LAMP-1 Chimeric to HIV-1 p55Gag in the Immunization of Neonate Mice Induces an Early Germinal Center Formation and AID Expression
Abstract
:1. Introduction
2. Material and Methods
2.1. Plasmids
2.2. Animals
2.3. Mice Immunization Protocol
2.4. Isolation of Mononuclear Cells
2.5. Detection of IFN-γ and Antibody-Secreting Cells by ELISPOT Assay
2.6. Detection of Anti-P24 IgG Antibodies through ELISA
2.7. TFH Cells and GC B Cell Characterization by Flow Cytometry
2.8. Immunofluorescence
2.9. Real-Time PCR
2.10. Anti-P24 Antibody Affinity by Microscale Thermophoresis
2.11. Statistical Analysis
3. Results
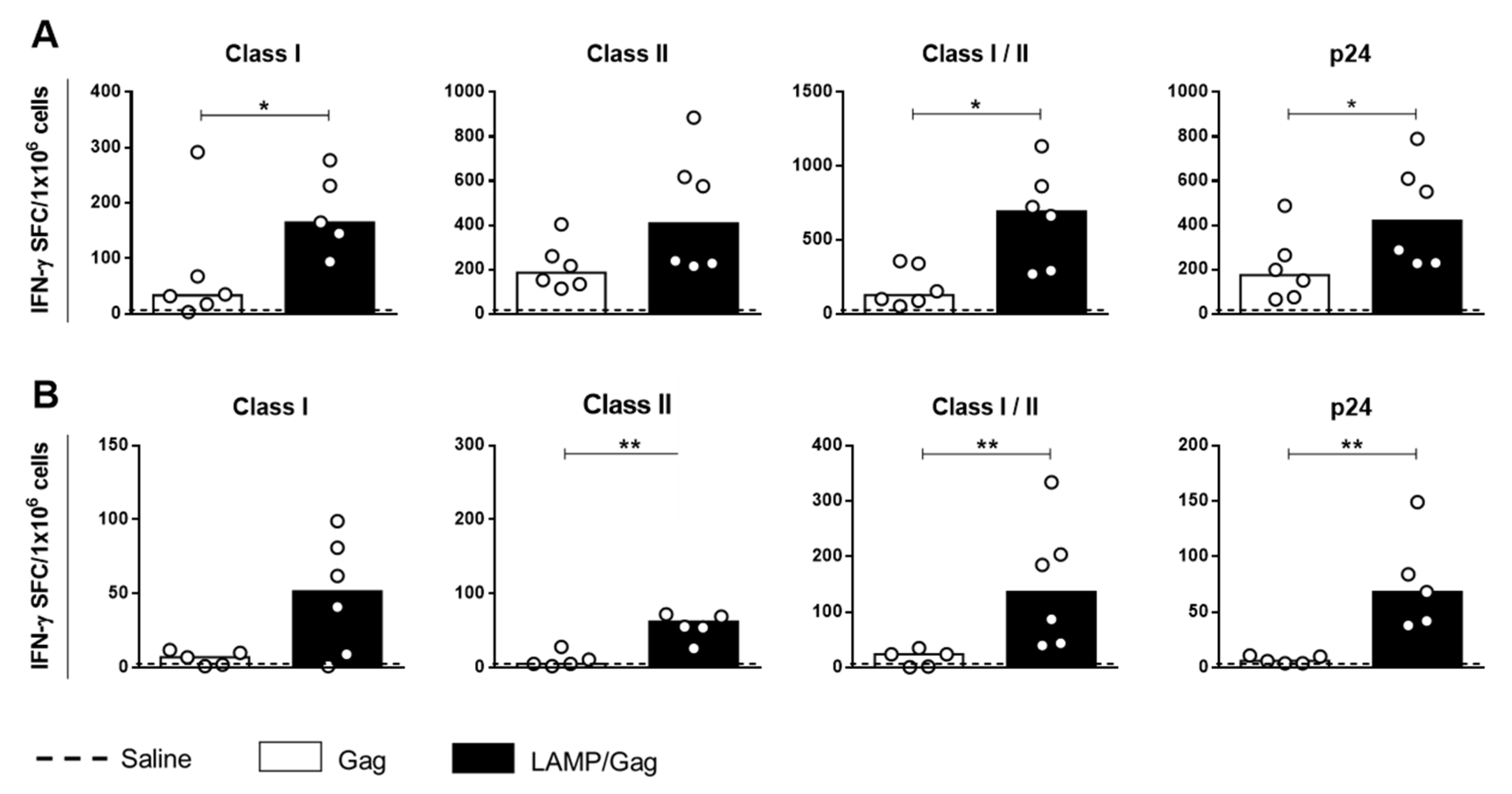
3.1. Neonatal Chimeric LAMP/Gag DNA Vaccine Enhances T-Cell Response
3.2. LAMP/Gag Vaccination of Neonates Generates High-Affinity Antibodies and Long-Lived Plasma Cells
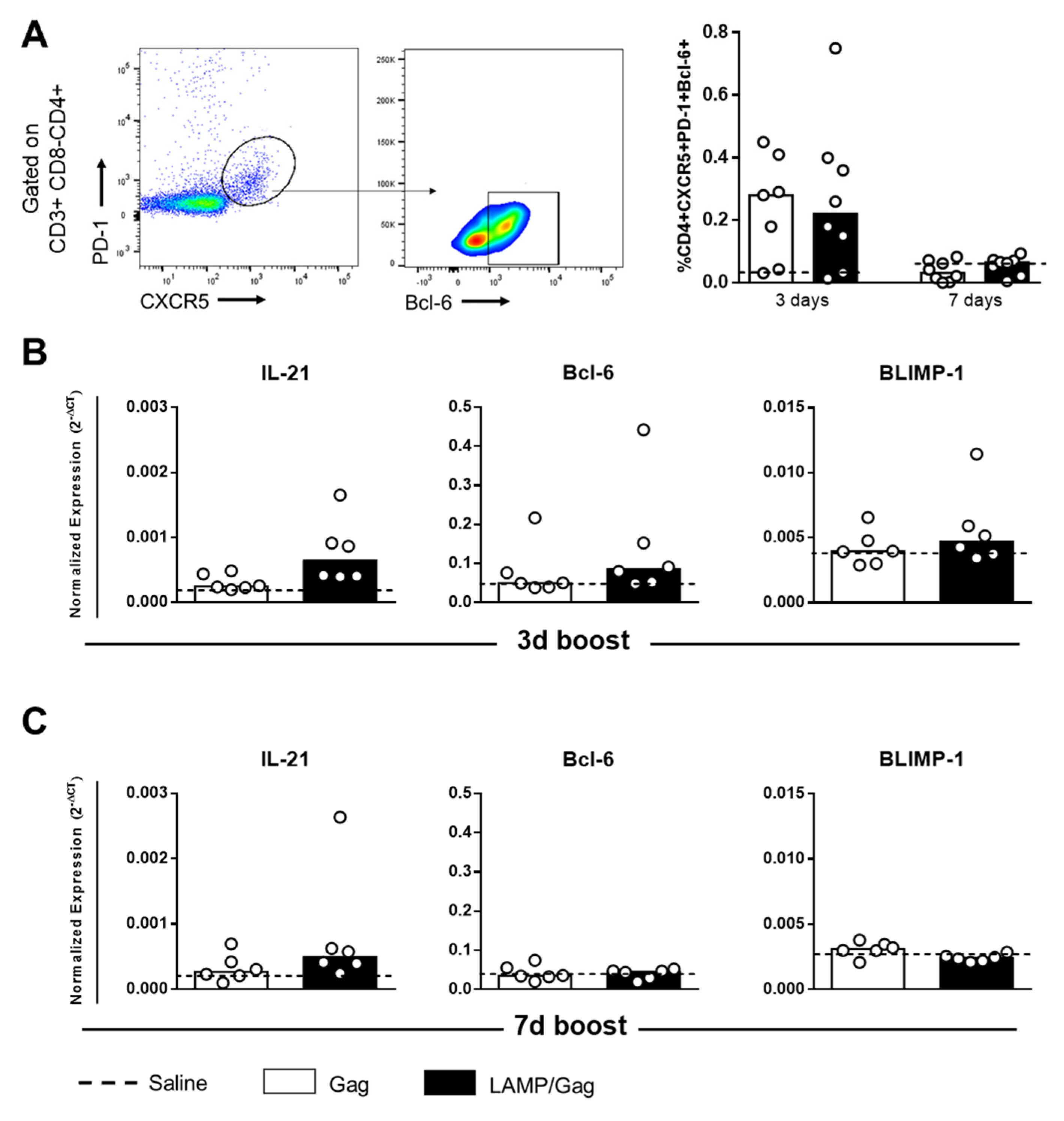
3.3. Neonatal LAMP/Gag Immunization Promotes Early TFH Cell Generation
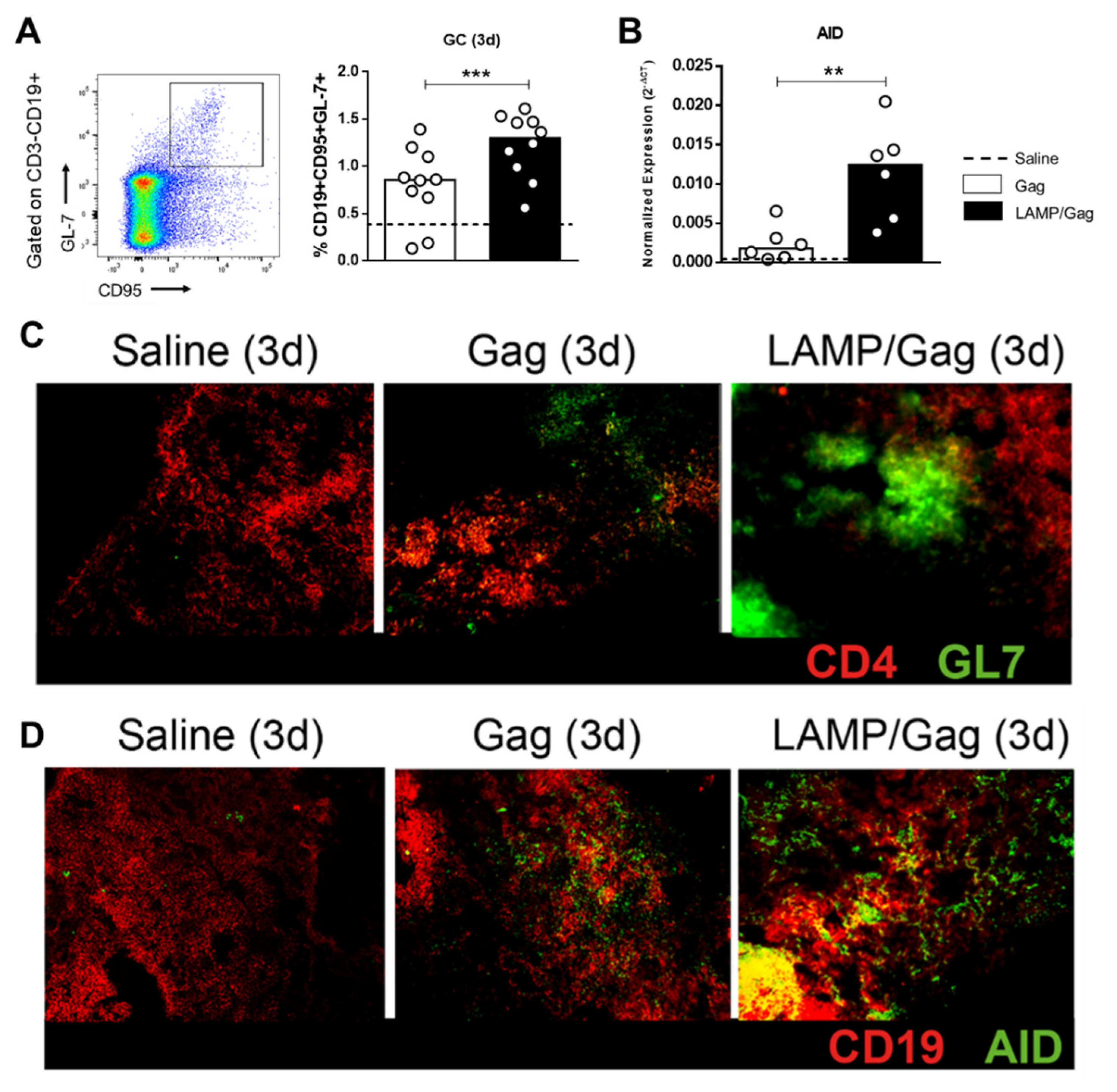
3.4. LAMP/Gag Induces Germinal Center Sites with Up-Regulation of AID Enzyme Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV&AIDS Statistics—Fact Sheet 2022. Available online: https://www.unaids.org/en/resources/fact-sheet#:~:text=38.4%20million%20%5B33.9%20million%E2%80%9343.8,AIDS%2Drelated%20illnesses%20in%202021 (accessed on 30 July 2022).
- de Brito, C.A.; Goldoni, A.L.; Sato, M.N. Immune adjuvants in early life: Targeting the innate immune system to overcome impaired adaptive response. Immunotherapy 2009, 1, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Goenka, A.; Kollmann, T.R. Development of immunity in early life. J. Infect. 2015, 71 (Suppl. 1), S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Goodnow, C.C.; Vinuesa, C.G.; Randall, K.L.; Mackay, F.; Brink, R. Control systems and decision making for antibody production. Nat. Immunol. 2010, 11, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Pihlgren, M.; Tougne, C.; Bozzotti, P.; Fulurija, A.; Duchosal, M.A.; Lambert, P.H.; Siegrist, C.A. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J. Immunol. 2003, 170, 2824–2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinicola, B.; Conti, M.G.; Terrin, G.; Sgrulletti, M.; Elfeky, R.; Carsetti, R.; Salinas, A.F.; Mortari, E.P.; Brindisi, G.; de Curtis, M.; et al. The Protective Role of Maternal Immunization in Early Life. Front. Pediatr. 2021, 9, 638871. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Kleijmeer, M.J.; Ossevoort, M.A.; van Veen, C.J.; van Hellemond, J.J.; Neefjes, J.J.; Kast, W.M.; Melief, C.J.; Geuze, H.J. MHC class II compartments and the kinetics of antigen presentation in activated mouse spleen dendritic cells. J. Immunol. 1995, 154, 5715–5724. [Google Scholar] [PubMed]
- Ruff, A.L.; Guarnieri, F.G.; Staveley-O’Carroll, K.; Siliciano, R.F.; August, J.T. The enhanced immune response to the HIV gp160/LAMP chimeric gene product targeted to the lysosome membrane protein trafficking pathway. J. Biol. Chem. 1997, 272, 8671–8678. [Google Scholar] [CrossRef] [Green Version]
- Arruda, L.B.; Sim, D.; Chikhlikar, P.R.; Maciel, M.; Akasaki, K.; August, J.T.; Marques, E.T. Dendritic cell-lysosomal-associated membrane protein (LAMP) and LAMP-1-HIV-1 gag chimeras have distinct cellular trafficking pathways and prime T and B cell responses to a diverse repertoire of epitopes. J. Immunol. 2006, 177, 2265–2275. [Google Scholar] [CrossRef] [Green Version]
- de Arruda, L.B.; Chikhlikar, P.R.; August, J.T.; Marques, E.T. DNA vaccine encoding human immunodeficiency virus-1 Gag, targeted to the major histocompatibility complex II compartment by lysosomal-associated membrane protein, elicits enhanced long-term memory response. Immunology 2004, 112, 126–133. [Google Scholar] [CrossRef]
- Marques, E.T.; Chikhlikar, P.; de Arruda, L.B.; Leao, I.C.; Lu, Y.; Wong, J.; Chen, J.S.; Byrne, B.; August, J.T. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J. Biol. Chem. 2003, 278, 37926–37936. [Google Scholar] [CrossRef] [Green Version]
- Chikhlikar, P.; de Arruda, L.B.; Maciel, M.; Silvera, P.; Lewis, M.G.; August, J.T.; Marques, E.T. DNA encoding an HIV-1 Gag/human lysosome-associated membrane protein-1 chimera elicits a broad cellular and humoral immune response in Rhesus macaques. PLoS ONE 2006, 1, e135. [Google Scholar] [CrossRef] [PubMed]
- Rigato, P.O.; Maciel, M.; Goldoni, A.L.; Piubelli, O.; de Brito, C.A.; Fusaro, A.E.; de Alencar, L.X.E.; August, T.; Marques, E.T.A.; Duarte, A.J.D.; et al. Immunization of neonatal mice with LAMP/p55 HIV gag DNA elicits robust immune responses that last to adulthood. Virology 2010, 406, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Rigato, P.O.; Maciel, M.; Goldoni, A.L.; Piubelli, O.G.; Orii, N.M.; Marques, E.T.; August, J.T.; Duarte, A.J.; Sato, M.N. Maternal LAMP/p55gagHIV-1 DNA immunization induces in utero priming and a long-lasting immune response in vaccinated neonates. PLoS ONE 2012, 7, e31608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldoni, A.L.; Maciel, M.; Rigato, P.O.; Piubelli, O.; de Brito, C.A.; Melo, A.; Marques, E.T.; August, J.T.; Duarte, A.J.; Sato, M.N. Mucosal and systemic anti-GAG immunity induced by neonatal immunization with HIV LAMP/gag DNA vaccine in mice. Immunobiology 2011, 216, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Maciel, M.; Cruz, F.D.S.P.; Cordeiro, M.T.; Da Motta, M.A.; Cassemiro, K.M.S.D.M.; Maia, R.D.C.C.; Figueiredo, R.; Galler, R.; Freire, M.D.S.; August, J.T.; et al. A DNA vaccine against yellow fever virus: Development and evaluation. PLoS Negl. Trop. Dis. 2015, 9, e0003693. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, F.F.; Viana, I.F.T.; Fischer, N.; Coêlho, D.F.; Silva, C.S.; Purificação, A.F.; Araújo, C.M.C.S.; Leite, B.H.S.; Durães-Carvalho, R.; Magalhães, T.; et al. Identification of a Zika NS2B epitope as a biomarker for severe clinical phenotypes. RSC Med. Chem. 2021, 12, 1525–1539. [Google Scholar] [CrossRef]
- Mata, M.; Travers, P.J.; Liu, Q.; Frankel, F.R.; Paterson, Y. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J. Immunol. 1998, 161, 2985–2993. [Google Scholar]
- Johnston, R.J.; Poholek, A.C.; DiToro, D.; Yusuf, I.; Eto, D.; Barnett, B.; Dent, A.L.; Craft, J.; Crotty, S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009, 325, 1006–1010. [Google Scholar] [CrossRef] [Green Version]
- Addo, M.M.; Yu, X.G.; Rathod, A.; Cohen, D.; Eldridge, R.L.; Strick, D.; Johnston, M.N.; Corcoran, C.; Wurcel, A.G.; Fitzpatrick, C.A.; et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 2003, 77, 2081–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, L.M.; Lima, J.F.; Cervantes, C.A.; Casseb, J.S.; Mendonça, M.; Duarte, A.J.; Sato, M.N. Increased frequency of circulating Tc22/Th22 cells and polyfunctional CD38(-) T cells in HIV-exposed uninfected subjects. Sci. Rep. 2015, 5, 13883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herath, S.; le Heron, A.; Colloca, S.; Patterson, S.; Tatoud, R.; Weber, J.; Dickson, G. Strain-dependent and distinctive T-cell responses to HIV antigens following immunisation of mice with differing chimpanzee adenovirus vaccine vectors. Vaccine 2016, 34, 4378–4385. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Kong, W.P.; Nabel, G.J. Enhanced breadth of CD4 T-cell immunity by DNA prime and adenovirus boost immunization to human immunodeficiency virus Env and Gag immunogens. J. Virol. 2005, 79, 8024–8031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.G.; Shang, H.; Addo, M.M.; Eldridge, R.L.; Phillips, M.N.; Feeney, M.E.; Strick, D.; Brander, C.; Goulder, P.J.; Rosenberg, E.S.; et al. Important contribution of p15 Gag-specific responses to the total Gag-specific CTL responses. AIDS 2002, 16, 321–328. [Google Scholar] [CrossRef]
- Buseyne, F.; Janvier, G.; Fleury, B.; Schmidt, D.; Rivière, Y. Multispecific and heterogeneous recognition of the gag protein by cytotoxic T lymphocytes (CTL) from HIV-infected patients: Factors other than the MHC control the epitopic specificities. Clin. Exp. Immunol. 1994, 97, 353–360. [Google Scholar] [CrossRef]
- Kaushik, S.; Vajpayee, M.; Wig, N.; Seth, P. Characterization of HIV-1 Gag-specific T cell responses in chronically infected Indian population. Clin. Exp. Immunol. 2005, 142, 388–397. [Google Scholar] [CrossRef]
- Chung, A.W.; Mabuka, J.M.; Ndlovu, B.; Licht, A.; Robinson, H.; Ramlakhan, Y.; Ghebremichael, M.; Reddy, T.; Goulder, P.J.R.; Walker, B.D.; et al. Viral control in chronic HIV-1 subtype C infection is associated with enrichment of p24 IgG1 with Fc effector activity. AIDS 2018, 32, 1207–1217. [Google Scholar] [CrossRef]
- Mastelic, B.; Kamath, A.T.; Fontannaz, P.; Tougne, C.; Rochat, A.F.; Belnoue, E.; Combescure, C.; Auderset, F.; Lambert, P.H.; Tacchini-Cottier, F.; et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J. Immunol. 2012, 189, 5764–5772. [Google Scholar] [CrossRef]
- Debock, I.; Jaworski, K.; Chadlaoui, H.; Delbauve, S.; Passon, N.; Twyffels, L.; Leo, O.; Flamand, V. Neonatal follicular Th cell responses are impaired and modulated by IL-4. J. Immunol. 2013, 191, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.S.; Deenick, E.K.; Batten, M.; Tangye, S.G. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 2012, 209, 1241–1253. [Google Scholar] [CrossRef] [Green Version]
- Petrovas, C.; Yamamoto, T.; Gerner, M.Y.; Boswell, K.L.; Wloka, K.; Smith, E.C.; Ambrozak, D.R.; Sandler, N.G.; Timmer, K.J.; Sun, X.; et al. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Invest. 2012, 122, 3281–3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassett, D.E.; Zhang, J.; Slifka, M.; Whitton, J.L. Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization. J. Virol. 2000, 74, 2620–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedegah, M.; Belmonte, M.; Epstein, J.E.; Siegrist, C.A.; Weiss, W.R.; Jones, T.R.; Lu, M.; Carucci, D.J.; Hoffman, S.L. Successful induction of CD8 T cell-dependent protection against malaria by sequential immunization with DNA and recombinant poxvirus of neonatal mice born to immune mothers. J. Immunol. 2003, 171, 3148–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capozzo, A.V.; Ramírez, K.; Polo, J.M.; Ulmer, J.; Barry, E.M.; Levine, M.M.; Pasetti, M.F. Neonatal immunization with a Sindbis virus-DNA measles vaccine induces adult-like neutralizing antibodies and cell-mediated immunity in the presence of maternal antibodies. J. Immunol. 2006, 176, 5671–5681. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Kampmann, B.; Mazmanian, S.K.; Marchant, A.; Levy, O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 2017, 46, 350–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muramatsu, M.; Kinoshita, K.; Fagarasan, S.; Yamada, S.; Shinkai, Y.; Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Keim, C.; Kazadi, D.; Rothschild, G.; Basu, U. Regulation of AID, the B-cell genome mutator. Genes Dev. 2013, 27, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.X.; Chaplin, D.D. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 1999, 17, 399–433. [Google Scholar] [CrossRef]
- Munguía-Fuentes, R.; Yam-Puc, J.C.; Silva-Sánchez, A.; Marcial-Juárez, E.; Gallegos-Hernández, I.A.; Calderón-Amador, J.; Randall, T.D.; Flores-Romo, L. Immunization of Newborn Mice Accelerates the Architectural Maturation of Lymph Nodes, But AID-Dependent IgG Responses Are Still Delayed Compared to the Adult. Front. Immunol. 2017, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Vono, M.; Eberhardt, C.S.; Mohr, E.; Auderset, F.; Christensen, D.; Schmolke, M.; Coler, R.; Meinke, A.; Andersen, P.; Lambert, P.H.; et al. Overcoming the Neonatal Limitations of Inducing Germinal Centers through Liposome-Based Adjuvants Including C-Type Lectin Agonists Trehalose Dibehenate or Curdlan. Front. Immunol. 2018, 9, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aradottir Pind, A.A.; Dubik, M.; Thorsdottir, S.; Meinke, A.; Harandi, A.M.; Holmgren, J.; del Giudice, G.; Jonsdottir, I.; Bjarnarson, S.P. Adjuvants Enhance the Induction of Germinal Center and Antibody Secreting Cells in Spleen and Their Persistence in Bone Marrow of Neonatal Mice. Front. Immunol. 2019, 10, 2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lofano, G.; Mancini, F.; Salvatore, G.; Cantisani, R.; Monaci, E.; Carrisi, C.; Tavarini, S.; Sammicheli, C.; Paccani, S.R.; Soldaini, E.; et al. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. J. Immunol. 2015, 195, 1617–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastelic Gavillet, B.; Eberhardt, C.S.; Auderset, F.; Castellino, F.; Seubert, A.; Tregoning, J.S.; Lambert, P.H.; de Gregorio, E.; del Giudice, G.; Siegrist, C.A. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J. Immunol. 2015, 194, 4836–4845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schussek, S.; Bernasconi, V.; Mattsson, J.; Wenzel, U.A.; Strömberg, A.; Gribonika, I.; Schön, K.; Lycke, N.Y. The CTA1-DD adjuvant strongly potentiates follicular dendritic cell function and germinal center formation, which results in improved neonatal immunization. Mucosal. Immunol. 2020, 13, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.M.; Forthal, D.N.; Harro, C.D.; Judson, F.N.; Mayer, K.H.; Para, M.F.; rgp120 HIV Vaccine Study Group. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 2005, 191, 654–665. [Google Scholar] [PubMed]
- Pitisuttithum, P.; Gilbert, P.; Gurwith, M.; Heyward, W.; Martin, M.; van Griensven, F.; Hu, D.; Tappero, J.W.; Choopanya, K.; Bangkok Vaccine Evaluation Group. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 2006, 194, 1661–1671. [Google Scholar] [CrossRef] [Green Version]
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef] [Green Version]
- Duerr, A.; Huang, Y.; Buchbinder, S.; Coombs, R.W.; Sanchez, J.; del Rio, C.; Casapia, M.; Santiago, S.; Gilbert, P.; Corey, L.; et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study). J. Infect. Dis. 2012, 206, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, G.E.; Allen, M.; Moodie, Z.; Churchyard, G.; Bekker, L.G.; Nchabeleng, M.; Mlisana, K.; Metch, B.; de Bruyn, G.; Latka, M.H.; et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: A double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect. Dis. 2011, 11, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Rolland, M.; Edlefsen, P.T.; Larsen, B.B.; Tovanabutra, S.; Sanders-Buell, E.; Hertz, T.; deCamp, A.C.; Carrico, C.; Menis, S.; Magaret, C.A.; et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 2012, 490, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Uritskiy, G.; Mahalingam, M.; Batra, H.; Chand, S.; Trinh, H.; Beck, C.; Shin, W.; AlSalmi, W.; Kijak, G.; et al. A genetic shift in an escaped transmitted/founder virus guides combinatorial vaccine design against HIV-1. bioRxiv 2021. [Google Scholar] [CrossRef]
- Norris, P.J.; Moffett, H.F.; Brander, C.; Allen, T.M.; O’Sullivan, K.M.; Cosimi, L.A.; Kaufmann, D.E.; Walker, B.D.; Rosenberg, E.S. Fine specificity and cross-clade reactivity of HIV type 1 Gag-specific CD4+ T cells. AIDS Res. Hum. Retrovir. 2004, 20, 315–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, F.M.E.; Oliveira, L.d.M.; Pietrobon, A.J.; Salles, É.M.d.; D’Império Lima, M.R.; Viana, I.F.T.; Lins, R.D.; Rigato, P.O.; Marques, E.T.d.A.; da Silva Duarte, A.J.; et al. LAMP-1 Chimeric to HIV-1 p55Gag in the Immunization of Neonate Mice Induces an Early Germinal Center Formation and AID Expression. Vaccines 2022, 10, 1246. https://doi.org/10.3390/vaccines10081246
Teixeira FME, Oliveira LdM, Pietrobon AJ, Salles ÉMd, D’Império Lima MR, Viana IFT, Lins RD, Rigato PO, Marques ETdA, da Silva Duarte AJ, et al. LAMP-1 Chimeric to HIV-1 p55Gag in the Immunization of Neonate Mice Induces an Early Germinal Center Formation and AID Expression. Vaccines. 2022; 10(8):1246. https://doi.org/10.3390/vaccines10081246
Chicago/Turabian StyleTeixeira, Franciane Mouradian Emidio, Luana de Mendonça Oliveira, Anna Julia Pietrobon, Érika Machado de Salles, Maria Regina D’Império Lima, Isabelle Freire Tabosa Viana, Roberto Dias Lins, Paula Ordonhez Rigato, Ernesto Torres de Azevedo Marques, Alberto José da Silva Duarte, and et al. 2022. "LAMP-1 Chimeric to HIV-1 p55Gag in the Immunization of Neonate Mice Induces an Early Germinal Center Formation and AID Expression" Vaccines 10, no. 8: 1246. https://doi.org/10.3390/vaccines10081246






