Efficacy of Commercial Infectious Bronchitis Vaccines against Canadian Delmarva (DMV/1639) Infectious Bronchitis Virus Infection in Layers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccines and Challenge Virus
2.2. Chickens
2.3. Experimental Procedures
2.3.1. Vaccination
2.3.2. Viral Challenge
2.3.3. Clinical Observations and Sample Collection
2.3.4. Quantification of Viral Genome Loads from Swabs and Tissues
2.3.5. Antibody-Mediated Immune Responses by Indirect ELISA
2.3.6. Cell-Mediated Immune Responses by Flow Cytometry
2.3.7. Histopathology
2.3.8. Statistical Analysis
3. Results
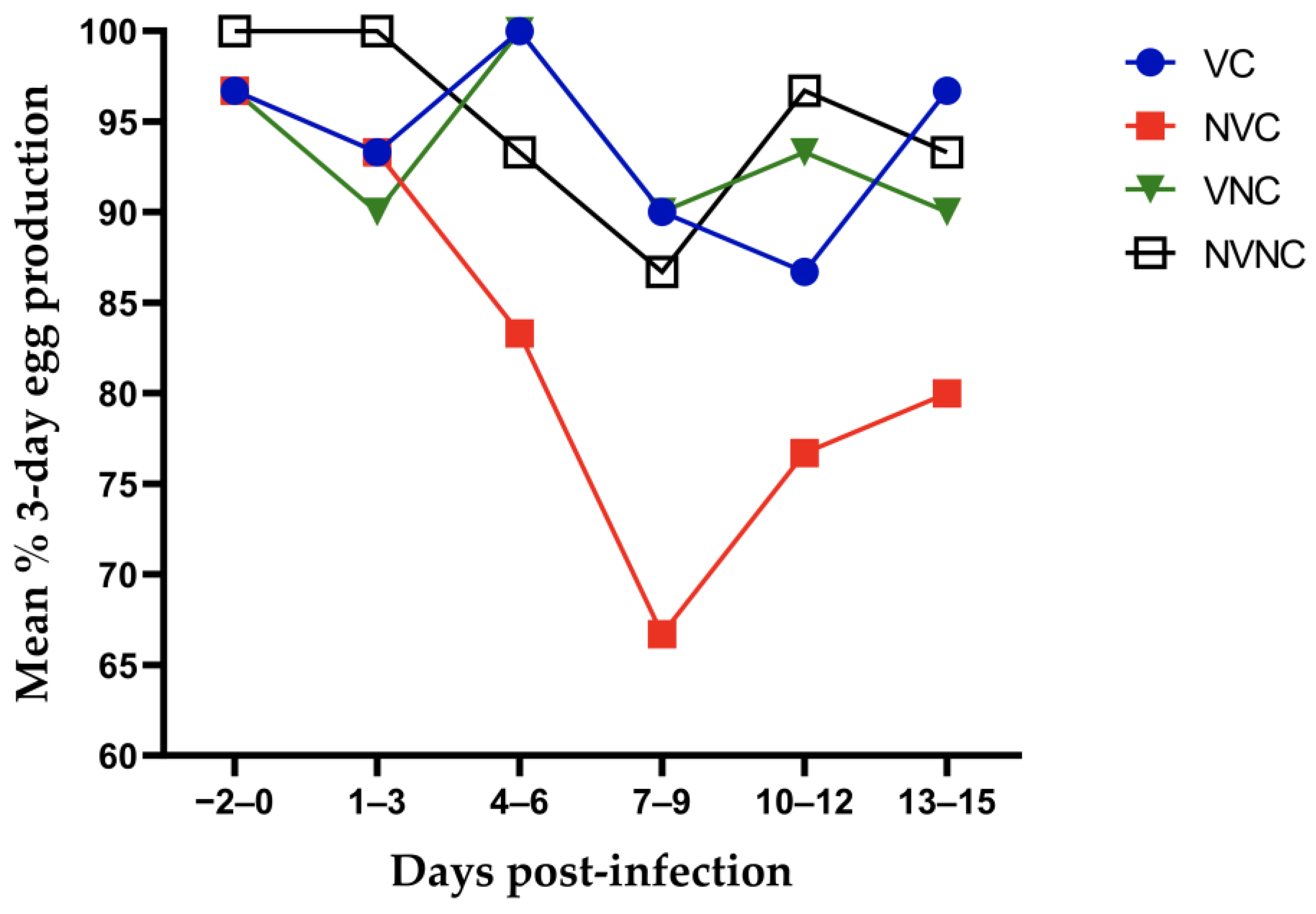
3.1. Clinical Observations and Egg Production
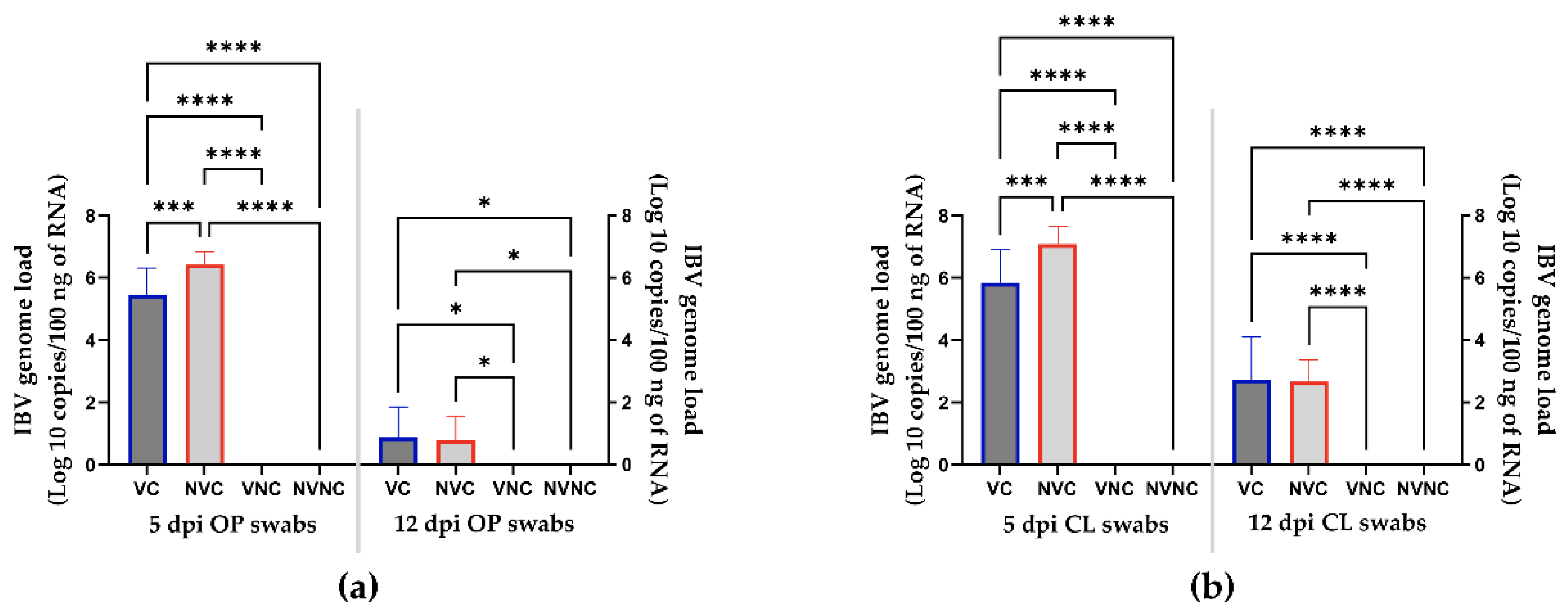
3.2. Viral Shedding
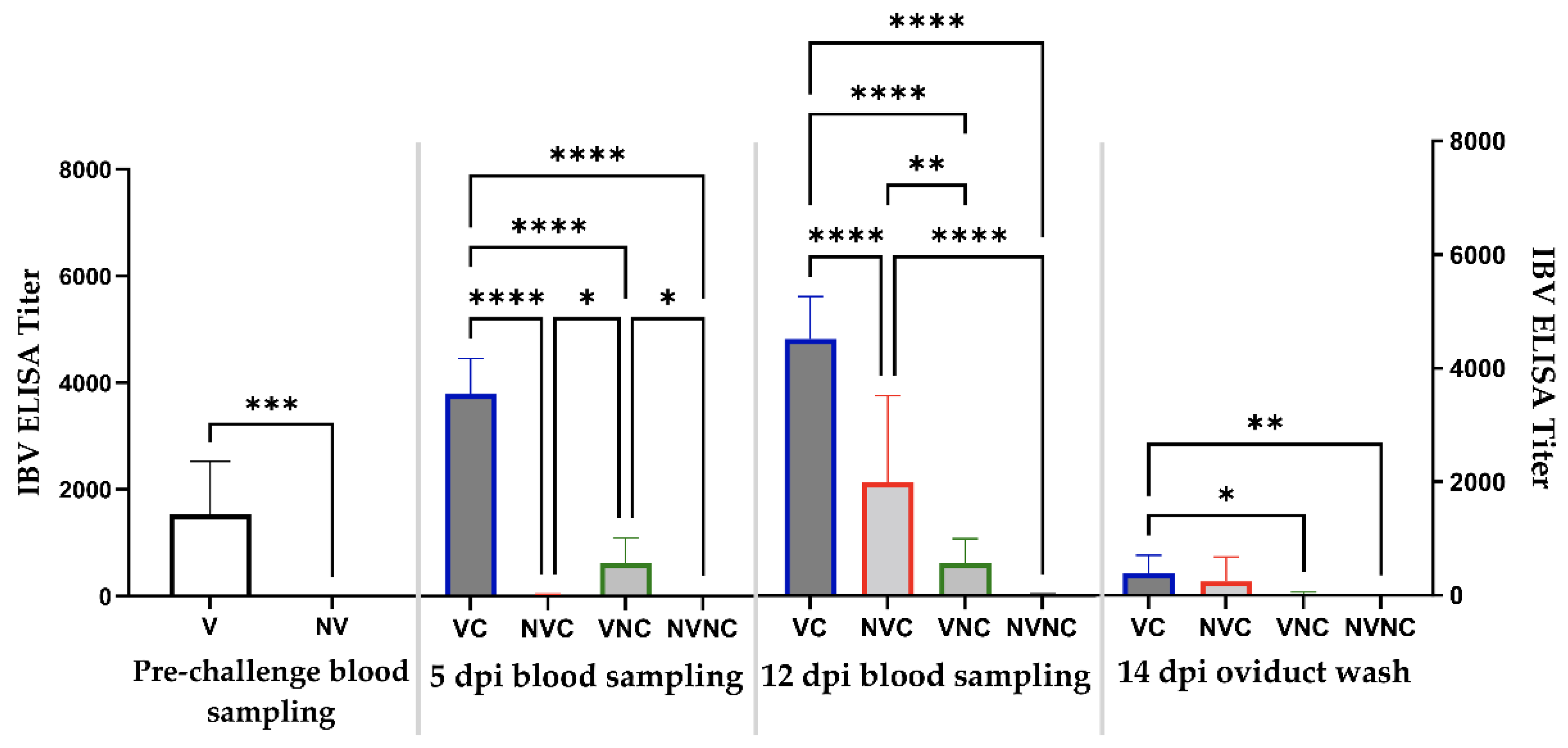
3.3. Anti-IBV Antibody Titer
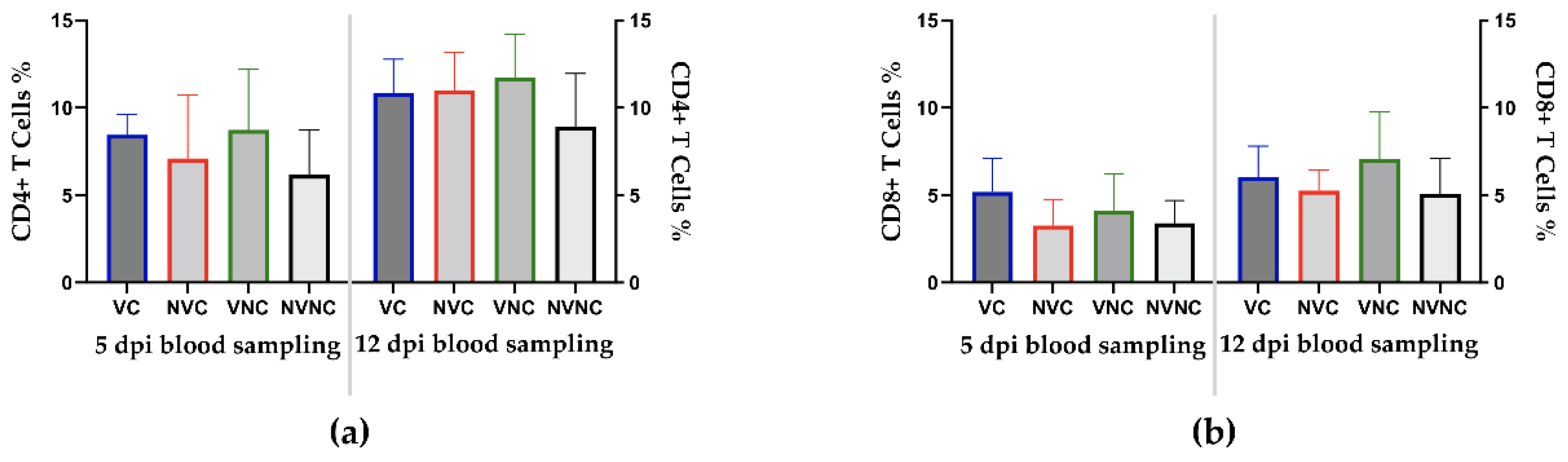
3.4. Peripheral Blood CD4+ and CD8+ T Cells
3.5. IBV Genome Loads in Tissues
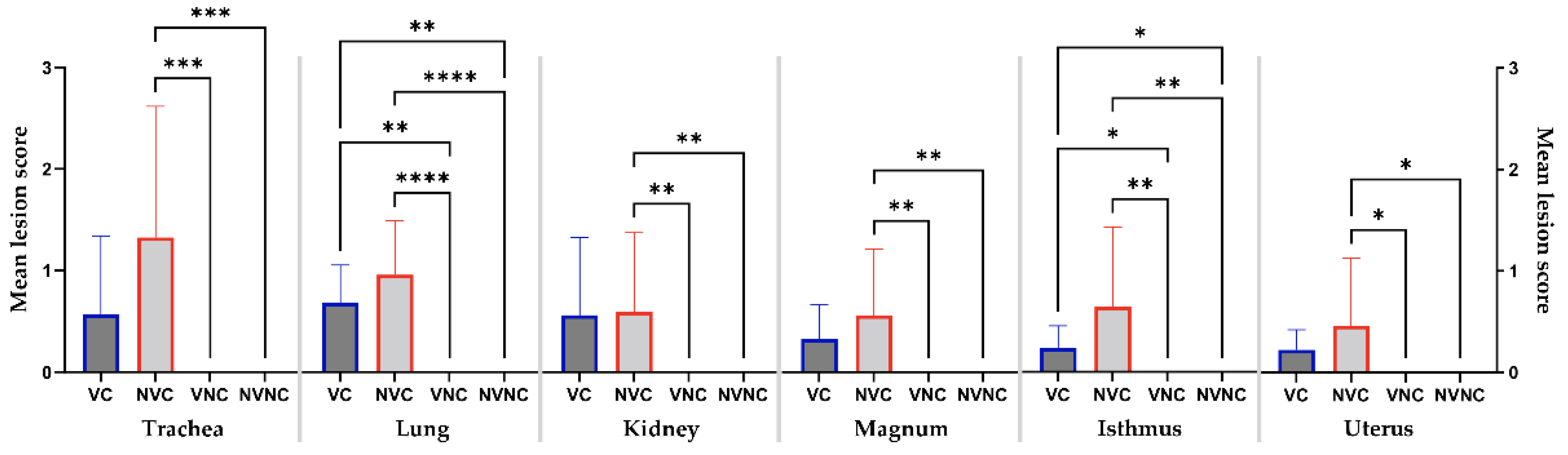
3.6. Histopathology
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickramasinghe, I.N.; van Beurden, S.J.; Weerts, E.A.; Verheije, M.H. The avian coronavirus spike protein. Virus Res. 2014, 194, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Chen, H.W. Infectious Bronchitis Virus Variants: Molecular Analysis and Pathogenicity Investigation. Int. J. Mol. Sci. 2017, 18, 2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, B. Vaccination against infectious bronchitis virus: A continuous challenge. Vet. Microbiol. 2017, 206, 137–143. [Google Scholar] [CrossRef]
- Raj, G.D.; Jones, R.C. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997, 26, 677–706. [Google Scholar] [CrossRef]
- Muneer, M.A.; Newman, J.A.; Halvorson, D.A.; Sivanandan, V.; Coon, C.N. Effects of avian infectious bronchitis virus (Arkansas strain) on vaccinated laying chickens. Avian Dis. 1987, 31, 820–828. [Google Scholar] [CrossRef]
- Chousalkar, K.K.; Roberts, J.R. Effects of Australian strains of infectious bronchitis virus on internal and external quality of hen eggs. Anim. Prod. Sci. 2009, 49, 162–169. [Google Scholar] [CrossRef]
- Hong, S.M.; Kwon, H.J.; Kim, I.H.; Mo, M.L.; Kim, J.H. Comparative genomics of Korean infectious bronchitis viruses (IBVs) and an animal model to evaluate pathogenicity of IBVs to the reproductive organs. Viruses 2012, 4, 2670–2683. [Google Scholar] [CrossRef] [Green Version]
- Sevoian, M.; Levine, P.P. Effects of Infectious Bronchitis on the Reproductive Tracts, Egg Production, and Egg Quality of Laying Chickens. Avian Dis. 1957, 1, 136–164. [Google Scholar] [CrossRef]
- Chousalkar, K.K.; Roberts, J.R.; Reece, R. Comparative histopathology of two serotypes of infectious bronchitis virus (T and n1/88) in laying hens and cockerels. Poult. Sci. 2007, 86, 50–58. [Google Scholar] [CrossRef]
- Crinion, R.A.; Ball, R.A.; Hofstad, M.S. Abnormalities in laying chickens following exposure to infectious bronchitis virus at one day old. Avian Dis. 1971, 15, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Benyeda, Z.; Mato, T.; Suveges, T.; Szabo, E.; Kardi, V.; Abonyi-Toth, Z.; Rusvai, M.; Palya, V. Comparison of the pathogenicity of QX-like, M41 and 793/B infectious bronchitis strains from different pathological conditions. Avian Pathol. 2009, 38, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parent, E.; Gagnon-Francoeur, A.; Lanthier, B.; Hébert, G.; Buczinski, S.; Boulianne, M. Diagnostic Accuracy of Ultrasonography to Detect False Layers in a Commercial Laying Flock Infected by an Infectious Bronchitis Virus Delmarva Genotype Causing Cystic Oviducts. Avian Dis. 2020, 64, 149–156. [Google Scholar] [CrossRef]
- Zhong, Q.; Hu, Y.X.; Jin, J.H.; Zhao, Y.; Zhao, J.; Zhang, G.Z. Pathogenicity of virulent infectious bronchitis virus isolate YN on hen ovary and oviduct. Vet. Microbiol. 2016, 193, 100–105. [Google Scholar] [CrossRef]
- Cook, J.K.; Orbell, S.J.; Woods, M.A.; Huggins, M.B. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999, 28, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.K.; Chesher, J.; Baxendale, W.; Greenwood, N.; Huggins, M.B.; Orbell, S.J. Protection of chickens against renal damage caused by a nephropathogenic infectious bronchitis virus. Avian Pathol. 2001, 30, 423–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Wit, J.J.; Cook, J.K. Factors influencing the outcome of infectious bronchitis vaccination and challenge experiments. Avian Pathol. 2014, 43, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Box, P.G.; Holmes, H.C.; Finney, P.M.; Froymann, R. Infectious bronchitis in laying hens: The relationship between haemagglutination inhibition antibody levels and resistance to experimental challenge. Avian Pathol. 1988, 17, 349–361. [Google Scholar] [CrossRef]
- de Wit, J.J.S.; Malo, A.; Cook, J.K.A. Induction of IBV strain-specific neutralizing antibodies and broad spectrum protection in layer pullets primed with IBV Massachusetts (Mass) and 793B vaccines prior to injection of inactivated vaccine containing Mass antigen. Avian Pathol. 2019, 48, 135–147. [Google Scholar] [CrossRef]
- de Wit, J.J.; Dijkman, R.; Guerrero, P.; Calvo, J.; Gonzalez, A.; Hidalgo, H. Variability in biological behaviour, pathogenicity, protectotype and induction of virus neutralizing antibodies by different vaccination programmes to infectious bronchitis virus genotype Q1 strains from Chile. Avian Pathol. 2017, 46, 666–675. [Google Scholar] [CrossRef] [Green Version]
- Amarasinghe, A.; Popowich, S.; De Silva Senapathi, U.; Abdul-Cader, M.S.; Marshall, F.; van der Meer, F.; Cork, S.C.; Gomis, S.; Abdul-Careem, M.F. Shell-Less Egg Syndrome (SES) Widespread in Western Canadian Layer Operations Is Linked to a Massachusetts (Mass) Type Infectious Bronchitis Virus (IBV) Isolate. Viruses 2018, 10, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.S.H.; Ojkic, D.; Coffin, C.S.; Cork, S.C.; van der Meer, F.; Abdul-Careem, M.F. Delmarva (DMV/1639) Infectious Bronchitis Virus (IBV) Variants Isolated in Eastern Canada Show Evidence of Recombination. Viruses 2019, 11, 1054. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.S.H.; Ali, A.; Buharideen, S.M.; Goldsmith, D.; Coffin, C.S.; Cork, S.C.; van der Meer, F.; Boulianne, M.; Abdul-Careem, M.F. Pathogenicity of the Canadian Delmarva (DMV/1639) Infectious Bronchitis Virus (IBV) on Female Reproductive Tract of Chickens. Viruses 2021, 13, 2488. [Google Scholar] [CrossRef]
- Hassan, M.S.H.; Najimudeen, S.M.; Ali, A.; Altakrouni, D.; Goldsmith, D.; Coffin, C.S.; Cork, S.C.; van der Meer, F.; Abdul-Careem, M.F. Immunopathogenesis of the Canadian Delmarva (DMV/1639) infectious bronchitis virus (IBV): Impact on the reproductive tract in layers. Microb. Pathog. 2022, 166, 105513. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Hy-Line W36 Commercial Layers Management Guide. Available online: https://www.hyline.com/literature/W-36 (accessed on 17 February 2020).
- Raj, G.D.; Jones, R.C. Local antibody production in the oviduct and gut of hens infected with a variant strain of infectious bronchitis virus. Vet. Immunol. Immunopathol. 1996, 53, 147–161. [Google Scholar] [CrossRef]
- Kameka, A.M.; Haddadi, S.; Kim, D.S.; Cork, S.C.; Abdul-Careem, M.F. Induction of innate immune response following infectious bronchitis corona virus infection in the respiratory tract of chickens. Virology 2014, 450–451, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Benyeda, Z.; Szeredi, L.; Mató, T.; Süveges, T.; Balka, G.; Abonyi-Tóth, Z.; Rusvai, M.; Palya, V. Comparative histopathology and immunohistochemistry of QX-like, Massachusetts and 793/B serotypes of infectious bronchitis virus infection in chickens. J. Comp. Pathol. 2010, 143, 276–283. [Google Scholar] [CrossRef]
- de Wit, J.J.; Nieuwenhuisen-van Wilgen, J.; Hoogkamer, A.; van de Sande, H.; Zuidam, G.J.; Fabri, T.H. Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388 (genotype QX) by maternally derived antibodies and by early vaccination. Avian Pathol. 2011, 40, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.S.H.; Abdul-Careem, M.F. Avian Viruses that Impact Table Egg Production. Animals 2020, 10, 1747. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Harada, M.; Horiuchi, T.; Yoshida, K.; Tanimura, C.; Nakamura, S.; Mase, M.; Suzuki, S. Serological studies of infectious bronchitis vaccines against Japanese field isolates of homologous and heterologous genotypes. J. Vet. Med. Sci. 2009, 71, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Collisson, E.W.; Pei, J.; Dzielawa, J.; Seo, S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev. Comp. Immunol. 2000, 24, 187–200. [Google Scholar] [CrossRef]
- Seo, S.H.; Collisson, E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 1997, 71, 5173–5177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chousalkar, K.K.; Cheetham, B.F.; Roberts, J.R. Detection of infectious bronchitis virus strain N1/88 from the oviduct and feces of experimentally infected vaccinated and unvaccinated hens. Poult. Sci. 2010, 89, 1603–1608. [Google Scholar] [CrossRef]
- De Wit, J.J. Detection of infectious bronchitis virus. Avian Pathol. 2000, 29, 71–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelb, J., Jr.; Ladman, B.S.; Pope, C.R.; Ruano, J.M.; Brannick, E.M.; Bautista, D.A.; Coughlin, C.M.; Preskenis, L.A. Characterization of nephropathogenic infectious bronchitis virus DMV/1639/11 recovered from Delmarva broiler chickens in 2011. Avian Dis. 2013, 57, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Buharideen, S.M.; Hassan, M.S.H.; Najimudeen, S.M.; Niu, D.; Czub, M.; Gomis, S.; Abdul-Careem, M.F. Immune Responses in Laying Hens after an Infectious Bronchitis Vaccination of Pullets: A Comparison of Two Vaccination Strategies. Vaccines 2021, 9, 531. [Google Scholar] [CrossRef]
- Ladman, B.S.; Pope, C.R.; Ziegler, A.F.; Swieczkowski, T.; Callahan, C.J.; Davison, S.; Gelb, J., Jr. Protection of chickens after live and inactivated virus vaccination against challenge with nephropathogenic infectious bronchitis virus PA/Wolgemuth/98. Avian Dis. 2002, 46, 938–944. [Google Scholar] [CrossRef] [Green Version]
- Gelb, J., Jr.; Wolff, J.B.; Moran, C.A. Variant serotypes of infectious bronchitis virus isolated from commercial layer and broiler chickens. Avian Dis. 1991, 35, 82–87. [Google Scholar] [CrossRef]
- Cavanagh, D.; Elus, M.M.; Cook, J.K. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian Pathol. 1997, 26, 63–74. [Google Scholar] [CrossRef] [Green Version]


| Tissue | Lesions |
|---|---|
| Trachea |
Loss of epithelial lining Loss of cilia Necrosis of epithelial lining Inflammatory cell infiltrations in the lamina propria |
| Lung |
Peri-bronchitis Inflammatory cell infiltrations in the interstitial tissue Circulatory disturbances (hyperemia, edema, and hemorrhage in the interstitial tissue |
| Kidney |
Necrosis of ducto-tubular epithelium Inflammatory cell infiltrations in the interstitial tissue Renal tubular dilatation |
| Oviduct (magnum, isthmus, and uterus) |
Epithelial cell necrosis Loss of cilia Tubular gland dilatation Lymphocyte infiltrations in lamina propria Edema in lamina propria |
| Groups | VC | NVC | Control Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Change (−) | Mild (+) | Moderate (++) | Severe (+++) | No Change (−) | Mild (+) | Moderate (++) | Severe (+++) | No Change (−) | Mild (+) | Moderate (++) | Severe (+++) | |
| Trachea | 6/10a | 1/10 | 3/10 | 0/10 | 1/10 | 4/10 | 2/10 | 3/10 | 10/10 | 0/10 | 0/10 | 0/10 |
| Lung | 0/10 | 8/10 | 2/10 | 0/10 | 010 | 5/10 | 4/10 | 1/10 | 10/10 | 0/10 | 0/10 | 0/10 |
| Kidney | 6/10 | 0/10 | 3/10 | 1/10 | 3/10 | 4/10 | 2/10 | 1/10 | 10/10 | 0/10 | 0/10 | 0/10 |
| Magnum | 4/10 | 6/10 | 0/10 | 0/10 | 2/10 | 7/10 | 0/10 | 1/10 | 10/10 | 0/10 | 0/10 | 0/10 |
| Isthmus | 2/8 | 6/8 | 0/8 | 0/8 | 1/7 | 5/7 | 0/7 | 1/7 | 10/10 | 0/10 | 0/10 | 0/10 |
| Uterus | 4/10 | 6/10 | 0/10 | 0/10 | 4/10 | 5/10 | 0/10 | 1/10 | 10/10 | 0/10 | 0/10 | 0/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.S.H.; Buharideen, S.M.; Ali, A.; Najimudeen, S.M.; Goldsmith, D.; Coffin, C.S.; Cork, S.C.; van der Meer, F.; Abdul-Careem, M.F. Efficacy of Commercial Infectious Bronchitis Vaccines against Canadian Delmarva (DMV/1639) Infectious Bronchitis Virus Infection in Layers. Vaccines 2022, 10, 1194. https://doi.org/10.3390/vaccines10081194
Hassan MSH, Buharideen SM, Ali A, Najimudeen SM, Goldsmith D, Coffin CS, Cork SC, van der Meer F, Abdul-Careem MF. Efficacy of Commercial Infectious Bronchitis Vaccines against Canadian Delmarva (DMV/1639) Infectious Bronchitis Virus Infection in Layers. Vaccines. 2022; 10(8):1194. https://doi.org/10.3390/vaccines10081194
Chicago/Turabian StyleHassan, Mohamed S. H., Sabrina M. Buharideen, Ahmed Ali, Shahnas M. Najimudeen, Dayna Goldsmith, Carla S. Coffin, Susan C. Cork, Frank van der Meer, and Mohamed Faizal Abdul-Careem. 2022. "Efficacy of Commercial Infectious Bronchitis Vaccines against Canadian Delmarva (DMV/1639) Infectious Bronchitis Virus Infection in Layers" Vaccines 10, no. 8: 1194. https://doi.org/10.3390/vaccines10081194
APA StyleHassan, M. S. H., Buharideen, S. M., Ali, A., Najimudeen, S. M., Goldsmith, D., Coffin, C. S., Cork, S. C., van der Meer, F., & Abdul-Careem, M. F. (2022). Efficacy of Commercial Infectious Bronchitis Vaccines against Canadian Delmarva (DMV/1639) Infectious Bronchitis Virus Infection in Layers. Vaccines, 10(8), 1194. https://doi.org/10.3390/vaccines10081194









