Effectiveness of Comirnaty® Vaccine and Correlates of Immunogenicity and Adverse Reactions: A Single-Center Prospective Case Series Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Vaccination Protocol
2.3. Antibody Testing
2.4. SARS-CoV-2 Virus Diagnosis
2.5. Assessment of BNT162b2 Vaccine-Related Adverse Reactions
2.6. Data Collection
2.6.1. Sociodemographic and Lifestyle
2.6.2. Physical Fitness
2.6.3. Clinical Characteristics
2.6.4. Vaccination
2.7. Data Management and Statistical Analysis
2.8. Ethical Considerations
3. Results
3.1. Sample and Lifestyle Characteristics
3.2. Physical Fitness Condition
3.3. Clinical Description
3.4. Vaccination Process
3.5. BNT162b2 Vaccine-Related Adverse Reactions
3.5.1. Local Reactions
3.5.2. Systemic Reactions
3.6. Antibody Level
3.7. Associations between Antibody Titer and Participant Characteristics
3.7.1. Age
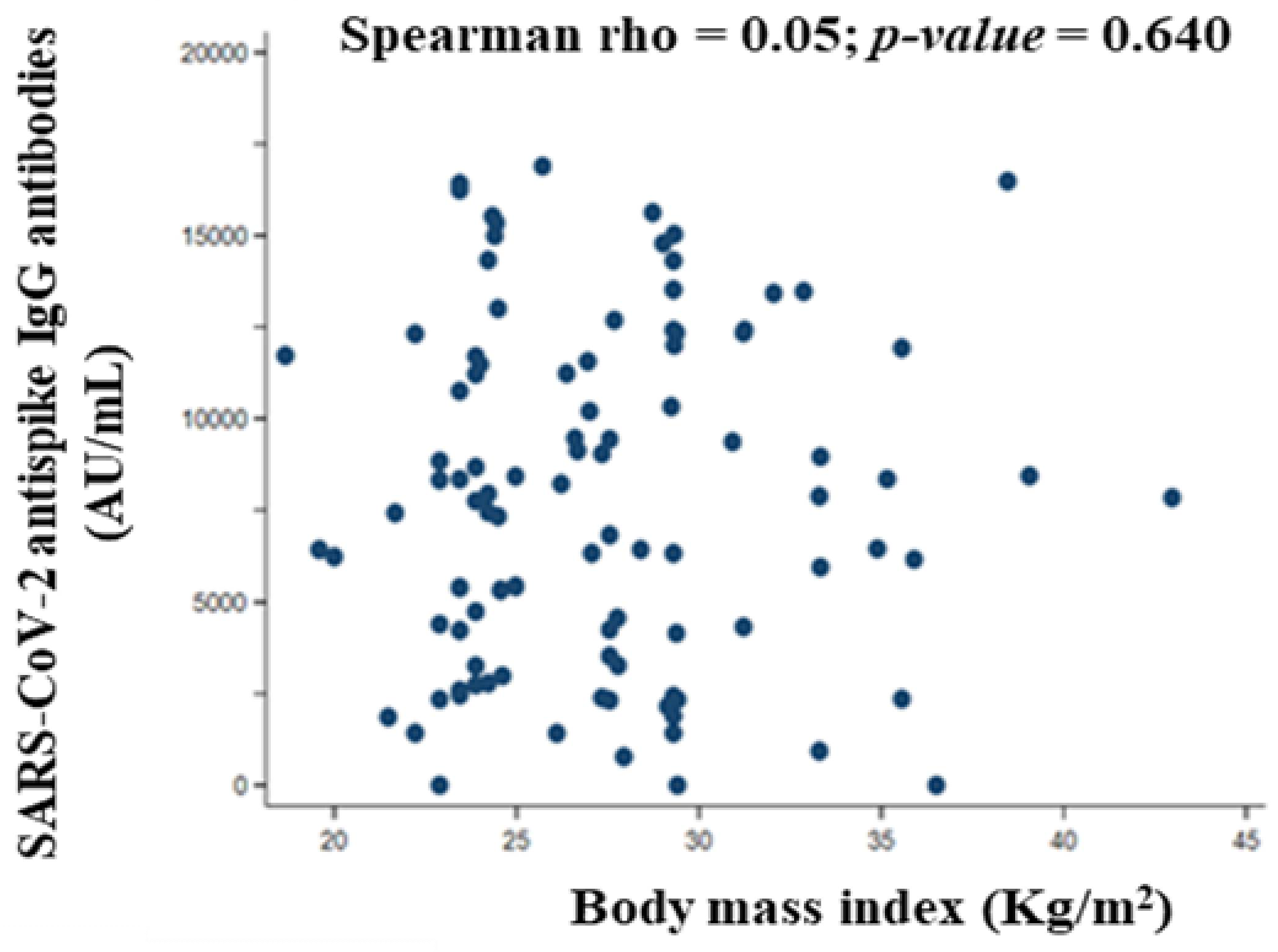
3.7.2. Body Mass Index
3.7.3. Number of Drugs and Specific Treatments
3.7.4. Blood Group
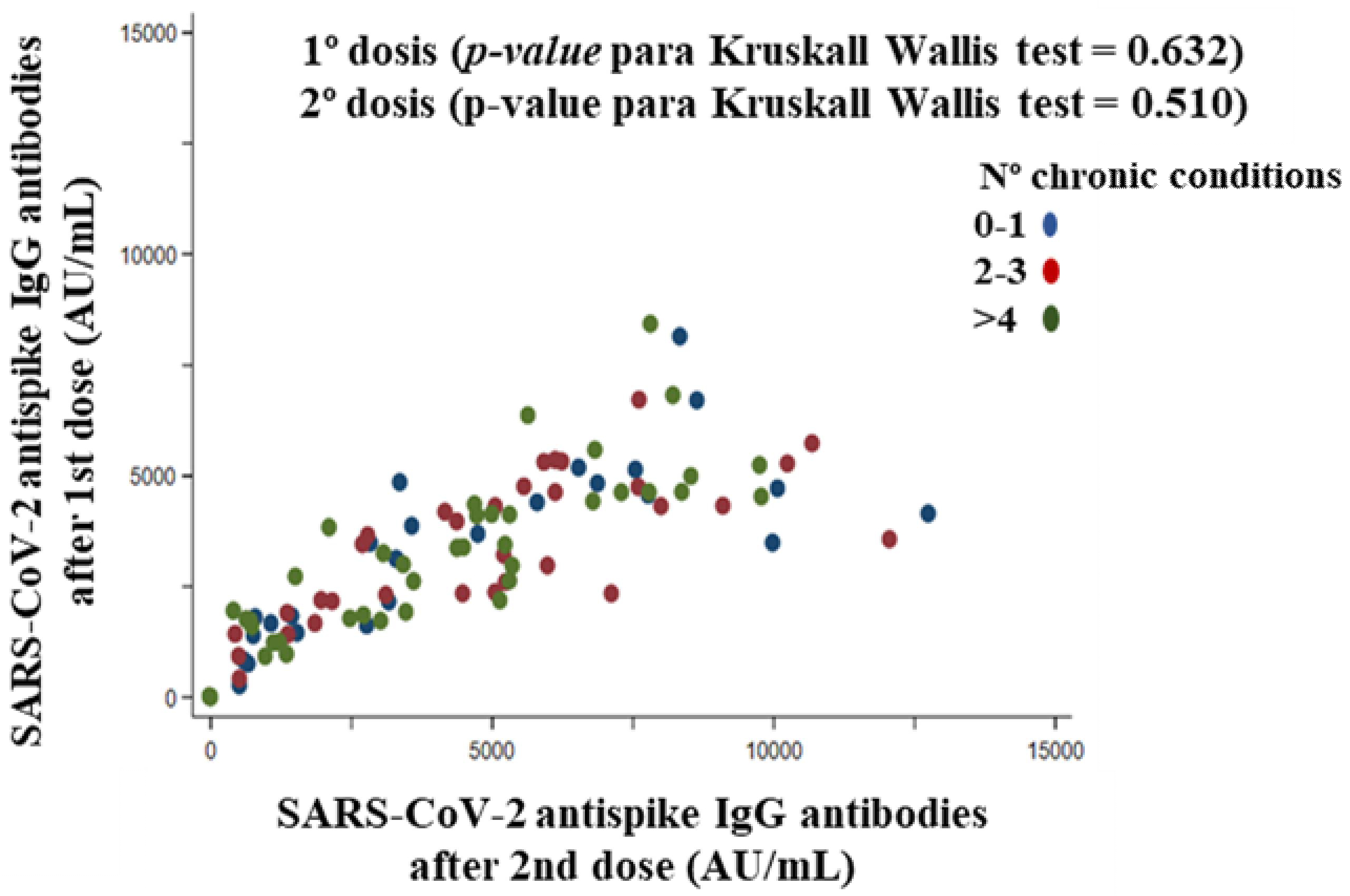
3.7.5. Chronic Conditions
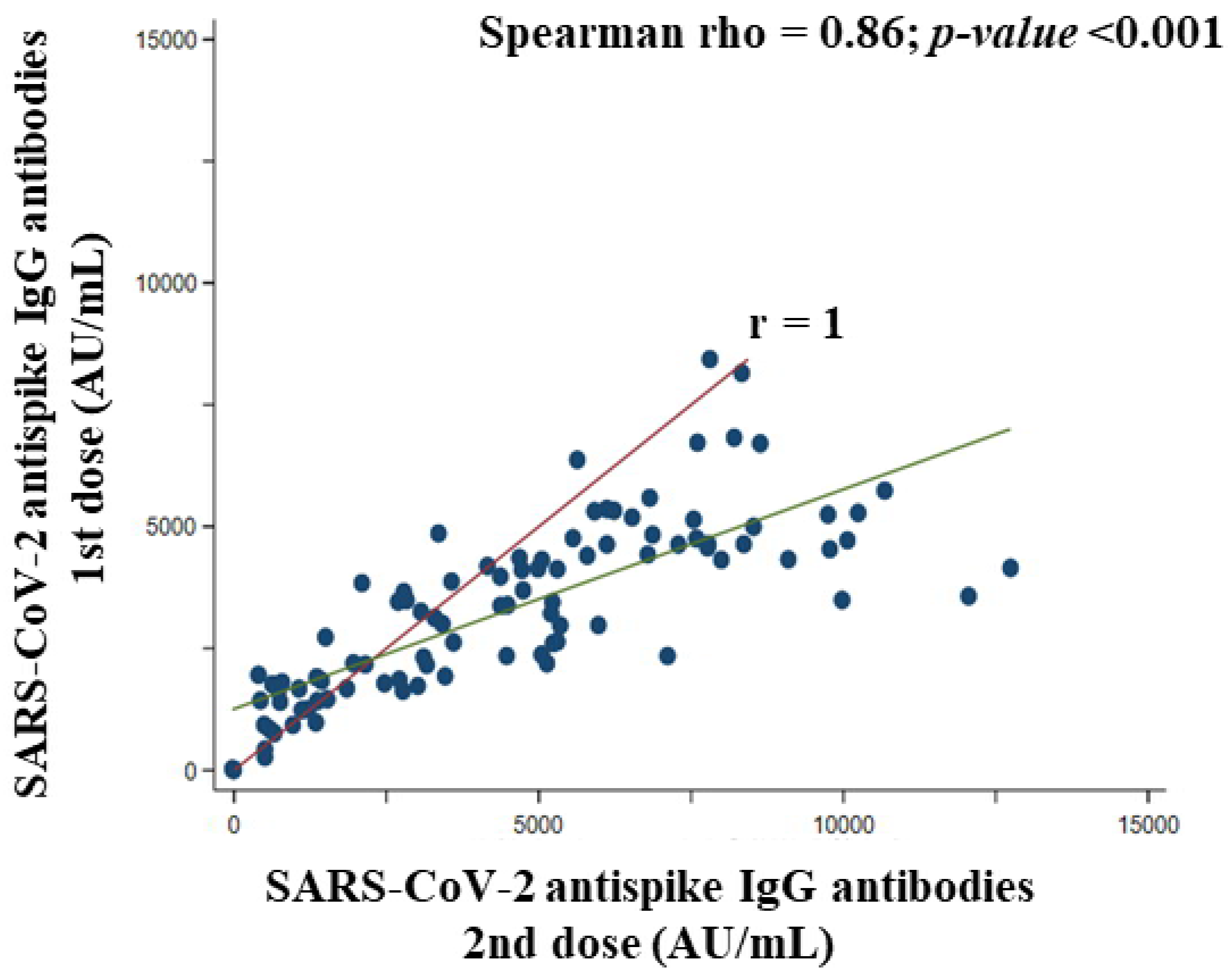
3.8. Association between the First and Second Doses of BNT162b2 Vaccine Concerning SARS-CoV-2 Anti Spike IgG Antibodies Titer in Participants
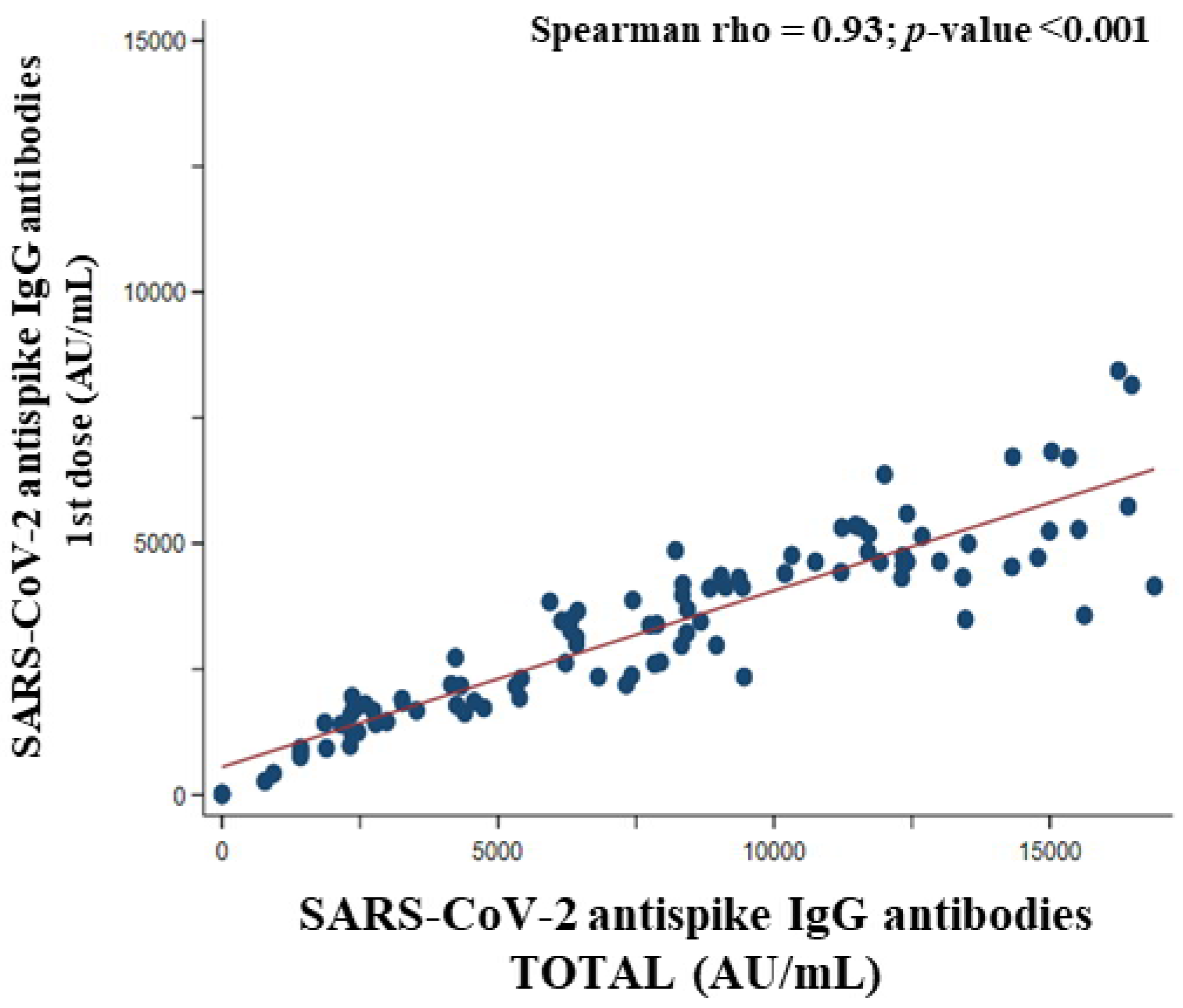
3.9. Association between SARS-CoV-2 Anti Spike IgG Antibodies Titer of the First Dose and Total SARS-CoV-2 Anti Spike IgG Antibodies Titer of the Participants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Human Ethics
References
- Fernández-Lázaro, D.; Sánchez-Serrano, N.; Mielgo-Ayuso, J.; García-Hernández, J.L.; González-Bernal, J.J.; Seco-Calvo, J. Long COVID a New Derivative in the Chaos of SARS-CoV-2 Infection: The Emergent Pandemic? J. Clin. Med. 2021, 10, 5799. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; González-Bernal, J.J.; Sánchez-Serrano, N.; Navascués, L.J.; Ascaso-Del-Río, A.; Mielgo-Ayuso, J. Physical Exercise as a Multimodal Tool for COVID-19: Could It Be Used as a Preventive Strategy? Int. J. Environ. Res. Public Health 2020, 17, 8496. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins Coronavirus Resource Center. COVID-19 Map. 2022. Available online: https://coronavirus.jhu.edu/map.html (accessed on 18 April 2022).
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Adams, D.P.; García Hernández, J.L.; González-Bernal, J.; González-Gross, M. Glycophosphopeptical AM3 Food Supplement: A Potential Adjuvant in the Treatment and Vaccination of SARS-CoV-2. Front. Immunol. 2021, 12, 98672. [Google Scholar] [CrossRef]
- Chiu, N.C.; Chi, H.; Tai, Y.L.; Peng, C.C.; Tseng, C.Y.; Chen, C.C.; Tan, B.F.; Lyn, C.Y. Impact of Wearing Masks, Hand Hygiene, and Social Distancing on Influenza, Enterovirus, and All-Cause Pneumonia During the Coronavirus Pandemic: Retrospective National Epidemiological Surveillance Study. J. Med. Internet Res. 2021, 22, e21257. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus disease (COVID-19): Herd immunity, Blockades, and COVID-19. Available online: https://www.who.int/news-room/q-a-detail/herd-immunity-lockdowns-and-covid-19 (accessed on 28 April 2022).
- World Health Organization. COVID-19 Vaccines. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (accessed on 28 April 2022).
- Anderson, R.M.; Vegvari, C.; Truscott, J.; Collyer, B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet 2020, 396, 1614–1616. [Google Scholar] [CrossRef]
- Kimura, I.; Kosugi, Y.; Wu, J.; Yamasoba, D.; Butlertanaka, E.P.; Tanaka, Y.L.; Liu, Y.; Shirakawa, K.; Kazuma, Y.; Nomura, R.; et al. SARS-CoV-2 Lambda variant exhibits higher infectivity and immune resistance. Cell Rep. 2022, 38, 110218. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Hoteit, R.; Yassine, H.M. Biological Properties of SARS-CoV-2 Variants: Epidemiological Impact and Clinical Consequences. Vaccines 2022, 10, 919. [Google Scholar] [CrossRef]
- García-García, D.; Morales, E.; Fonfría, E.S.; Vigo, I.; Bordehore, C. Caveats on COVID-19 herd immunity threshold: The Spain case. Sci. Rep. 2022, 12, 598. [Google Scholar] [CrossRef]
- Spanish Agency of Medicines and Health Products (AEMPS). Information on Authorized Vaccines. Available online: https://www.aemps.gob.es/la-aemps/ultima-informacion-de-la-aemps-acerca-del-covid-19/vacunas-contra-la-covid-19/informacion-de-vacunas-autorizadas/ (accessed on 3 May 2022).
- Altawalah, H. Antibody Responses to Natural SARS-CoV-2 Infection or after COVID-19 Vaccination. Vaccines 2021, 9, 910. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, K.; Shelling, A.N.; Wu, Z. Nanotechnology-Enabled COVID-19 mRNA Vaccines. Encyclopedia 2021, 1, 773–780. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- The Ministry of Health, Government of Spain. COVID-19 Vaccination. 2022. Available online: https://www.vacunacovid.gob.es/ (accessed on 5 May 2022).
- The Ministry of Health, Government of Spain. COVID-19 Vaccination Strategy in Spain. 2022. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/vacunaCovid19.htm (accessed on 5 May 2022).
- CARE guidelines for CAse Reports. Available online: https://www.care-statement.org/ (accessed on 19 July 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez, G.M.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Bayram, A.; Demirbakan, H.; Günel Karadeniz, P.; Erdoğan, M.; Koçer, I. Quantitation of antibodies against SARS-CoV-2 spike protein after two doses of CoronaVac in healthcare workers. J. Med. Virol. 2021, 93, 5560–5567. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Gómez, N.; Sánchez-Serrano, N.; Alaoui Sosse, A.; Aldea-Mansilla, C. Emergency Standardization for SARS-CoV-2 virus Diagnosis by Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) in a COVID-19 pandemic situation. Madr. J. Public Health 2020, 3, 1–11. [Google Scholar]
- Caixàs, A.; Villaró, M.; Arraiza, C.; Montalvá, J.C.; Lecube, A.; Fernández-García, J.M.; Coriogi, R.; Bellido, D.; Llisterrik, J.L.; Tinahones, F.J. SEEDO-SEMERGEN consensus document on continuous care of obesity between Primary Care and Specialist Hospital Units 2019. Med. Clin. (Barcelona) 2020, 155, 267e1–267e11. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [Green Version]
- Gould, D.; Crichton, N. Information point: Visual Analogue Scale (VAS). J. Clin. Nurse 2001, 10, 697–706. [Google Scholar] [CrossRef]
- Bohannon, R.W. Dynamometer measurements of hand-grip strength predict multiple outcomes. Percept. Mot. Ski. 2001, 93, 323–328. [Google Scholar] [CrossRef]
- Gálvez Cano, M.; Varela Pinedo, L.F.; Helver Chávez, J.; Cieza, Z. Correlation of the Get-Up-And-Go Test With The Tinetti Test when assessing the risk for falls in elderly persons. Acta. Med. Peruana 2010, 27, 8–11. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Personalized vaccinology: A review. Vaccine 2018, 36, 5350–5357. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- Arregocés-Castillo, L.; Fernández-Niño, J.; Rojas-Botero, M.; Palacios-Clavijo, A.; Galvis-Pedraza, M.; Rincón-Medrano, L.; Pinto-Álvarez, M.; Ruiz-Gómez, F.; Trejo-Valdivia, B. Effectiveness of COVID-19 vaccines in older adults in Colombia: A retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022, 3, e242–e252. [Google Scholar] [CrossRef]
- Kissling, E.; Hooiveld, M.; Sandonis Martín, V.; Martínez-Baz, I.; William, N.; Vilcu, A.M.; Mazagatos, C.; Domegan, L.; de Lusignan, S.; Meijer, A.; et al. Vaccine effectiveness against symptomatic SARS-CoV-2 infection in adults aged 65 years and older in primary care: I-MOVE-COVID-19 project, Europe, December 2020 to May 2021. Eurosurveillance 2021, 26, 2100670. [Google Scholar] [CrossRef]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of COVID-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef]
- Mitsunaga, T.; Ohtaki, Y.; Seki, Y.; Yoshioka, M.; Mori, H.; Suzuka, M.; Mashiko, S.; Takeda, S.; Mashiko, K. The evaluation of factors affecting antibody response after administration of the BNT162b2 vaccine: A prospective study in Japan. PeerJ 2021, 9, e12316. [Google Scholar] [CrossRef]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Goncalves-Mendes, N.; Talvas, J.; Dualé, C.; Guttmann, A.; Corbin, V.; Marceau, G.; Sapin, V.; Brachet, P.; Evrard, B.; Laurichesse, H.; et al. Impact of Vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: A randomized placebo-controlled trial. Front. Immunol. 2019, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Kashi, D.S.; Oliver, S.J.; Wentz, L.M.; Roberts, R.; Carswell, A.T.; Tang, J.C.Y.; Jackson, S.; Izard, R.M.; Allan, D.; Rhodes, L.E.; et al. Vitamin D and the hepatitis B vaccine response: A prospective cohort study and a randomized, placebo-controlled oral vitamin D 3 and simulated sunlight supplementation trial in healthy adults. Eur. J. Nutr. 2021, 60, 475–491. [Google Scholar] [CrossRef]
- Lalor, M.K.; Floyd, S.; Gorak-Stolinska, P.; Weir, R.E.; Blitz, R.; Branson, K.; Fine, P.E.; Dockrell, H.M. BCG vaccination: A role for vitamin D? PLoS ONE 2011, 6, e16709. [Google Scholar] [CrossRef]
- Perez-Araluce, R.; Martinez-Gonzalez, M.A.; Fernández-Lázaro, C.I.; Bes-Rastrollo, M.; Gea, A.; Carlos, S. Mediterranean diet and the risk of COVID-19 in the ‘Seguimiento Universidad de Navarra’ cohort. Clin. Nutr. 2021, in press. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. 2022. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 May 2022).
- Coppack, S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Asher, A.; Tintle, N.L.; Myers, M.; Lockshon, L.; Bacareza, H.; Harris, W.S. Blood omega-3 fatty acids and death from COVID-19: A pilot study. Prostaglandins Leukot Essent Fat. Acids 2021, 166, 102250. [Google Scholar] [CrossRef] [PubMed]
- Winberger, B.; Herndler-Brandstetter, D.; Schwanninger, A.; Weiskopf, D.; Grubeck-Loebenstein, B. Biology of immune responses to vaccines in elderly persons. Clin. Infect. Dis. 2008, 46, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Herzog Tzarfati, K.; Gutwein, O.; Apel, A.; Rahimi-Levene, N.; Sadovnik, M.; Harel, L.; Benveniste-Levkovitz, P.; Bar Chaim, A.; Koren-Michowitz, M. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am. J. Hematol. 2021, 96, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Bacova, B.; Kohutova, Z.; Zubata, I.; Gaherova, L.; Kucera, P.; Heizer, T.; Mikesova, M.; Karel, T.; Novak, J. Cellular and humoral immune response to SARS-CoV-2 mRNA vaccines in patients treated with either Ibrutinib or Rituximab. Clin. Exp. Med. 2022, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Domínguez Ortega, C.; Nerea Sánchez-Serrano, N.; Beddar Chaib, F.; Jerves Donoso, D.; Jiménez-Callejo, E.; Rodríguez-García, S. Convalescent Plasma Therapy, Therapeutic Formulations of Repurposed Drugs in 20th Century Epidemics against COVID-19: A Systematic Review. Pharmaceutics 2022, 14, 1020. [Google Scholar] [CrossRef]
- Muñiz-Diaz, E.; Llopis, J.; Parra, R.; Roig, I.; Ferrer, G.; Grifols, J.; Millán, A.; Ene, G.; Ramiro, L. Relationship between the ABO blood group and COVID-19 susceptibility, severity, and mortality in two cohorts of patients. Blood Transfus. 2021, 19, 54–63. [Google Scholar]
- Ewald, D.R.; Sumner, S.C.J. Blood Type Biochemistry and Human Disease. Wiley Interdiscip. Rev. 2016, 8, 517–535. [Google Scholar] [CrossRef] [Green Version]
- Zietz, M.; Zucker, J.; Tatonetti, N.P. Associations between blood type and COVID-19 infection, intubation, and death. Nat. Commun. 2020, 11, 5761. [Google Scholar] [CrossRef]
- Ray, J.G.; Schull, M.J.; Vermeulen, M.J.; Park, A.L. Association Between ABO and Rh Blood Groups and SARS-CoV-2 Infection or Severe COVID-19 Illness: A Population-Based Cohort Study. Ann. Intern. Med. 2021, 174, 308–315. [Google Scholar] [CrossRef]
- Kim, Y.; Latz, C.A.; DeCarlo, C.S.; Lee, S.; Png, C.Y.M.; Kibrik, P.; Sung, E.; Alabi, O.; Dua, A. Relationship between blood type and outcomes following COVID-19 infection. Semin. Vasc. Surg. 2021, 34, 125–131. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Dunning, A.J.; Diaz Granados, C.A.; Voloshen, T.; Hu, B.; Landolfi, V.A.; Talbot, H.K. Correlates of Protection against Influenza in the Elderly: Results from an Influenza Vaccine Efficacy Trial. Clin. Vaccine Immunol. 2016, 23, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xia, H.; Zou, J.; Weaver, S.C.; Swanson, K.A.; Cai, H.; Cutler, M.; Cooper, D.; Muik, A.; et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature 2021, 596, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A.; et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Gravenstein, S.; Upshaw, C.M.; Hooton, J.W.; Krause, P.; Drinka, P.; Bleackley, R.C. Granzyme B: A marker of risk for influenza in institutionalized older adults. Vaccine 2001, 9, 3744–3751. [Google Scholar] [CrossRef]
- Silva-Cayetano, A.; Foster, W.S.; Innocentin, S.; Belij-Rammerstorfer, S.; Spencer, A.J.; Burton, O.T.; Fra-Bidó, S.; Le Lee, J.; Thakur, N.; Conceicao, C.; et al. A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice. Med 2021, 2, 243–262.e8. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Setayeshgar, S.; Zou, M.; Prystajecky, N.; Tyson, J.R.; Sbihi, H.; Fjell, C.D.; Galanis, E.; Naus, M.; Patrick, D.M.; et al. Comparative single-dose mRNA and ChAdOx1 vaccine effectiveness against SARS-CoV-2, including variants of concern: Test-negative design, British Columbia, Canada. J. Infect. Dis. 2021, 27, jiac023. [Google Scholar]
- European Medicines Agency. COVID-19 Vaccines: Key Facts. 2022. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-key-facts (accessed on 10 May 2022).
- Kitagawa, H.; Kaiki, Y.; Sugiyama, A.; Nagashima, S.; Kurisu, A.; Nomura, T.; Omori, K.; Akita, T.; Shigemoto, N.; Tanaka, J.; et al. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan. J. Infect. Chemother. 2022, 28, 576–581. [Google Scholar] [CrossRef]
- Saita, M.; Yan, Y.; Ito, K.; Sasano, H.; Seyama, K.; Naito, T. Reactogenicity following two doses of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers in Japan. J. Infect. Chemother. 2022, 28, 116–119. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19 Vaccine Boosters. 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html (accessed on 10 May 2022).
- Riad, A.; Sağıroğlu, D.; Üstün, B.; Pokorná, A.; Klugarová, J.; Attia, S.; Klugar, M. Prevalence and Risk Factors of CoronaVac Side Effects: An Independent Cross-Sectional Study among Healthcare Workers in Turkey. J. Clin. Med. 2021, 10, 2629. [Google Scholar] [CrossRef] [PubMed]
- Klugar, M.; Riad, A.; Mekhemar, M.; Conrad, J.; Buchbender, M.; Howaldt, H.P.; Attia, S. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers. Biology 2021, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Full Cohort (n = 106) | Institutionalized Patients (n = 73) | Healthcare Workers (n = 33) | p-Value |
|---|---|---|---|---|
| Sociodemographic and Lifestyle | ||||
| Gender, n (%) | <0.001 | |||
| Male | 37 (34.9) | 35 (47.9) | 2 (6.1) | |
| Female | 69 (65.1) | 38 (52.1) | 31 (93.9) | |
| Age (years), mean (SD) | 73.3 (19.1) | 84.3 (7.6) | 48.8 (12.8) | <0.001 |
| Nationality, n (%) | 0.034 | |||
| Spanish | 104 (98.1) | 73 (100.0) | 31 (93.9) | |
| Other | 2 (1.9) | 0 | 2 (6.1) | |
| 1 Body mass index (BMI), mean (SD) | 27.2 (5.2) | 27.3 (4.0) | 26.9 (7.1) | 0.751 |
| Smoker, n (%) | 19 (17.9) | 6 (8.2) | 13 (39.4) | <0.001 |
| Non-Smoker | 25 (23.6) | 15 (20.5) | 10 (30.3) | |
| Never Smoker | 62 (58.5) | 52 (71.2) | 10 (30.3) | |
| 2 Trichopoulou’s MedDiet score, mean (SD) | 10.2 (1.8) | 10.1 (1.5) | 10.3 (2.4) | 0.610 |
| 3 Self-perceived health status g (%), mean (SD) | 79.4 (16.1) | 76.9 (15.7) | 84.8 (15.8) | 0.019 |
| Physical Fitness | ||||
| 4 Manual pressure dynamometry (kg/cm2), mean (SD) | ||||
| Dominant hand | 20.4 (13.9) | 16.1 (11.7) | 30 (13.7) | <0.001 |
| Non-dominant hand | 17.6 (12.3) | 13 (8.9) | 28 (12.5) | <0.001 |
| 5 Get-Up-And-Go Test (seconds), n (%), | ||||
| Yes (>30 seg) | 24 (22.6) | 24 (32.9) | 0 | |
| No | 77 (72.6) | 44 (60.3) | 33 (100.0) | --- |
| Disabled | 5 (4.7) | 5 (6.9) | 0 | |
| Clinics | ||||
| Known allergies, n (%) | 0.963 | |||
| Yes | 26 (24.5) | 18 (24.7) | 8 (24.2) | |
| No | 80 (75.5) | 55 (75.3) | 25 (75.8) | |
| 6 Previously passed COVID-19 infection, n (%) | 0.231 | |||
| Yes | 0 | 0 | 0 | |
| No | 106 (100) | 73 (100) | 33 (100) | |
| Chronic conditions, n (%) | ||||
| Arterial hypertension | 47 (44.3) | 38 (52.1) | 9 (27.3) | 0.017 |
| Obesity | 35 (33.0) | 28 (38.4) | 7 (21.2) | 0.082 |
| Insulin-dependent diabetes mellitus | 24 (22.6) | 20 (27.4) | 4 (12.1) | 0.082 |
| 7 Respiratory | 15 (14.2) | 9 (12.3) | 6 (18.2) | 0.423 |
| Cancer | 28 (26.4) | 15 (20.5) | 13 (39.4) | 0.042 |
| 8 Cardiovascular | 35 (33.0) | 30 (41.1) | 5 (15.2) | 0.009 |
| Usual treatment, n (%) | ||||
| Antihypertensives | 51 (48.1) | 39 (53.4) | 12 (36.4) | 0.104 |
| Anticoagulants | 25 (23.6) | 25 (34.2) | 0 | <0.001 |
| Immunosuppressants | 1 (0.9) | 1 (1.4) | 0 | 0.499 |
| Anxiolytics/Sedatives | 54 (50.9) | 52 (71.2) | 2 (6.1) | <0.001 |
| Lipid lowering agents | 11 (10.4) | 11 (15.1) | 0 | 0.018 |
| Antidiabetics | 15 (14.2) | 15 (20.5) | 0 | 0.005 |
| Cardiovascular | 50 (47.2) | 47 (64.4) | 3 (9.1) | <0.001 |
| Use of oxygen therapy, n (%) | 0.728 | |||
| Currently | 1 (0.9) | 1 (1.4) | 0 | |
| Previous/Occasional | 5 (4.7) | 3 (4.1) | 2 (6.1) | |
| Never | 100 (94.3) | 69 (94.5) | 31 (93.9) | |
| Vital signs, mean (SD) | ||||
| Blood pressure | ||||
| SBP (mmHg) | 126 (15.0) | 127 (15.0) | 123 (15.0) | |
| DBT (mmHg) | 71.3 (13.4) | 70.1 (14.7) | 73.9 (9.4) | |
| Heart rate (bpm) | 75.1 (11.7) | 74.3 (12.3) | 76.9 (10.1) | |
| Temperature (°C) | 35.8 (0.5) | 35.9 (0.4) | 35.7 (0.5) | |
| Oxygen saturation (%) | 96.9 (1.7) | 96.4 (1.6) | 98 (1.3) |
| Type | Symptom | Presence of Symptoms | Symptoms Score | |||
|---|---|---|---|---|---|---|
| Participants (n = 106) | 1 | 2 | 3 | 4 | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Local reactions | Injection site Pain | 47 (44.3) | 43 (90.9) | 4 (9.1) | - | - |
| Injection site Redness | 20 (18.8) | 20 (100.0) | - | - | - | |
| Injection site Swelling | 20 (18.8) | 18 (90.4) | 2 (9.6) | - | - | |
| Systemic reactions | Chills or shivering | 18 (17.0) | 16 (89.4) | 2 (10.3) | - | - |
| Fatigue or tiredness | 24 (22.5) | 18 (75.1) | 5 (20.8) | 1 (4.1) | - | |
| Muscle aches or pains | 30 (28.2) | 23 (76.9) | 6 (19.8) | 1 (3.3) | - | |
| Headache | 30 (28.2) | 22 (73.5) | 8 (26.5) | - | - | |
| Joint pains | 15 (14.0) | 14 (93.6) | 1 (6.4) | - | - | |
| Vomiting or Nauseous | 9 (8.4) | 8 (93.7) | 1 (6.3) | - | - | |
| Diarrhea | 11 (10.0) | 7 (64.1) | 4 (35.9) | - | - | |
| Fever (≥38.0 °C) | 4 (3.2) | 2 (40.6) | 1 (29.7) | 1 (29.7) | - | |
| Type | Symptom | Presence of Symptoms | Symptoms Score | |||
|---|---|---|---|---|---|---|
| Participants (n = 106) | 1 | 2 | 3 | 4 | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Local reactions | Injection site Pain | 23 (21.6) | 20 (87.1) | 2 (8.3) | 1 (4.6) | - |
| Injection site Redness | 19 (17.9) | 18 (94.9) | 1 (5.1) | - | - | |
| Injection site Swelling | 14 (13.3) | 14 (100) | - | - | - | |
| Systemic reactions | Chills or shivering | 14 (13.3) | 11 (78.9) | 3 (21.1) | - | - |
| Fatigue or tiredness | 16 (15.0) | 12 (75.4) | 2 (12.3) | 2 (12.3) | - | |
| Muscle aches or pains | 19 (17.9) | 11 (58.1) | 7 (36.9) | 1 (5.0) | - | |
| Headache | 22 (20.7) | 14 (63.7) | 6 (27.1) | 2 (9.2) | - | |
| Joint pains | 11 (10.3) | 5 (45.6) | 3 (27.2) | 3 (27.2) | - | |
| Vomiting or Nauseous | 5 (4.6) | 4 (80.4) | 1 (19.6) | - | - | |
| Diarrhea | 7 (6.5) | 5 (72.3) | 2 (27.7) | - | - | |
| Fever (≥38.0 °C) | 1 (0.9) | 1 (100.0) | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Garrosa, M.; Sánchez-Serrano, N.; Garrosa, E.; Jiménez-Callejo, E.; Pardo Yanguas, M.D.; Mielgo-Ayuso, J.; Seco-Calvo, J. Effectiveness of Comirnaty® Vaccine and Correlates of Immunogenicity and Adverse Reactions: A Single-Center Prospective Case Series Study. Vaccines 2022, 10, 1170. https://doi.org/10.3390/vaccines10081170
Fernández-Lázaro D, Garrosa M, Sánchez-Serrano N, Garrosa E, Jiménez-Callejo E, Pardo Yanguas MD, Mielgo-Ayuso J, Seco-Calvo J. Effectiveness of Comirnaty® Vaccine and Correlates of Immunogenicity and Adverse Reactions: A Single-Center Prospective Case Series Study. Vaccines. 2022; 10(8):1170. https://doi.org/10.3390/vaccines10081170
Chicago/Turabian StyleFernández-Lázaro, Diego, Manuel Garrosa, Nerea Sánchez-Serrano, Evelina Garrosa, Elena Jiménez-Callejo, María Dolores Pardo Yanguas, Juan Mielgo-Ayuso, and Jesús Seco-Calvo. 2022. "Effectiveness of Comirnaty® Vaccine and Correlates of Immunogenicity and Adverse Reactions: A Single-Center Prospective Case Series Study" Vaccines 10, no. 8: 1170. https://doi.org/10.3390/vaccines10081170
APA StyleFernández-Lázaro, D., Garrosa, M., Sánchez-Serrano, N., Garrosa, E., Jiménez-Callejo, E., Pardo Yanguas, M. D., Mielgo-Ayuso, J., & Seco-Calvo, J. (2022). Effectiveness of Comirnaty® Vaccine and Correlates of Immunogenicity and Adverse Reactions: A Single-Center Prospective Case Series Study. Vaccines, 10(8), 1170. https://doi.org/10.3390/vaccines10081170








