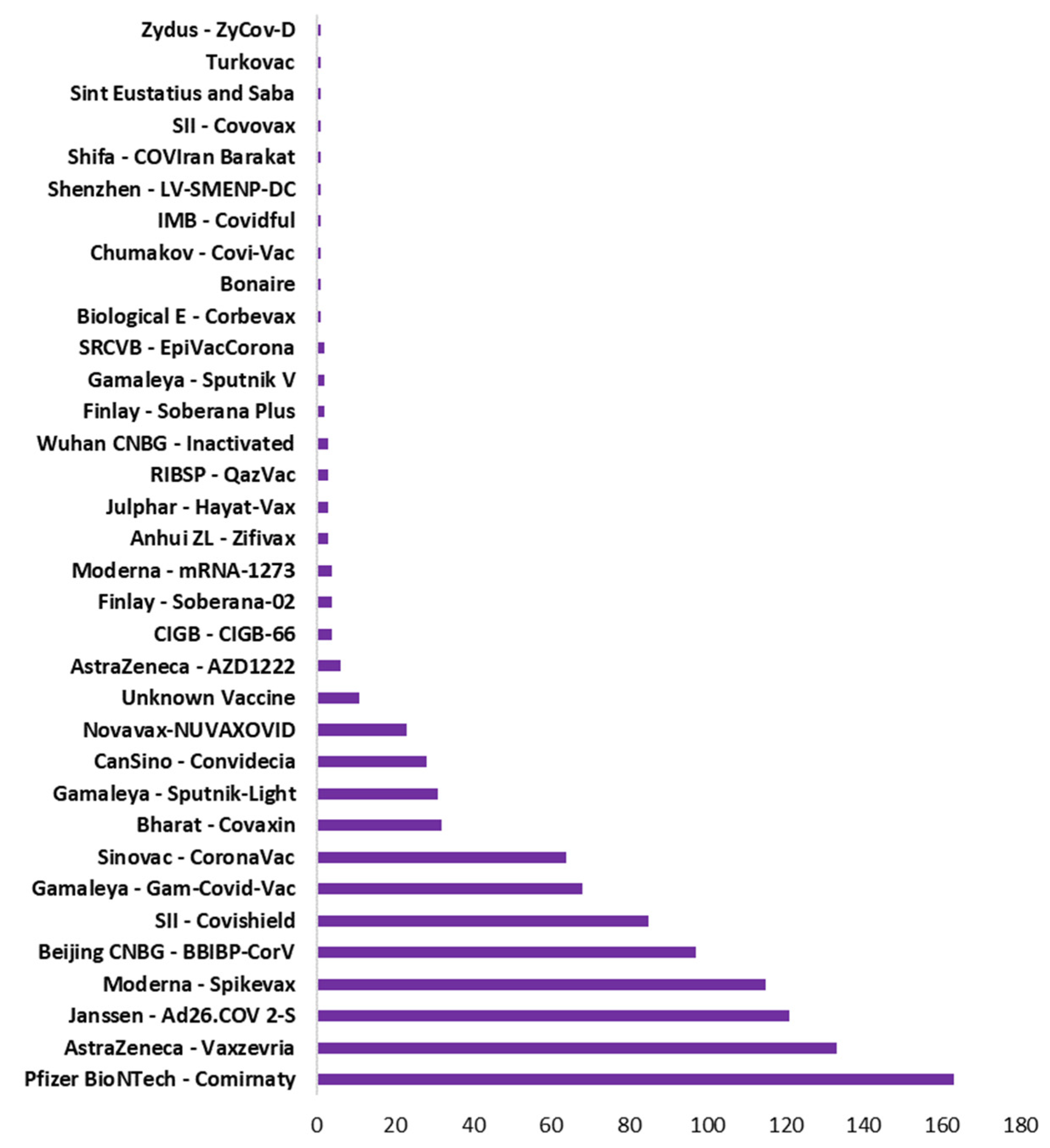
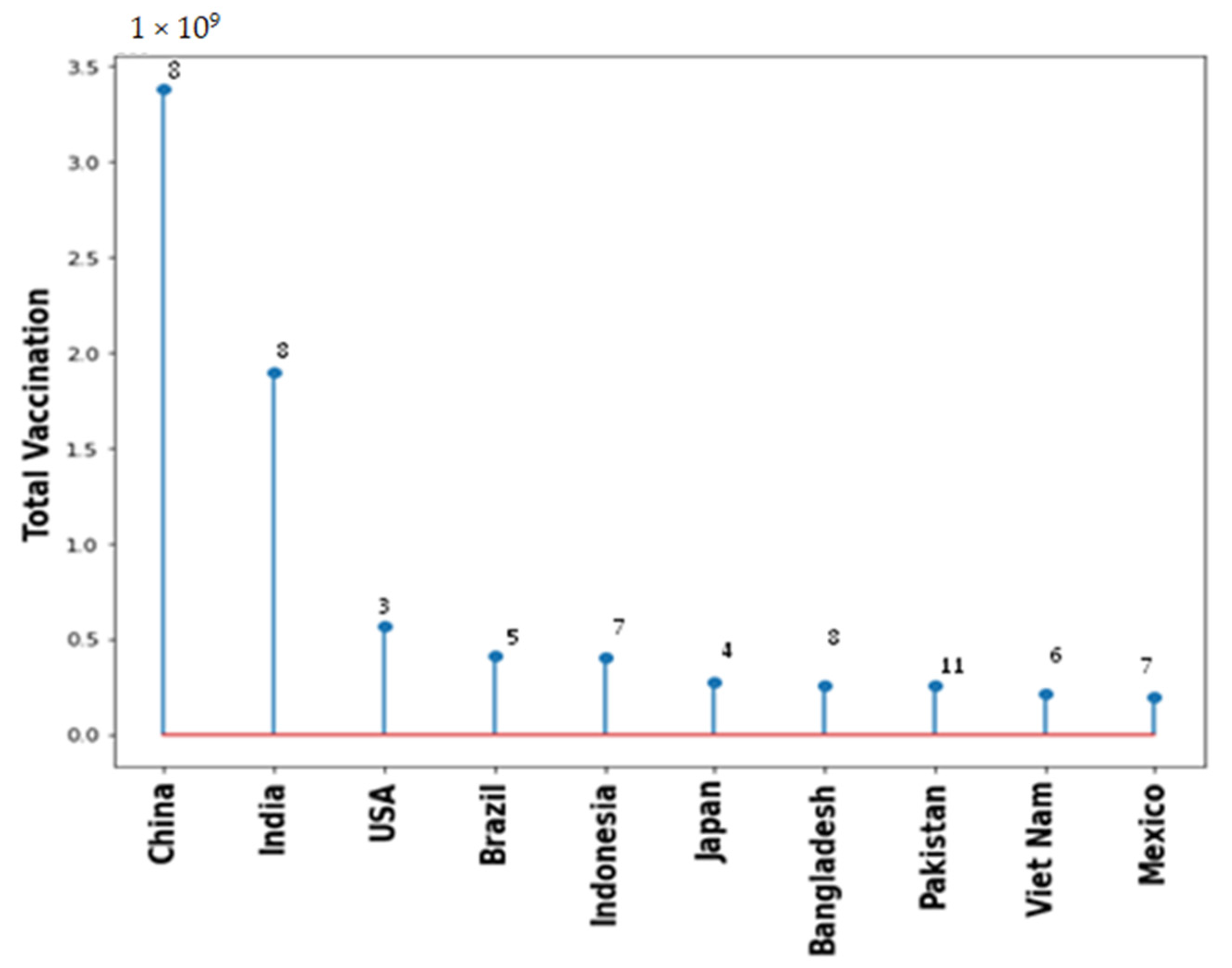
4.7. Systemic Health Complications Post COVID-19 Vaccination
Vaccination drive had been rapidly growing across all countries. Figure 5 shows the type of vaccine and number of countries used that vaccine. Pfizer BioNTech has been used maximally in 163 countries. However, AstraZeneca, Janssen and Moderna also used in 100+ countries. Figure 6 shows the top 10 countries based on total number vaccine dose on total population. China, India, and USA are the leaders in vaccination drive. India and China both used 8 different vaccines while USA used 3, as shown in Figure 6. The COVID-19 vaccines developed by the Pfizer and Moderna relies on the use of a combination of mRNA and nanoparticles for the efficient delivery of vaccines [104,105]. While, the vaccines developed by AstraZeneca, Gam-COVID-Vac and Johnson and Johnson relies on the recombinant DNA carried in a non-replicating adenovirus vector [106,107]. Both of these vaccine types produce the spike protein of SARS-CoV-2 against which neutralizing antibodies are produced within host body. The results obtained from the phase III clinical trials revealed that both the Pfizer as well as Moderna mRNA vaccines gave about 90–95% protection against COVID-19. Whereas the vaccines containing recombinant adenoviral vector exhibited a slightly lower degree of protection (70% and 91%, respectively). Both the vaccine types induce the generation of significant levels of neutralizing antibodies as well as the virus specific T cells detectable in the patient’s blood within 2–4 weeks of the administration [108,109,110]. These results were obtained from the clinical trials involving more than 100,000 volunteers from the global population. Although, the data obtained from the clinical trials ensures the safety of COVID-19 vaccines, numerous case studies have described serious complications with the involvement of multiple organs such as heart, brain and vascular system after vaccine administration [111,112,113,114,115,116,117,118,119,120].
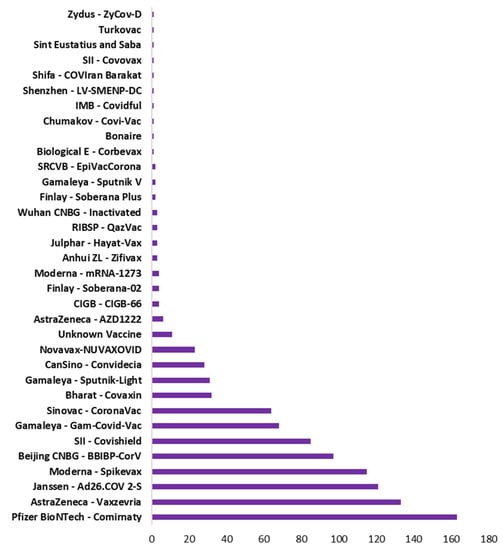
Figure 5.
Type of vaccine with total number of countries used that vaccine.
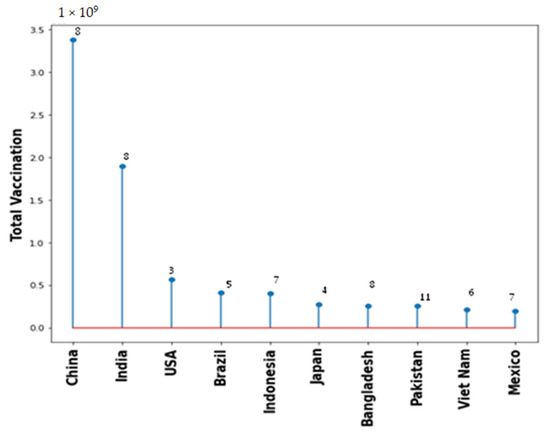
Figure 6.
Total vaccination for the top 10 countries with number of types of different vaccine used.
4.8. Cardiac Complications
Various case reports showed the induction of myocarditis post administration of COVID-19 mRNA vaccines. These are categorized by the Center for Disease Control and Prevention as acute myocarditis, confirmed myocarditis and probable myocarditis [120]. The data obtained from CDC confirmed the likelihood of myopericarditis in younger males which becomes predominant following the second mRNA vaccine dose compared to the first. Various theories have been proposed to understand the mechanism behind this association; (1) erratic activation of innate immune response by the mRNA of vaccine, (2) similarity between COVID-19 spike protein encoded by the mRNA vaccine and the cardiac proteins; (3) generation of hyperimmune response due to induction of high antibody titers, and (4) autoimmune response by the vaccine induced antibodies in patients with compromised cardiac health [121,122,123,124].
4.9. Myopathies
In COVID-19 patients severe damage and loss of skeletal muscles has been reported which may lead to a severe form of myopathy further complicating the disease outcome. Although, the mechanism underlying this condition is not revealed yet, it is believed that the virus induced cytokine storm causes the damage to muscle cells [125]. The condition of rhabdomyolysis which is a life threatening condition caused by muscle breakdown and death has been detected several days post the onset of COVID-19 symptoms [126]. This conditions correlates well with levels of the inflammatory markers [127].
Rhabdomyolysis has been previously observed as a rare case of complication due to vaccination in case of the influenza A H1N1 vaccine as well as a recombinant zoster vaccine [128]. Recently, severe case of rhabdomyolysis was observed in a young COVID-19 patient post administration of AstraZeneca COVID vaccine. Patient also had a deficiency of carnitine palmitoyltransferase II [129]. Another such case was observed in a male patient of 21 years having the asthma history who have received the first dose of Pfizer vaccine [130]. In a latest case, the patient administered with 2 doses of Pfizer mRNA vaccine developed anti-neutrophil cytoplasmic antibody associated vasculitis as well as the Pauci-Immune Crescentic Glomerulonephritis. However, the patient recovered post appropriate treatment [131].
4.10. Hematological Complications
In a few patients who received the recombinant adenoviral vector vaccine (chAdOx1-nCov-19) encoding for the viral spike protein developed the life-threatening conditions of thrombosis and thrombocytopenia. It was thought to be due to the induction of antibody response against the platelet factor 4 by the vaccine [132,133]. Other more common and less adverse side effects include headache, diarrhea, arthralgia, and fever which are generally self-limiting.
In the year, 2021 a case study was reported of an old man (68 years) with an extremely severe attack of rhabdomyolysis 9-day post administration of first vaccine dose. This patient displayed multiple organ failure with damage to the liver, kidney, lung and bone marrow. The presence of elevated inflammatory markers was an indicative of the cytokine storm. The patient was administered with the immune-suppressive therapy targeted against multiple organs consisting of steroids, anakinra and eculizumab. Although the patient responded to the treatment indicated by the restored levels of the creatine phosphokinase but eventually he succumbed to the accelerated pace of deterioration and died after 48 days of vaccination [134].
Recently, administration of adenoviral vector COVID-19 vaccine has shown to induce coagulopathy in patients [135]. Additionally, two cases of cerebral venous sinus thrombosis were reported probably due to the autoantibodies against the platelet factor-4 indicating a probable immunological disorder [136].
Due to several recent reports, the development of a new syndrome following the administration of mRNA vaccine has been put forward termed as ‘Vaccine Induced Immune Thrombocytopenia’ [137,138,139,140,141]. A recent autopsy report from 58-year-old patient post 13 days of his first dose of viral vector vaccine indicated the presence of above-mentioned syndrome. The patient displayed multiple microthrombi in the heart, lung, kidney and choroid plexus [136]. The aggravation of immune thrombocytopenic purpura has also been reported post administration of mRNA vaccines. Scottish database shows the probable association between the viral vector vaccine and thrombocytopenic purpura [142,143].
4.11. Multisystem Inflammatory Syndrome (MIS)
The BNT162b2 mRNA vaccine produced by the Pfizer-BioNTech showed high efficiency with less side effects in clinical studies. In a case study reported in April 2021, a young male (20 year) displayed an early onset of nausea, non-bloody emesis, diffuse myalgias, abdominal pain on the day of vaccine administration. The next day, he showed signs of fatigue and pustules on the chest, face and back. After 48 h post vaccination, the patient exhibited hematuria as well as dysuria. The patient has no history of COVID-19 infection, was not on any medications or recreational drugs as well as no background of allergies. The patient received vaccinations in the childhood without any allergic responses. His two siblings also received both doses of BNT162b2 vaccines without any health complications [144]. Laboratory evaluations revealed the presence of systemic inflammatory response such as elevated levels of erythrocyte sedimentation rate, lactate dehydrogenase, C-reactive protein. Cytokine evaluation depicted high levels of IL-6, IL-10, IL-13. Blood examination revealed lymphopenia, enhanced granulocytes. The patient showed symptoms of systemic inflammation, acute hearing loss, pericardial effusion, progressive kidney injury, systemic capillary leak, multifocal ischemic strokes.
4.12. Hematophagocytic Lymphohistiocytosis (HLH)
Another critical complication caused by COVID-19 vaccine is the hemophagocytic lymphohistiocytosis (HLH) observed in a 43-year-old farmer from China. HLH is a life threatening hyper-inflammatory condition induced due to the erratic activation of macrophages and TC lymphocytes that can potentially lead to multi-organ failure. The patient developed vomiting, malaise and consistent high fever immediately post administration of the inactivated SARS-CoV-2 vaccine (first dose). The preliminary body examination indicated the presence of pancytopenia, increased levels of triglyceride, decreased fibrinogen levels, elevated levels of serum ferritin, low cytotoxicity levels of Natural Killer (NK) cells and the presence of the DNA from Epstein-Barr Virus (EBV) and bone marrow hemophagocytosis. In this patient, the immune response induced by COVID-19 vaccine caused the development of HLH with a background of the chronic EBV infection. The mentioned study highlights the importance of screening for the presence of chronic EBV or other viral infection before the vaccine administration [145].
4.13. Neurological Complications
Towards the end of 2020, several reports indicating the development of neurological complications post COVID-19 vaccination started emerging. The first report of two patients who developed transverse myelitis upon receiving viral vector vaccine. Till now, out of the 9442 adverse events post immunization with mRNA vaccines, 254 were showing neurological complications [114]. In Mexico, a nationwide study involving 704,003 patients who received first dose of BNT162b2 mRNA was undertaken to evaluate the adverse events. It was observed that from a total of 33 adverse events, 17 showed neurological complications such as seizures, Guillain-Barre Syndrome, exacerbation of lumbar radiculopathy, acute transverse myelitis. Whereas the proportion of non-serious neurological complications were about 600 from a total of 100,000 vaccine administered patients [146]. The most common side effects observed after COVID-19 vaccination are myalgia (60%) and headache (68%) [147].
5. Vaccination Impact on Long Term COVID
Long-term COVID-19 incidence is minimized by vaccination, which prevents people from being infected in the first instance. The injections may help guard against the disease by reducing the amount of time the virus has complete freedom in the body. Bar-Ilan University in Safed, Israel conducted a test on 3000 people and surveyed their long term COVID-19 condition that is the major indicator for the organ failure at the later stage. This study showed that vaccinated participants had 54% less chances of headaches, 64% less probability for fatigue and 68% less likely for the muscle pain [148].
6. Gender Based Difference of Vaccination
There is a limited amount of gender-specific studies of the efficacy and adverse effects of the COVID19 vaccination. Baden et al. reported that vaccine efficacy was similar on male and female [149] while Bignucolo et al. showed higher efficacy on male population compared to female population [150]. Despite the minimal risk of infection in the reproductive system, vaccination has been linked to pregnancy difficulties in various studies [151,152,153,154,155,156,157,158,159,160,161]. Omeish et al., 2021 presented a cross-sectional study that showed gender-related differences in adverse effects after vaccination [162]. In this study, there was a significant difference between male (85.8%) and female (92.3%) participants’ side effects following the first vaccine dosage, however this difference was not significant following the second dose. Johnson and Johnson vaccine also reported for its rare blood-clotting side effect that has affected predominantly female recipients. However, the published findings were insufficiently conclusive, suggesting the need for more gender-specific research on diverse geographic populations to determine the post-vaccination adverse effects on male and female populations.
7. Vaccination and Multiorgan Failure: Case Studies
7.1. CS-1
One fatal incidence of MIS-A (multi system inflammatory syndrome in adults) occurred after a complete COVID-19 vaccination in a patient who had a prior spontaneous SARS-CoV-2 infection detected 6 weeks before the beginning of MIS-A symptoms. Cases of MIS-A comprised three days of fever, laboratory evidence of inflammation, neurologic and mucocutaneous clinical symptoms, and severe cardiac disease that included systemic hypotension advancing to cardiogenic shock. Capillaritis and multiorgan microvascular thrombosis histopathologic findings in conjunction with clinical symptoms and laboratory results are consistent with MIS-A. On physical examination, significant blood loss may suggest a diffuse intravascular coagulation–type image in which diffuse micro thrombosis depleted platelets and clotting factors. Although significant cardiac impairment in the context of large fluid volumes and intraperitoneal hemorrhage may have led to multiorgan failure, an etiology of clinical deterioration was likely multifaceted [163].
7.2. CS-2
This study presents the first instance of HLH in a healthy person upon receiving COVID-19 vaccination. After first dose of the virus inactivated vaccine, a Chinese farmer (43 year) had lethargy, nausea, and a prolonged high fever. Pancytopenia, elevated lipid, and reduced fibrinogen were observed during the initial examination. Further testing revealed high blood levels of ferritin levels, decreased NK cell mediated cytotoxicity, and the presence of EBV DNA. Hemophagocytosis was detected in the bone marrow. As a result, HLH was verified. HLH is a potentially fatal hyperinflammatory illness characterized by abnormally activated macrophages and cytotoxic T lymphocytes that can lead to multiple organ failure. According to the findings, it is critical to rule out the existence of active or subclinical EBV infection (or other common viruses) before administering these vaccines [145].
7.3. CS-3
In this instance, a White lady (48 year) arrived at the emergency room with complaint of 3-day long malaise and stomach discomfort. A first assessment at another hospital revealed slight anemia and significant thrombocytopenia. The peripheral blood smear revealed a significant decrease in the count of platelets, with the occasional presence of schistocytes. Further studies revealed reduced fibrinogen levels in addition to the prolonged activated partial thromboplastin time and a significant increase in d-dimer, indicating disseminated intravascular coagulation. The viral RNA was not found using RT-PCR test from the nasopharyngeal swab sample. Following the report of newer headache onset, CT scan indicated the presence of cerebral venous sinus thrombosis. Even after the administration of unfractionated heparin, patient still had increasing levels of microthrombi with hemorrhagic stroke as observed using the magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) of the brain. Another round of angiography revealed some new thrombi in hepatic and splenic veins. Furthermore, the investigation also revealed the administration of Ad26.COV2. S vaccine 14 days before the beginning of onset [164].
7.4. CS-4
On 19 April 2021, a healthy old man (68 year) with no risk factors received the first dose of adenoviral vaccines. He denied any further drug assumption. Since the patient was concerned about adverse effects associated to the surgery, blood tests were conducted 15 days before the vaccination, and it was confirmed to be normal. He was taken to the emergency hospital after 9 days due to dyspnea, acute malaise, severe stomach discomfort, myalgia, and difficulties ambulating. Even though the patient was receiving steroid therapy, his body temperature remained at 36.2 °C and did not display fever. During clinical assessment, patient had a mild clouding of the sensory system, as well as weakness in all four limbs but no significant localized neurological abnormalities. Tests performed within a few hours of symptoms onset showed increased serum levels of alanine aminotransferase, aspartate aminotransferase, creatine phosphokinase, creatinine, blood urea nitrogen, lactic acid. Inflammatory markers were high, and there was a quick and substantial rise in CK and myoglobin. The patient was moved to the critical care unit due to the fast advancement of multi-organ failure. Other typical causes of rhabdomyolysis were ruled out, including trauma, vigorous activity, recent surgery, alcohol use, toxins, autoimmune illnesses, and malignancies [134].
7.5. CS-5
This research examined clinical characteristics of 11 individuals in Europe (Austria and Germany) who developed thrombosis or thrombocytopenia after receiving adenoviral vector-based vaccine. To detect the presence of antibodies against platelet factor 4 (PF4)-heparin, ELISA was used, and for the detection of platelet-activating antibodies, a modified (PF4-enhanced) platelet-activation test was used. Here, 9 individuals showed cerebral venous thrombosis, 3 displayed splanchnic-vein thrombosis, 3 had pulmonary embolism, and 4 had various thromboses. Out of these 11 individuals, 6 patients died. In addition, 5 individuals were diagnosed with disseminated intravascular coagulation. Heparin was not administered to any patient before the onset of symptoms. All individuals who were positive for anti-PF4–heparin also showed the platelet activation. In 2 individuals, further tests with PF4–heparin antibodies revealed PF4-dependent platelet activation. This substantiates Immune thrombotic thrombocytopenia can occur in the uncommon case of ChAdOx1 nCov-19 vaccination [132].
8. Vaccinated and Non-Vaccinated
It is now widely accepted that vaccinated people are at lower risk compared to non-vaccinated individuals. A study at Bar-Ilan University, Israel suggested that dose of vaccine (Pfizer-BioNTech) produced high scale safety from long term COVID syndrome than the unvaccinated individuals. However, the study is not yet been peer reviewed [165]. Another study compared the health complications and several different parameters on vaccinated and unvaccinated individuals. This study showed that asymptomatic infection was found on 18.2% of the first dose vaccinated individuals while it was 12% to the corresponding unvaccinated population. In contrast to asymptomatic infection, hospitalization was higher on unvaccinated population by 6.8%. Similarly, more than five disease symptoms were reported in 22.2% of vaccinated population while this number went to 31.4% on unvaccinated population [166]. Another study from Israel, 5526 unvaccinated population data infected from COVID was collected and found that 4.6 per 100,000 were hospitalized while it was 0.3 per 100,000 individuals on vaccinated population. However, for vaccinated population only 596 cases were available. Similarly, the data of death cases was 0.6 per 100,000 on unvaccinated population and 0.1 per 100,000 on vaccinated cases [167].
A recent study conducted from June 2021 to January 2022 where 4,079,234 individuals (age group 12–17) were administered with mRNA vaccine. Multisystemic inflammatory syndrome (MIS-C) were reported in 9 cases that could directly relate with the multi organ dysfunction. Out of these 9 cases, 8 were male and 3 had evidence for past SARS-CoV2 infection. The reporting rate of MIS-C was 1.1 per million doses of vaccine while it was 113 on the same age group infected by SARS-CoV-2. This study is still not peer-reviewed but showed the prevalence of MIS-C cases following mRNA vaccine in children [168].
9. Conclusions
After the detection of the first case of COVID-19, several complications ranging from mild to severe associated with the viral pathogenesis have been reported. Many of these complications resulted in the multiple organ dysfunction and failure. Due to the emergency of developing an effective therapeutic regime, several researchers tried their hands on creating COVID-19 vaccines which included the use of viral mRNA, envelope glycoproteins, DNA etc. The rapidly increasing number of deaths or severe deformities in survived patients literally forced the healthcare systems across the globe to test the efficacy of these vaccine candidates in clinical trials. Following this, these vaccines were made available to the governments of various countries without testing for their long-term complications. This assurgency created explicit usage of vaccines in different age groups many of which showed serious complications leading to multiple organ failure. Thus, although COVID-19 vaccines have been instrumental in bringing down the rate of mortality and disease transmission, various moderate to serious complications continue to emerge from various parts of the globe eventually leading to multi organ failure. This situation demands the immediate attention and in-depth studies to evaluate the immediate and long-term complications of various vaccine types in people from different age group and also various approaches for the betterment of the existing vaccines. Case studies are reported where the major complications including multiorgan failure have emerged after the vaccination. However, there is no study to establish strong correlation between vaccination and multiorgan failure. The biggest case series was performed in Austria where 11 patients were reported for thrombosis or thrombocytopenia after vaccination leading to multiorgan failure. Multiple organs, including the heart, lungs, kidneys, lymphocytes, testes, nervous system, and liver, are reportedly equipped with ACE-2 receptors for SARS-CoV2. Consequently, SARS-CoV2 can infect these organs by adhering to ACE-2 receptor-bearing cells. Lung fibrosis, which was found to be prevalent in severe COVID patients, might indirectly impact the damage to other organs. However, direct correlation between the fibrosis and observation of SARS-CoV2 RNA is still debatable. Dormant form of viruses in these fibrous tissues are also not reported. This concludes, in SARS-CoV2 infection, fibrosis may indirectly cause damage to other organs via affecting oxygen supply, cytokine storm, dysregulated immunological responses, coagulation failure, and infiltration of inflammatory cells.
Overall, this review presents a detailed study on COVID-19 infection and its complications including multi organ failure in vaccinated and non-vaccinated individuals. Moreover, current available data is not sufficiently large to draw any strong conclusion on instituting any relation between vaccination and multiorgan failure due to small sample size and lack of evidence-based reports. Moreover, the biological and pathological reasons behind these major complications are still not completely understood and more future studies on this aspect would provide greater details.
Author Contributions
Conceptualization, A.A.R., M.A.B., A.A.M., S.A. (Saad Alhumaid) and H.A.; methodology, A.A.R., J.A. and S.A. (Salma AlBahrani); resources, J.M.A.-J., M.G., M.A., Y.N.A., S.A.A. and H.N.A.; data curation, H.A., A.A. and M.A.; writing—original draft preparation, A.A.R., A.A.M., M.A., M.G., W.A.A., A.A.A., Y.N.A. and H.N.A.; writing—review and editing, A.A.R., A.A.M., S.A. (Sultan Alotaibi), K.H. and M.-H.T.; supervision, A.A.R., S.A. (Saad Alhumaid); project administration, A.A.R, M.-H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors want to thank King Abdulaziz City for Science and Technology (KACST) for support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Godfred-Cato, S.; Bryant, B.; Leung, J.; Oster, M.E.; Conklin, L.; Abrams, J.; Roguski, K.; Wallace, B.; Prezzato, E.; Koumans, E.H.; et al. COVID-19–Associated Multisystem Inflammatory Syndrome in Children—United States, March–July 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Dufort, E.M.; Koumans, E.H.; Chow, E.J.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Toubiana, J.; Poirault, C.; Corsia, A.; Bajolle, F.; Fourgeaud, J.; Angoulvant, F.; Debray, A.; Basmaci, R.; Salvador, E.; Biscardi, S.; et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: Prospective observational study. BMJ 2020, 369, m2094. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE2. Circ. Res. 2020, 128, 1323–1326. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, X.; Wei, X.; Li, J.; Yang, J.; Tan, H.; Zhu, J.; Zhang, Q.; Wu, J.; Liu, L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 2020, 92, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020, 116, 1097–1100. [Google Scholar] [CrossRef] [Green Version]
- Lam, T.T.-Y.; Jia, N.; Zhang, Y.-W.; Shum, M.H.-H.; Jiang, J.-F.; Zhu, H.-C.; Tong, Y.-G.; Shi, Y.-X.; Ni, X.-B.; Liao, Y.-S.; et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danser, A.H.J.; Epstein, M.; Batlle, D. Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension 2020, 75, 1382–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nagata, N.; Shirato, K.; Kawase, M.; Takeda, M.; Taguchi, F. Efficient Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein by the Transmembrane Protease TMPRSS2. J. Virol. 2010, 84, 12658–12664. [Google Scholar] [CrossRef] [Green Version]
- Wrapp, D.; De Vlieger, D.; Corbett, K.S.; Torres, G.M.; Wang, N.; Van Breedam, W.; Roose, K.; van Schie, L.; Hoffmann, M.; Pöhlmann, S.; et al. Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell 2020, 181, 1436–1441. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 202, 756–759. [Google Scholar] [CrossRef]
- Channappanavar, R.; Zhao, J.; Perlman, S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014, 59, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Wang, G.-Q. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Brown, J.Q.; Heide, R.S.V. Pulmonary and Cardiac Pathology in COVID-19: The First Autopsy Series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Tian, L.; Qiang, T.; Liang, C.; Ren, X.; Jia, M.; Zhang, J.; Li, J.; Wan, M.; YuWen, X.; Li, H.; et al. RNA-dependent RNA polymerase (RdRp) inhibitors: The current landscape and repurposing for the COVID-19 pandemic. Eur. J. Med. Chem. 2021, 213, 113201. [Google Scholar] [CrossRef] [PubMed]
- CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis); Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Elhabyan, A.; Elyaacoub, S.; Sanad, E.; Abukhadra, A.; Elhabyan, A.; Dinu, V. The role of host genetics in susceptibility to severe viral infections in humans and insights into host genetics of severe COVID-19: A systematic review. Virus Res. 2020, 289, 198163. [Google Scholar] [CrossRef]
- Russo, R.; Andolfo, I.; Lasorsa, V.A.; Iolascon, A.; Capasso, M. Genetic Analysis of the Coronavirus SARS-CoV-2 Host Protease TMPRSS2 in Different Populations. Front. Genet. 2020, 11, 872. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Huang, H.; Li, D.; Gu, D.; Lu, X.; Zhang, Z.; Liu, L.; Liu, T.; Liu, Y.; et al. Relationship between the ABO Blood Group and the COVID-19 Susceptibility. Clin. Infect. Dis. 2020, 73, 328–331. [Google Scholar] [CrossRef]
- The Severe COVID-19 GWAS Group. Genomewide Association Study of Severe COVID-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.X.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Petersen, E.L.; Goßling, A.; Adam, G.; Aepfelbacher, M.; Behrendt, C.-A.; Cavus, E.; Cheng, B.; Fischer, N.; Gallinat, J.; Kühn, S.; et al. Multi-organ assessment in mainly non-hospitalized individuals after SARS-CoV-2 infection: The Hamburg City Health Study COVID programme. Eur. Heart J. 2022, 43, 1124–1137. [Google Scholar] [CrossRef]
- Takahashi, T.; Wong, P.; Ellingson, M.K.; Lucas, C.; Klein, J.; Israelow, B.; Silva, J.; Oh, J.E.; Mao, T.; Tokuyama, M.; et al. Sex differences in immune responses to SARS-CoV-2 that underlie disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020, 14, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, L.; Wang, F.-S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Mao, R.; Qiu, Y.; He, J.-S.; Tan, J.-Y.; Li, X.-H.; Liang, J.; Shen, J.; Zhu, L.-R.; Chen, Y.; Iacucci, M.; et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, L.; Li, J.; Cheng, X.; Yang, J.; Tian, C.; Zhang, Y.; Huang, S.; Liu, Z.; Cheng, J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin. Gastroenterol. Hepatol. 2020, 18, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.-Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Lax, S.F.; Skok, K.; Zechner, P.; Kessler, H.H.; Kaufmann, N.; Koelblinger, C.; Vander, K.; Bargfrieder, U.; Trauner, M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome: Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann. Intern. Med. 2020, 173, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Hu, L.; Zhang, Y.; Han, W.; Lu, Z.; Ke, A.; Zhou, J.; Shi, G.; Fang, N.; Fan, J.; et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. Genomics 2020. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [Green Version]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Patel, V.B.; Parajuli, N.; Fan, D.; Basu, R.; Wang, Z.; Ramprasath, T.; Kassiri, Z.; Penninger, J.M.; Oudit, G.Y. Heterozygote loss of ACE2 is sufficient to increase the susceptibility to heart disease. J. Mol. Med. 2014, 92, 847–858. [Google Scholar] [CrossRef]
- Sama, I.E.; Ravera, A.; Santema, B.T.; van Goor, H.; ter Maaten, J.M.; Cleland, J.G.F.; Rienstra, M.; Friedrich, A.W.; Samani, N.J.; Ng, L.L.; et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur. Heart J. 2020, 41, 1810–1817. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020, 323, 2427. [Google Scholar] [CrossRef]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Alabed, M.; Temsah, M.-H.; Al Heialy, S.; Hamid, Q.; Halwani, R. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther.-Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.-H.; Li, B.; Zheng, X.-S.; Yang, X.-L.; Hu, B.; Wang, Y.-Y.; Xiao, G.-F.; Yan, B.; Shi, Z.-L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.-L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [Green Version]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Uhal, B.D.; Li, X.; Xue, A.; Gao, X.; Abdul-Hafez, A. Regulation of alveolar epithelial cell survival by the ACE-2/angiotensin 1–7/ Mas axis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 301, L269–L274. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Guo, F.; Liu, K.; Wang, H.; Rao, S.; Yang, P.; Jiang, C. Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2. Virus Res. 2008, 136, 8–15. [Google Scholar] [CrossRef]
- Haga, S.; Nagata, N.; Okamura, T.; Yamamoto, N.; Sata, T.; Yamamoto, N.; Sasazuki, T.; Ishizaka, Y. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Res. 2010, 85, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, I.; Bertram, S.; Herzog, P.; Pfefferle, S.; Steffen, I.; Muench, M.O.; Simmons, G.; Hofmann, H.; Kuri, T.; Weber, F.; et al. Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. J. Virol. 2010, 84, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.P.; Look, D.C.; Tan, P.; Shi, L.; Hickey, M.; Gakhar, L.; Chappell, M.C.; Wohlford-Lenane, C.; McCray, P.B. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 297, L84–L96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Nicholls, J.M.; Chen, Y.-G. Severe Acute Respiratory Syndrome-associated Coronavirus Nucleocapsid Protein Interacts with Smad3 and Modulates Transforming Growth Factor-β Signaling. J. Biol. Chem. 2008, 283, 3272–3280. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Revilla, J.; Deierborg, T.; Venero, J.L.; Boza-Serrano, A. Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider. Front. Immunol. 2020, 11, 2069. [Google Scholar] [CrossRef]
- Colarusso, C.; Maglio, A.; Terlizzi, M.; Vitale, C.; Molino, A.; Pinto, A.; Vatrella, A.; Sorrentino, R. Post-COVID-19 Patients Who Develop Lung Fibrotic-like Changes Have Lower Circulating Levels of IFN-β but Higher Levels of IL-1α and TGF-β. Biomedicines 2021, 9, 1931. [Google Scholar] [CrossRef]
- Ling, T.Y.; Kuo, M.D.; Li, C.L.; Yu, A.L.; Huang, Y.H.; Wu, T.J.; Lin, Y.C.; Chen, S.H.; Yu, J. Identification of pulmonary Oct-4 + stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc. Natl. Acad. Sci. USA 2006, 103, 9530–9535. [Google Scholar] [CrossRef] [Green Version]
- Zietz, M.; Zucker, J.; Tatonetti, N.P. Testing the association between blood type and COVID-19 infection, intubation, and death. Nat. Commun. 2020, 13, 5761. [Google Scholar] [CrossRef]
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.-D.; Sacco, C.; Bertuzzi, A.; et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020, 191, 9–14. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; Ding, Y.; Lu, W.; Wang, J. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Damage After 2019-nCoV Infection. Front. Med. 2021. [Google Scholar] [CrossRef]
- Li, Z.; Wu, M.; Yao, J.; Guo, J.; Liao, X.; Song, S.; Li, J.; Duan, G.; Zhou, Y.; Wu, X.; et al. Caution on Kidney Dysfunctions of COVID-19 Patients. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D.; Abate, M.; et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney impairment is associated with in-hospital death of COVID-19 patients. Kidney 2020. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Wang, R.; Feng, Z.; Tan, Y.; Wang, H.; Wang, C.; Liu, L.; Liu, Y.; Liu, Y.; et al. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Nat. Commun. 2020, 12, 2506. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Jin, J.-M.; Bai, P.; He, W.; Wu, F.; Liu, X.-F.; Han, D.-M.; Liu, S.; Yang, J.-K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Shastri, A.; Wheat, J.; Agrawal, S.; Chaterjee, N.; Pradhan, K.; Goldfinger, M.; Kornblum, N.; Steidl, U.; Verma, A.; Shastri, J. Delayed clearance of SARS-CoV-2 in male compared to female patients: High ACE2 expression in testes suggests possible existence of gender-specific viral reservoirs. MedRxiv 2020. [CrossRef]
- Wang, Z.; Xu, X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells 2020, 9, 920. [Google Scholar] [CrossRef] [Green Version]
- Bukowska, A.; Spiller, L.; Wolke, C.; Lendeckel, U.; Weinert, S.; Hoffmann, J.; Bornfleth, P.; Kutschka, I.; Gardemann, A.; Isermann, B.; et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp. Biol. Med. 2017, 242, 1412–1423. [Google Scholar] [CrossRef]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Bilaloglu, S.; Aphinyanaphongs, Y.; Jones, S.; Iturrate, E.; Hochman, J.; Berger, J.S. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA 2020, 324, 799. [Google Scholar] [CrossRef]
- Rahi, M.S.; Jindal, V.; Reyes, S.-P.; Gunasekaran, K.; Gupta, R.; Jaiyesimi, I. Hematologic disorders associated with COVID-19: A review. Ann. Hematol. 2021, 100, 309–320. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Oldenburg, J.; Klamroth, R.; Langer, F.; Albisetti, M.; von Auer, C.; Ay, C.; Korte, W.; Scharf, R.E.; Pötzsch, B.; Greinacher, A. Erratum: Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hämostaseologie 2021, 41, e1. [Google Scholar] [CrossRef]
- Morais, S.; Cruz, E. Trombose, Hemorragia e Trombocitopenia Induzidas pelas Vacinas contra a COVID-19: Protocolo de Atuação. Acta Méd. Port. 2021, 34, 625. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: SARS-CoV-2 mRNA Vaccines and the Possible Mechanism of Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT). Med. Sci. Monit. 2021, 27, e932899-1–e932899-2. [Google Scholar] [CrossRef]
- Malhotra, H.S.; Gupta, P.; Prabhu, V.; Kumar Garg, R.; Dandu, H.; Agarwal, V. COVID-19 vaccination-associated myelitis. QJM Int. J. Med. 2021, 114, 591–593. [Google Scholar] [CrossRef]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202. [Google Scholar] [CrossRef]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 6, 1196. [Google Scholar] [CrossRef]
- Marshall, M.; Ferguson, I.D.; Lewis, P.; Jaggi, P.; Gagliardo, C.; Collins, J.S.; Shaughnessy, R.; Caron, R.; Fuss, C.; Corbin, K.J.E.; et al. Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination. Pediatrics 2021, 148, e2021052478. [Google Scholar] [CrossRef]
- Starekova, J.; Bluemke, D.A.; Bradham, W.S.; Grist, T.M.; Schiebler, M.L.; Reeder, S.B. Myocarditis Associated with mRNA COVID-19 Vaccination. Radiology 2021, 301, E409–E411. [Google Scholar] [CrossRef]
- Román, G.C.; Gracia, F.; Torres, A.; Palacios, A.; Gracia, K.; Harris, D. Acute Transverse Myelitis (ATM):Clinical Review of 43 Patients With COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events With the ChAdOx1 nCoV-19 Vaccine (AZD1222). Front. Immunol. 2021, 12, 653786. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef]
- Khedkar, P.H.; Patzak, A. SARS-CoV-2: What do we know so far? Acta Physiol. 2020, 229, e13470. [Google Scholar] [CrossRef]
- Muthukumar, A.; Narasimhan, M.; Li, Q.-Z.; Mahimainathan, L.; Hitto, I.; Fuda, F.; Batra, K.; Jiang, X.; Zhu, C.; Schoggins, J.; et al. In-Depth Evaluation of a Case of Presumed Myocarditis After the Second Dose of COVID-19 mRNA Vaccine. Circulation 2021, 144, 487–498. [Google Scholar] [CrossRef]
- Moran, E.M.; Mastaglia, F.L. Cytokines in immune-mediated inflammatory myopathies: Cellular sources, multiple actions and therapeutic implications. Clin. Exp. Immunol. 2014, 178, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Rivas-García, S.; Bernal, J.; Bachiller-Corral, J. Rhabdomyolysis as the main manifestation of coronavirus disease 2019. Rheumatology 2020, 59, 2174–2176. [Google Scholar] [CrossRef]
- Chan, K.H.; Slim, J. Rhabdomyolysis as Potential Late Complication Associated with COVID. Emerg. Infect. Dis. 2020, 26, 2535. [Google Scholar] [CrossRef]
- Rajaratnam, N.; Govil, S.; Patel, R.; Ahmed, M.; Elias, S. Rhabdomyolysis after recombinant zoster vaccination: A rare adverse reaction. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 145–146. [Google Scholar] [CrossRef]
- Tan, A.; Stepien, K.M.; Narayana, S.T.K. Carnitine palmitoyltransferase II deficiency and post-COVID vaccination rhabdomyolysis. QJM Int. J. Med. 2021, 114, 596–597. [Google Scholar] [CrossRef]
- Nassar, M.; Chung, H.; Dhayaparan, Y.; Nyein, A.; Acevedo, B.J.; Chicos, C.; Zheng, D.; Barras, M.; Mohamed, M.; Alfishawy, M.; et al. COVID-19 vaccine induced rhabdomyolysis: Case report with literature review. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102170. [Google Scholar] [CrossRef]
- Hakroush, S.; Tampe, B. Case Report: ANCA-Associated Vasculitis Presenting With Rhabdomyolysis and Pauci-Immune Crescentic Glomerulonephritis After Pfizer-BioNTech COVID-19 mRNA Vaccination. Front. Immunol. 2021, 12, 762006. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Luciano, P.Q.; Binatti, R.; Sodré, A.R.; Zajac, S.R.; Marson, F.A.L.; Ortega, M.M. Vaccine-induced immune thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccine in an older patient: Minireview and a case report. J. Infect. Public Health 2022, 15, 638–642. [Google Scholar] [CrossRef]
- Cirillo, E.; Esposito, C.; Giardino, G.; Azan, G.; Fecarotta, S.; Pittaluga, S.; Ruggiero, L.; Barretta, F.; Frisso, G.; Notarangelo, L.D.; et al. Case Report: Severe Rhabdomyolysis and Multiorgan Failure After ChAdOx1 nCoV-19 Vaccination. Front. Immunol. 2022, 13, 845496. [Google Scholar] [CrossRef]
- Markus, H.S. Ischaemic stroke can follow COVID-19 vaccination but is much more common with COVID-19 infection itself. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1142. [Google Scholar] [CrossRef]
- Mehta, P.R.; Apap Mangion, S.; Benger, M.; Stanton, B.R.; Czuprynska, J.; Arya, R.; Sztriha, L.K. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination—A report of two UK cases. Brain. Behav. Immun. 2021, 95, 514–517. [Google Scholar] [CrossRef]
- Al-Mayhani, T.; Saber, S.; Stubbs, M.J.; Losseff, N.A.; Perry, R.J.; Simister, R.J.; Gull, D.; Jäger, H.R.; Scully, M.A.; Werring, D.J. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1247–1248. [Google Scholar] [CrossRef]
- Fanni, D.; Saba, L.; Demontis, R.; Gerosa, C.; Chighine, A.; Nioi, M.; Suri, J.S.; Ravarino, A.; Cau, F.; Barcellona, D.; et al. Vaccine-induced severe thrombotic thrombocytopenia following COVID-19 vaccination: A report of an autoptic case and review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5063–5069. [Google Scholar] [CrossRef]
- Wolf, M.E.; Luz, B.; Niehaus, L.; Bhogal, P.; Bäzner, H.; Henkes, H. Thrombocytopenia and Intracranial Venous Sinus Thrombosis after “COVID-19 Vaccine AstraZeneca” Exposure. J. Clin. Med. 2021, 10, 1599. [Google Scholar] [CrossRef]
- Suresh, P.; Petchey, W. ChAdOx1 nCOV-19 vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis (CVST). BMJ Case Rep. 2021, 14, e243931. [Google Scholar] [CrossRef]
- Blauenfeldt, R.A.; Kristensen, S.R.; Ernstsen, S.L.; Kristensen, C.C.H.; Simonsen, C.Z.; Hvas, A. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J. Thromb. Haemost. 2021, 19, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.R.; Shi, T.; Vasileiou, E.; Katikireddi, S.V.; Kerr, S.; Moore, E.; McCowan, C.; Agrawal, U.; Shah, S.A.; Ritchie, L.D.; et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021, 27, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Toom, S.; Wolf, B.; Avula, A.; Peeke, S.; Becker, K. Familial thrombocytopenia flare-up following the first dose of mRNA -1273 COVID-19 vaccine. Am. J. Hematol. 2021, 96, E134–E135. [Google Scholar] [CrossRef]
- Kahn, B.; Apostolidis, S.A.; Bhatt, V.; Greenplate, A.R.; Kallish, S.; LaCava, A.; Lucas, A.; Meyer, N.J.; Negoianu, D.; Ogdie, A.R.; et al. Multisystem Inflammation and Organ Dysfunction After BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccination. Crit. Care Explor. 2021, 3, e0578. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.V.; Hu, Y. Hemophagocytic lymphohistiocytosis after COVID-19 vaccination. J. Hematol. Oncol. J. Hematol. Oncol. 2021, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Hernández-Vanegas, L.E.; Núñez, I.; Hernández-Valdivia, N.; Carrillo-García, D.A.; Michel-Chávez, A.; Galnares-Olalde, J.A.; Carbajal-Sandoval, G.; del Mar Saniger-Alba, M.; et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: A nationwide descriptive study. Clin. Immunol. 2021, 229, 108786. [Google Scholar] [CrossRef] [PubMed]
- Bhopal, S.S.; Olabi, B.; Bhopal, R. Vaccines for COVID-19: Learning from ten phase II trials to inform clinical and public health vaccination programmes. Public Health 2021, 193, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Barzilay, R.; Moore, T.M.; Greenberg, D.M.; DiDomenico, G.E.; Brown, L.A.; White, L.K.; Gur, R.C.; Gur, R.E. Resilience, COVID-19-related stress, anxiety and depression during the pandemic in a large population enriched for healthcare providers. Transl. Psychiatry 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef]
- Bignucolo, A.; Scarabel, L.; Mezzalira, S.; Polesel, J.; Cecchin, E.; Toffoli, G. Sex Disparities in Efficacy in COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2021, 9, 825. [Google Scholar] [CrossRef]
- Breslin, N.; Baptiste, C.; Gyamfi-Bannerman, C.; Miller, R.; Martinez, R.; Bernstein, K.; Ring, L.; Landau, R.; Purisch, S.; Friedman, A.M.; et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am. J. Obstet. Gynecol. MFM 2020, 2, 100118. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Q.; Zheng, D.; Jiang, H.; Wei, Y.; Zou, L.; Feng, L.; Xiong, G.; Sun, G.; Wang, H.; et al. Clinical Characteristics of Pregnant Women with COVID-19 in Wuhan, China. N. Engl. J. Med. 2020, 382, e100. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ 2020, 369, m2107. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Cruz-Melguizo, S.; Adrien, M.; Fuentes, L.; Marin, E.; Perez-Medina, T. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet. Gynecol. Scand. 2020, 99, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, R.; Chen, J.; Xie, Y.; Zhang, S.; Wang, X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int. J. Gynaecol. Obstet. 2020, 150, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, J.; Fan, C.; Juan, J.; Yu, X.; Li, J.; Feng, L.; Li, C.; Chen, H.; Qiao, Y.; et al. Coronavirus disease 2019 in pregnant women: A report based on 116 cases. Am. J. Obstet. Gynecol. 2020, 223, 111.e1–111.e14. [Google Scholar] [CrossRef]
- Al-Matary, A.; Almatari, F.; Al-Matary, M.; AlDhaefi, A.; Alqahtani, M.H.S.; Alhulaimi, E.A.; AlOtaiby, S.; Almehiny, K.; John, L.S.; Alanazi, F.S.; et al. Clinical outcomes of maternal and neonate with COVID-19 infection-Multicenter study in Saudi Arabia. J. Infect. Public Health 2021, 14, 702–708. [Google Scholar] [CrossRef]
- Damar Çakırca, T.; Torun, A.; Hamidanoğlu, M.; Portakal, R.D.; Ölçen, M.; Çakırca, G.; Haksever, M. COVID-19 infection in pregnancy: A single center experience with 75 cases. Ginekol. Pol. 2021. [Google Scholar] [CrossRef]
- Keita, H.; James, A.; Bouvet, L.; Herrmann, E.; Le Gouez, A.; Mazoit, J.X.; Mercier, F.J.; Benhamou, D.; Obstetric Anaesthesia COVID-19 Collaboration Network. Clinical, obstetrical and anaesthesia outcomes in pregnant women during the first COVID-19 surge in France: A prospective multicentre observational cohort study. Anaesth. Crit. Care Pain Med. 2021, 40, 100937. [Google Scholar] [CrossRef]
- Nayak, M.K.; Panda, S.K.; Panda, S.S.; Rath, S.; Ghosh, A.; Mohakud, N.K. Neonatal outcomes of pregnant women with COVID-19 in a developing country setup. Pediatr. Neonatol. 2021, 62, 499–505. [Google Scholar] [CrossRef]
- Sahin, D.; Tanacan, A.; Erol, S.A.; Anuk, A.T.; Yetiskin, F.D.Y.; Keskin, H.L.; Ozcan, N.; Ozgu-Erdinc, A.S.; Eyi, E.G.Y.; Yucel, A.; et al. Updated experience of a tertiary pandemic center on 533 pregnant women with COVID-19 infection: A prospective cohort study from Turkey. Int. J. Gynaecol. Obstet. 2021, 152, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Omeish, H.; Najadat, A.; Al-Azzam, S.; Tarabin, N.; Abu Hameed, A.; Al-Gallab, N.; Abbas, H.; Rababah, L.; Rabadi, M.; Karasneh, R.; et al. Reported COVID-19 vaccines side effects among Jordanian population: A cross sectional study. Hum. Vaccin. Immunother. 2022, 18, 1981086. [Google Scholar] [CrossRef]
- Grome, H.N.; Threlkeld, M.; Threlkeld, S.; Newman, C.; Martines, R.B.; Reagan-Steiner, S.; Whitt, M.A.; Gomes-Solecki, M.; Nair, N.; Fill, M.M.; et al. Fatal Multisystem Inflammatory Syndrome in Adult after SARS-CoV-2 Natural Infection and COVID-19Vaccination. Emerg. Infect. Dis. 2021, 27, 2914–2918. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef] [PubMed]
- Kreier, F. Long-COVID symptoms less likely in vaccinated people, Israeli data say. Nature 2022. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Ouldali, N.; Bagheri, H.; Salvo, F.; Antona, D.; Pariente, A.; Leblanc, C.; Tebacher, M.; Micallef, J.; Levy, C.; Cohen, R.; et al. Multisystemic inflammatory syndrome following COVID-19 mRNA vaccine in children: A national post-authorization pharmacovigilance study. medRxiv 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).