Modelling the Economic Impact of lnfluenza Vaccine Programs with the Cell-Based Quadrivalent Influenza Vaccine and Adjuvanted Trivalent Influenza Vaccine in Canada
Abstract
:1. Introduction
2. Methods
2.1. Model Structure and Parameters
2.2. Scenarios
2.3. Sensitivity Analysis
3. Results
3.1. Epidemiological Impact
3.2. Economic Impact
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Government of Canada. Flu (Influenza): For Health Professinals. 2021. Available online: https://www.canada.ca/en/public-health/services/diseases/flu-influenza/health-professionals.html (accessed on 16 May 2022).
- Schanzer, D.L.; McGeer, A.; Morris, K. Statistical estimates of respiratory admissions attributable to seasonal and pandemic influenza for Canada. Influenza Other Respir. Viruses 2013, 7, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Schanzer, D.L.; Sevenhuysen, C.; Winchester, B.; Mersereau, T. Estimating influenza deaths in Canada, 1992–2009. PLoS ONE 2013, 8, e80481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Influenza (Seasonal) Fact Sheet. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 16 May 2022).
- Tanner, A.R.; Dorey, R.B.; Brendish, N.J.; Clark, T.W. Influenza vaccination: Protecting the most vulnerable. Eur. Respir. Rev. 2021, 30, 159. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada National Advisory Committee on Immunization (NACI). Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2020–2021. 2020. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2020-2021/naci-2020-2021-seasonal-influenza-stmt-eng.pdf (accessed on 16 May 2022).
- Government of Canada. Public Funding for Influenza Vaccination by Province/Territory (as of August 2020). 2020. Available online: https://www.canada.ca/en/public-health/services/provincial-territorial-immunization-information/public-funding-influenza-vaccination-province-territory.html (accessed on 16 May 2022).
- Dugan, H.L.; Henry, C.; Wilson, P.C. Aging and influenza vaccine-induced immunity. Cell Immunol. 2020, 348, 103998. [Google Scholar] [CrossRef]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- McLean, H.Q.; Thompson, M.G.; Sundaram, M.E.; Kieke, B.A.; Gaglani, M.; Murthy, K.; Piedra, P.A.; Zimmerman, R.K.; Nowalk, M.P.; Raviotta, J.M.; et al. Influenza vaccine effectiveness in the United States during 2012–2013: Variable protection by age and virus type. J. Infect. Dis. 2015, 211, 1529–1540. [Google Scholar] [CrossRef] [Green Version]
- Ohmit, S.E.; Thompson, M.G.; Petrie, J.G.; Thaker, S.N.; Jackson, M.L.; Belongia, E.A.; Zimmerman, R.K.; Gaglani, M.; Lamerato, L.; Spencer, S.M.; et al. Influenza vaccine effectiveness in the 2011-2012 season: Protection against each circulating virus and the effect of prior vaccination on estimates. Clin. Infect. Dis. 2014, 58, 319–327. [Google Scholar] [CrossRef]
- Doroshenko, A.; Lee, N.; MacDonald, C.; Zelyas, N.; Asadi, L.; Kanji, J.N. Decline of Influenza and Respiratory Viruses with COVID-19 Public Health Measures: Alberta, Canada. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2021; Volume 96, pp. 3042–3052. [Google Scholar]
- Skowronski, D.M.; Zou, M.; Sabaiduc, S.; Murti, M.; Olsha, R.; Dickinson, J.A.; Gubbay, J.B.; Croxen, M.A.; Charest, H.; Jassem, A.; et al. Interim estimates of 2019/20 vaccine effectiveness during early-season co-circulation of influenza A and B viruses, Canada, February 2020. Euro Surveill. 2020, 25, 2000103. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.C.; Zost, S.J.; Thompson, A.J.; Oyen, D.; Nycholat, C.M.; McBride, R.; Paulson, J.C.; Hensley, S.E.; Wilson, I.A. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017, 13, e1006682. [Google Scholar] [CrossRef] [Green Version]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Perez, S.D.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef] [Green Version]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Sylvester, G.C.; Mansi, J.A. Relative Effectiveness of the Cell-derived Inactivated Quadrivalent Influenza Vaccine Versus Egg-derived Inactivated Quadrivalent Influenza Vaccines in Preventing Influenza-related Medical Encounters during the 2018–2019 Influenza Season in the United States. Clin. Infect. Dis. 2021, 73, e692–e698. [Google Scholar]
- Boikos, C.; Sylvester, G.C.; Sampalis, J.S.; Mansi, J.A. Relative Effectiveness of the Cell-Cultured Quadrivalent Influenza Vaccine Compared to Standard, Egg-derived Quadrivalent Influenza Vaccines in Preventing Influenza-like Illness in 2017–2018. Clin. Infect. Dis. 2020, 71, e665–e671. [Google Scholar] [CrossRef]
- Bart, S.; Cannon, K.; Herrington, D.; Mills, R.; Forleo-Neto, E.; Lindert, K.; Mateen, A.A. Immunogenicity and safety of a cell culture-based quadrivalent influenza vaccine in adults: A Phase III, double-blind, multicenter, randomized, non-inferiority study. Hum. Vaccines Immunother. 2016, 12, 2278–2288. [Google Scholar] [CrossRef] [Green Version]
- Hartvickson, R.; Cruz, M.; Ervin, J.; Brandon, D.; Forleo-Neto, E.; Dagnew, A.F.; Chandra, R.; Lindert, K.; Mateen, A.A. Non-inferiority of mammalian cell-derived quadrivalent subunit influenza virus vaccines compared to trivalent subunit influenza virus vaccines in healthy children: A phase III randomized, multicenter, double-blind clinical trial. Int. J. Infect. Dis. 2015, 41, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.H.; Hilsky, Y.; Mould-Quevedo, J. The Epidemiological and Economic Impact of a Cell-Based Quadrivalent Influenza Vaccine in Adults in the US: A Dynamic Modeling Approach. Vaccines 2021, 9, 1095. [Google Scholar] [CrossRef]
- Baguelin, M.; Flasche, S.; Camacho, A.; Demiris, N.; Miller, E.; Edmunds, W.J. Assessing optimal target populations for influenza vaccination programmes: An evidence synthesis and modelling study. PLoS Med. 2013, 10, e1001527. [Google Scholar] [CrossRef]
- Thommes, E.W.; Chit, A.; Meier, G.C.; Bauch, C.T. Examining Ontario’s universal influenza immunization program with a multi-strain dynamic model. Vaccine 2014, 32, 5098–5117. [Google Scholar] [CrossRef]
- World Health Organization. FluNet, Global Influenza Surveillance and Response System (GISRS). 2021. Available online: https://www.who.int/tools/flunet (accessed on 16 May 2022).
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczyk, R.; Massari, M.; Salmaso, S.; Tomba, G.S.; Wallinga, J.; et al. Social Contacts and Mixing Patterns Relevant to the Spread of Infectious Diseases. PLoS Med. 2008, 5, e74. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States. 2020. Available online: https://www.cdc.gov/flu/about/burden/past-seasons.html (accessed on 16 May 2022).
- Rajaram, S.; Suphaphiphat, P.; Van Boxmeer, J.; Haag, M.; Leav, B.; Iheanacho, I.; Kistler, K.; De Lejarazu, R.O. Retrospective Assessment of the Antigenic Similarity of Egg-Propagated and Cell Culture-Propagated Reference Influenza Viruses as Compared with Circulating Viruses across Influenza Seasons 2002–2003 to 2017–2018. Int. J. Environ. Res. Public Health 2020, 17, 5423. [Google Scholar] [CrossRef]
- Canadian Sentinel Practitioner Surveillance Network (SPSN). Influenza Vaccine Effectiveness Estimates 2004–2005 to 2019–2020 Seasons. 2020. Available online: http://www.bccdc.ca/resource-gallery/Documents/StatisticsandResearch/Publications/Epid/InfluenzaandRespiratory/SPSN_VE_By_Year_Table.pdf (accessed on 16 May 2022).
- Divino, V.; Anupindi, V.R.; DeKoven, M.; Mould-Quevedo, J.; Pelton, I.S.; Postma, M.J.; Levin, M.J.; Anupindi, V.R.; DeKoven, M.; Mould-Quevedo, J.; et al. A Real-World Clinical and Economic Analysis of Cell-Derived Quadrivalent Influenza Vaccine Compared to Standard Egg-Derived Quadrivalent Influenza Vaccines during the 2019–2020 Influenza Season in the United States. In Open Forum Infect. Dis.; Oxford University Press: Cary, NC, USA, 2022; Volume 9, p. ofab604. [Google Scholar]
- Krishnarajah, G.; Divino, V.; Postma, M.J.; Pelton, S.I.; Anupindi, V.R.; DeKoven, M.; Mould-Quevedo, J. Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018-19 Influenza Season in the United States. Vaccines 2021, 9, 80. [Google Scholar] [CrossRef]
- Divino, V.; Krishnarajah, G.; Pelton, S.I.; Mould-Quevedo, J.; Anupindi, V.R.; DeKoven, M.; Postma, M.J. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017-18 influenza season. Vaccine 2020, 38, 6334–6343. [Google Scholar] [CrossRef]
- Fisman, D.N.; Tuite, A.R. Estimation of the health impact and cost-effectiveness of influenza vaccination with enhanced effectiveness in Canada. PLoS ONE 2011, 6, e27420. [Google Scholar] [CrossRef] [Green Version]
- Quebec Government. List of Vaccines Offered in Pharmacies and Covered under the Conditions of the Quebec Immunization Program 2021. Available online: https://www.ramq.gouv.qc.ca/sites/default/files/documents/liste-vaccins-pqi-2021-03-11-fr.pdf (accessed on 16 May 2022).
- Moran, K.; Maaten, S.; Guttmann, A.; Northrup, D.; Kwong, J.C. Influenza vaccination rates in Ontario children: Implications for universal childhood vaccination policy. Vaccine 2009, 27, 2350–2355. [Google Scholar] [CrossRef]
- Czaja, C.A.; Miller, L.; Alden, N.; Wald, H.L.; Cummings, C.N.; Rolfes, M.A.; Anderson, E.J.; Bennett, N.M.; Billing, L.M.; Chai, S.J.; et al. Age-Related Differences in Hospitalization Rates, Clinical Presentation, and Outcomes Among Older Adults Hospitalized with Influenza—U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET). In Open Forum Infectious Diseases; Oxford University Press: Cary, NC, USA, 2019; Volume 6, p. ofz225. [Google Scholar]
- Chung, J.R.; Rolfes, M.A.; Flannery, B.; Prasad, P.; O’Halloran, A.; Garg, S.; Fry, A.M.; Singleton, J.A.; Patel, M.; Reed, C.; et al. Effects of Influenza Vaccination in the United States During the 2018–2019 Influenza Season. Clin. Infect. Dis. 2020, 71, e368–e376. [Google Scholar] [CrossRef]
- Panda, A.; Qian, F.; Mohanty, S.; Van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef]
- Reber, A.J.; Chirkova, T.; Kim, J.H.; Cao, W.; Biber, R.; Shay, D.K.; Sambhara, S. Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population. Aging Dis. 2012, 3, 68–90. [Google Scholar]
- Coleman, B.L.; Sanderson, R.; Haag, M.D.M.; McGovern, I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir. Viruses 2021, 15, 813–823. [Google Scholar] [CrossRef]
- Wilkinson, K.; Wei, Y.; Szwajcer, A.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M.; Mahmud, S.M. Efficacy and safety of high-dose influenza vaccine in elderly adults: A systematic review and meta-analysis. Vaccine 2017, 35, 2775–2780. [Google Scholar] [CrossRef]
- Public Health England. The National Influenza Immunisation Programme 2021 to 2022. 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1018779/Influenza_vaccination_information_document_for_healthcare_practitioners.pdf (accessed on 18 February 2022).
- Australian Government DoH. Statement on the Administation of Seasonal Influenza Vaccines in 2022. Available online: https://www.health.gov.au/resources/publications/atagi-advice-on-seasonal-influenza-vaccines-in-2022 (accessed on 7 March 2022).
- Ortiz de Lejarazu-Leonardo, R.; Montomoli, E.; Wojcik, R.; Christopher, S.; Mosnier, A.; Pariani, E.; Trilla Garcia, A.; Fickenscher, H.; Gärtner, B.C.; Jandhyala, R.; et al. Estimation of Reduction in Influenza Vaccine Effectiveness Due to Egg-Adaptation Changes-Systematic Literature Review and Expert Consensus. Vaccines 2021, 9, 1255. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Luo, Y.; Ackerson, B.; Tanenbaum, H.C.; Sy, L.S.; Gandhi, A.; Tseng, H.F. Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017–2018. Vaccine 2019, 37, 5807–5811. [Google Scholar] [CrossRef]
- DeMarcus, L.; Shoubaki, L.; Federinko, S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017-2018 influenza season. Vaccine 2019, 37, 4015–4021. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.T. On sample sizes to estimate the protective efficacy of a vaccine. Stat. Med. 1988, 7, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Comber, L.; Murchu, E.O.; Jordan, K.; Hawkshaw, S.; Marshall, L.; O’Neill, M.; Teljeur, C.; Ryan, M.; Carnahan, A.; Martín, J.J.P.; et al. Systematic review of the efficacy, effectiveness and safety of high-dose seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals ≥18 years of age. Rev. Med. Virol. 2022, e2330. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L. Influenza Vaccines for Older Adults: GRADE Summary. Advisory Committee on Immunization Practices 23 February 2022. Available online: https://www.cdc.gov/vaccines/acip/meetings/slides-2022-02-23-24.html (accessed on 7 March 2022).

| Year | Matching Assumptions | Absolute QIVe Vaccine Effectiveness per Influenza Strain and per Year | rVE QIVc When Egg- Adapted * | rVE HD-QIV-aTIV When Egg-Adapted * | rVE HD-QIV-aTIV When Matched | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A/ H1N1 | A/ H3N2 | BVIC | BYAM | A/H1N1 | A/H3N2 | BVIC | BYAM | A/ H3N2 | A/H3N2 | A and B | |
| 2012 | M | U | M | U | 59% (53–65%) | 41% (37–45%) | 68% (61–75%) | 68% (61–75%) | 15.6% (7–20%) | 9% (7.20–10%) | 24% (9.70–36%) |
| 2013 | M | U | U | M | 71% (64–78%) | 66% (59–73%) | 72% (65–79%) | 72% (65–79%) | |||
| 2014 | M | U | U | M | 9% (8–10%) | 9% (8–10%) | 9% (8–10%) | 9% (8–10%) | |||
| 2015 | M | M | U | M | 43% (39–47%) | 44% (40–48%) | 50% (45–55% | 50% (45–55% | |||
| 2016 | M | U | M | U | 36% (32–40%) | 36% (32–40%) | 72% (65–79%) | 72% (65–79%) | |||
| 2017 | M | U | M | U | 58% (52–64%) | 14% (13–15% | 46% (41–51%) | 46% (41–51%) | |||
| 2018 | M | M | M | U | 67% (60–74%) | 17% (15–19%) | 72% (65–79%) | 72% (65–79%) | |||
| 2019 | M | U | M | U | 43% (39–47%) | 50% (45–55% | 65% (59–72%) | 65% (59–72%) | |||
| Age Group | Hospitalization a | ICU b | Mechanical Ventilation c | ECMO c | Death b | GP Visit Costs d (CAD) | ED Costs e (CAD) | Hospitalization Costs (CAD) | ICU Cost (CAD) | ICU and Mechanical Ventilation Cost (CAD) | ICU and ECMO (CAD) | QALY per Case | Death Discounted (5%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–4 Y | 0.089% | 12% | 81% | 4.00% | 1% | 52.61 | 313.43 | 5103 | 33,242 | 50,411 | 151,726 | 0.985 | 18.53 |
| 5–19 Y | 0.018% | 11% | 81% | 4.00% | 2% | 44.86 | 286.79 | 6075 | 28,654 | 50,552 | 235,899 | 0.985 | 18.15 |
| 20–64 Y | 0.033% | 23% | 81% | 4.00% | 10% | 44.14 | 314.42 | 9557 | 20,239 | 61,290 | 96,211 | 0.98 | 15.14 |
| 65+ | 0.132% | 16% | 81% | 4.00% | 19% | 56.29 | 389.74 | 11,894 | 22,164 | 57,084 | 95,684 | 0.97 | 2.41 |
| Absolute Value | Difference vs. Reference Scenario | ||||||
|---|---|---|---|---|---|---|---|
| Reference Scenario (QIVe for All) | QIVe (6 m to 64 y) + aTIV for ≥65 y | QIVe (6 m to 64 y) + HD-QIV for ≥65 y | QIVc (6 m to 64 y) + aTIV for ≥65 y | QIVe (6 m to 64 y) + aTIV for ≥65 y | QIVe (6 m to 64 y) + HD-QIV for ≥65 y | QIVc (6 m to 64 y) + aTIV for ≥65 y | |
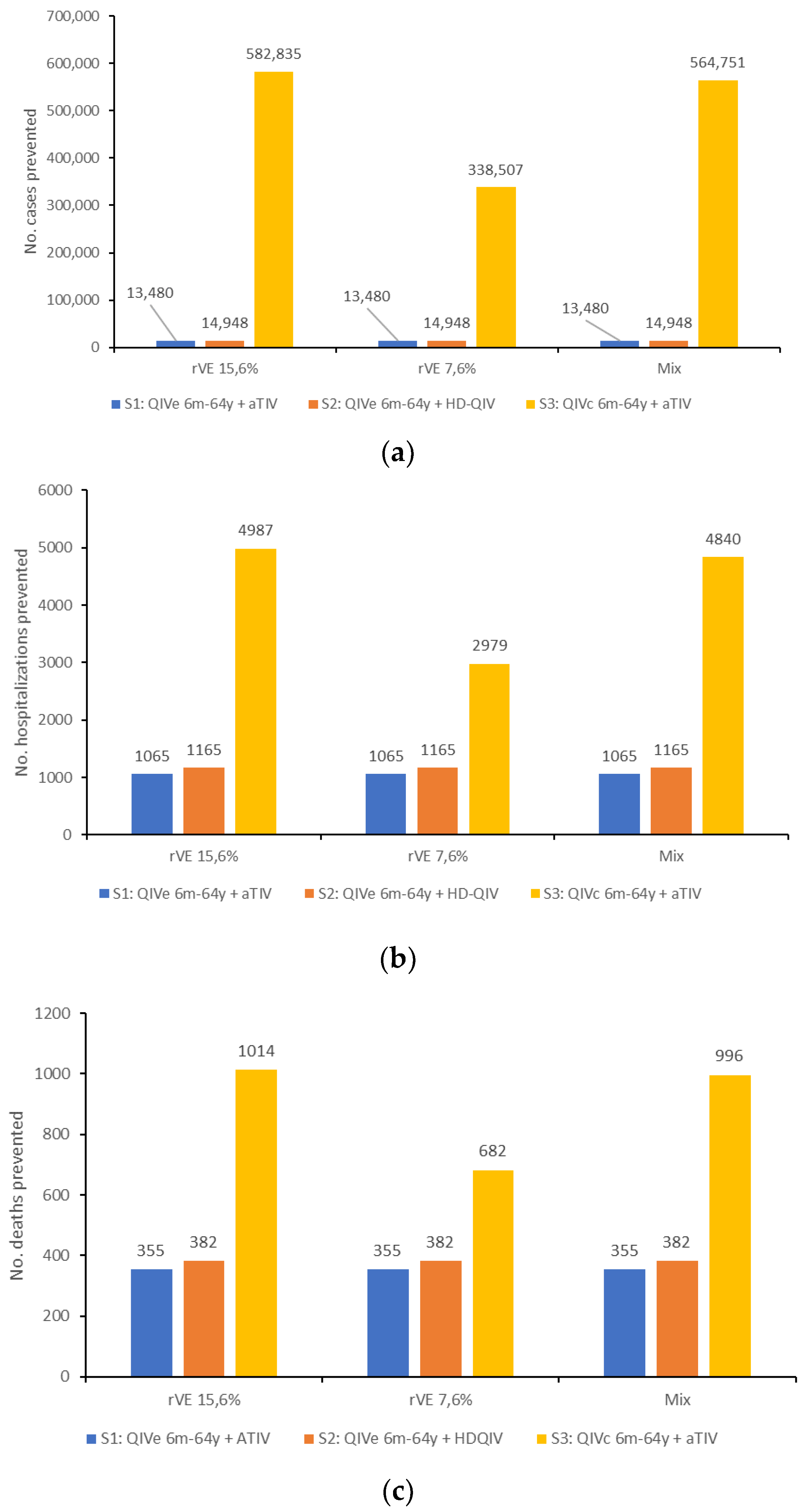
| Symptomatic influenza cases | 2,793,715 | 2,691,577 | 2,681,540 | 2,210,880 | −102,138 | −112,175 | −582,835 |
| GP consultations | 382,372 | 368,892 | 367,424 | 308,371 | −13,480 | −14,948 | −74,001 |
| ED consultations | 95,593 | 92,223 | 91,856 | 77,093 | −3370 | −3737 | −18,500 |
| Total number of hospitalizations | 22,835 | 21,770 | 21,670 | 17,848 | −1065 | −1165 | −4987 |
| Total number of ICU hospitalizations | 4575 | 4376 | 4357 | 3585 | −199 | −218 | −990 |
| Total number of deaths | 3379 | 3024 | 2997 | 2365 | −355 | −382 | −1014 |
| Cost of influenza vaccine (CAD) | 201,324,565 | 203,130,422 | 501,893,579 | 325,232,342 | 1,805,857 | 300,569,014 | 123,907,777 |
| Cost of influenza vaccine (discounted) (CAD) | 170,782,959 | 172,314,862 | 425,754,654 | 275,893,514 | 1,531,903 | 254,971,695 | 105,110,555 |
| Cost of medical consultations (CAD) | 17,381,968 | 16,735,367 | 16,666,491 | 13,972,659 | −646,601 | −715,477 | −3,409,309 |
| Cost of hospitalizations (CAD) | 458,359,891 | 437,223,795 | 435,228,411 | 358,292,003 | −21,136,096 | −23,131,480 | −100,067,888 |
| Total medical cost (CAD) | 475,741,859 | 453,959,161 | 451,894,901 | 372,264,662 | −21,782,698 | −23,846,958 | −103,477,197 |
| Total medical cost (discounted) (CAD) | 401,650,258 | 383,744,102 | 381,901,843 | 313,426,249 | −17,906,156 | −19,748,415 | −88,224,009 |
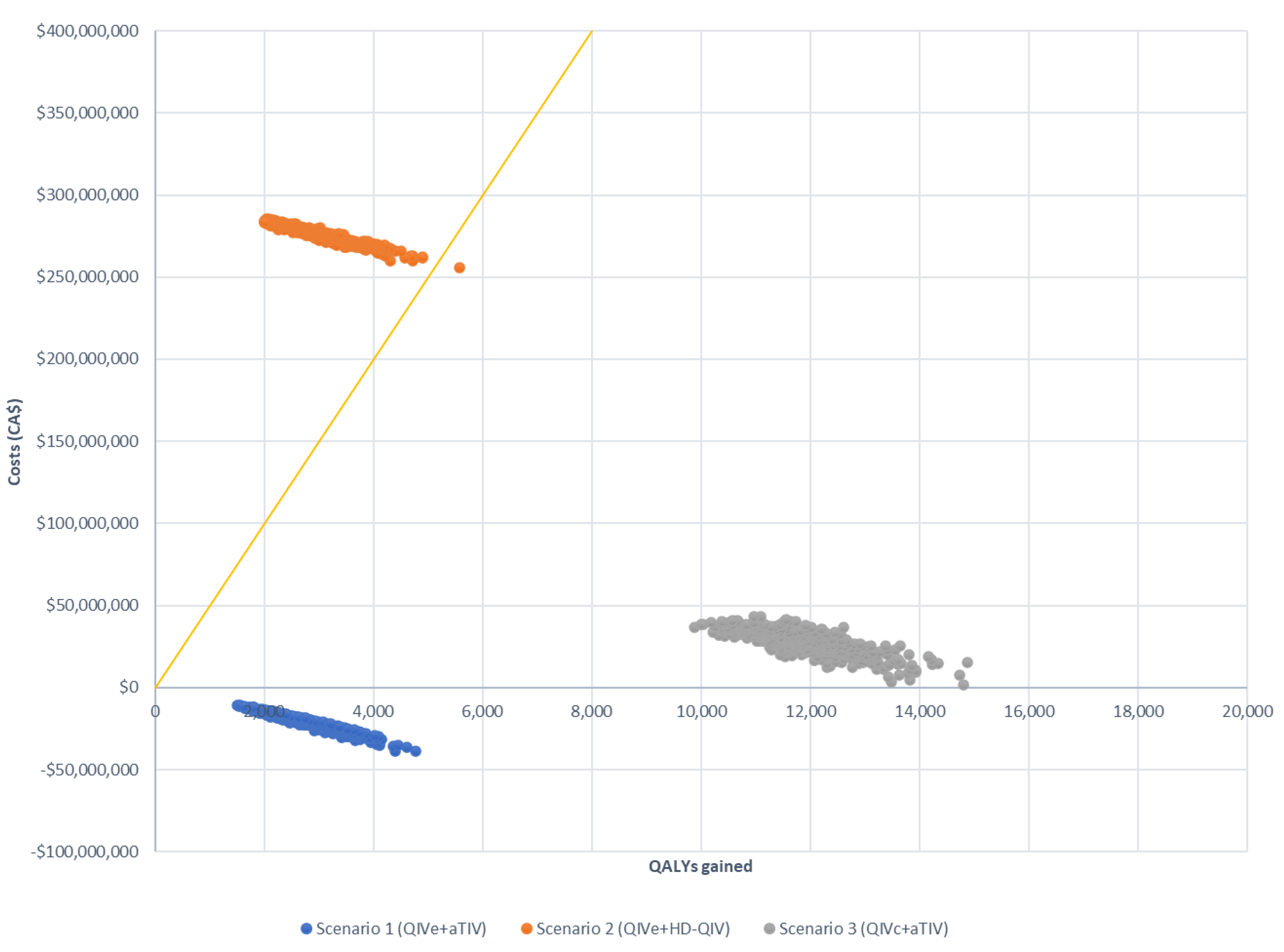
| Total direct cost (vaccine, medical, and hospitalizations) (CAD) | 677,066,424 | 657,089,584 | 953,788,480 | 697,497,004 | −19,976,840 | 276,722,056 | 20,430,580 |
| Total direct cost (discounted) (CAD) | 572,433,217 | 556,058,964 | 807,656,497 | 589,319,763 | −16,374,253 | 235,223,280 | 16,886,546 |
| QALY loss from symptomatic cases | 48,901 | 46,849 | 46,657 | 38,340 | −2052 | −2244 | −10,561 |
| QALY loss from deaths | 17,150 | 15,993 | 15,901 | 12,666 | −1157 | −1249 | −4484 |
| Total QALY loss | 66,051 | 62,842 | 62,559 | 51,006 | −3209 | −3492 | −15,045 |
| Total QALY loss (discounted) | 55,804 | 53,164 | 52,911 | 42,958 | −2640 | −2893 | −12,846 |
| Scenario | S1: QIVe 6 m–64 y + aTIV | S2: QIVe 6 m–64 y + HD-QIV | S3: QIVc 6 m–64 y + aTIV |
|---|---|---|---|
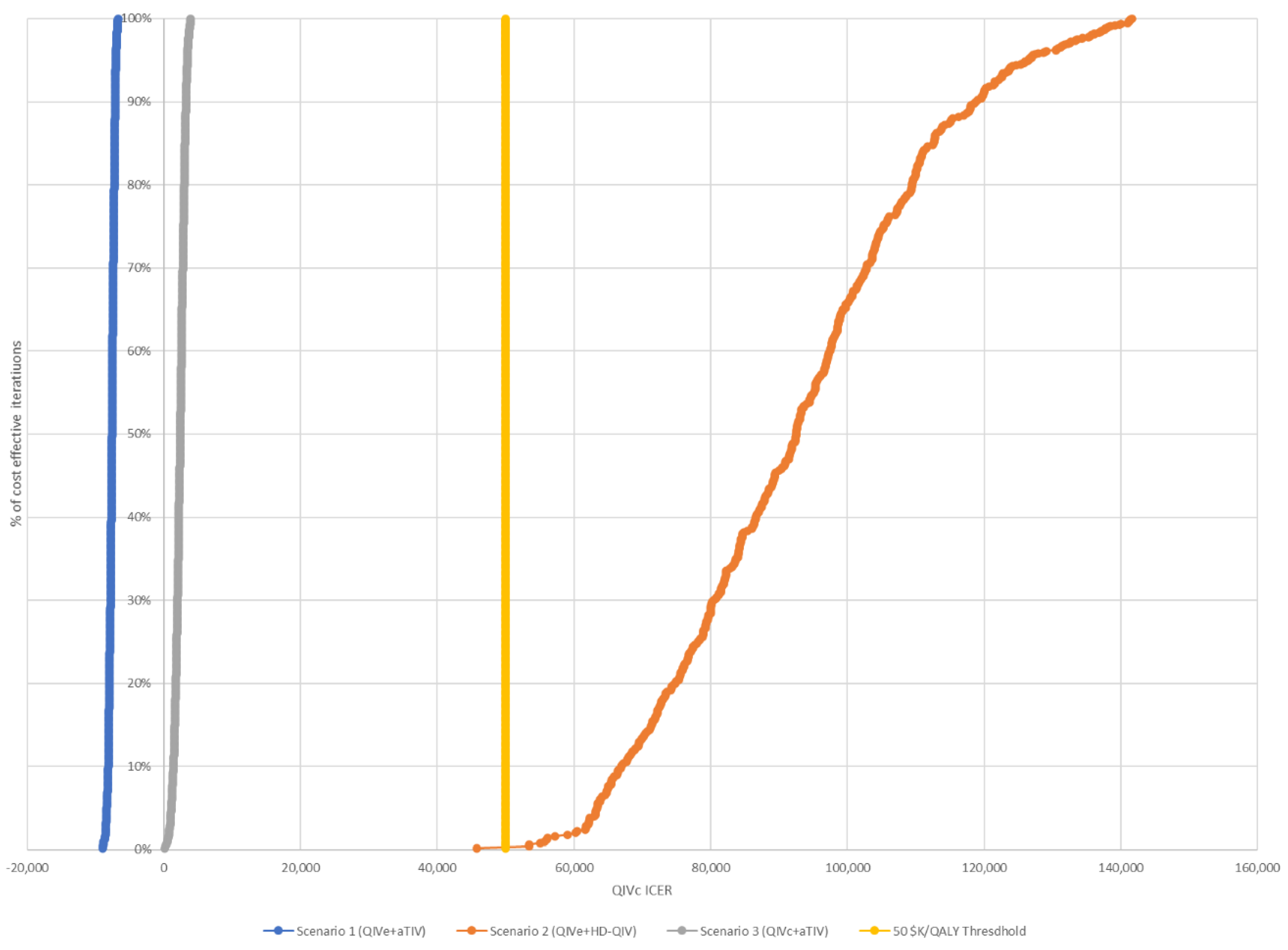
| rVE 15.6% | Cost-saving | CAD 81,300/QALY | CAD 1300/QALY |
| rVE 7.6% | Cost-saving | CAD 81,300/QALY | CAD 6900/QALY |
| Mix a | Cost-saving | CAD 81,300/QALY | CAD 1500/QALY |
| Parameter | Scenario | ICER per QALY Gained (Median) | Lower Bound 95% CI | Upper Bound 95% CI |
|---|---|---|---|---|
| VEa | QIVe 6 m-64 y + aTIV ≥ 65 y | Dominant strategy | Dominant strategy | Dominant strategy |
| QIVe 6 m-64 y + HD-QIV ≥ 65 y | CAD 92,994 | CAD 68,503 | CAD 140,674 | |
| QIVc 6 m-64 y + aTIV ≥ 65 y | CAD 1475 | CAD 431 | CAD 2904 | |
| Season Profileb | QIVe 6 m-64 y + aTIV ≥ 65 y | Dominant strategy | Dominant strategy | Dominant strategy |
| QIVe 6 m-64 y + HD-QIV ≥ 65 y | CAD 89,805 | CAD 81,517 | CAD 98,930 | |
| QIVc 6 m-64 y + aTIV ≥ 65 y | CAD 2479 | CAD 1160 | CAD 4845 | |
| VE and Season Profile | QIVe 6 m-64 y + aTIV ≥ 65 y | Dominant strategy | Dominant strategy | Dominant strategy |
| QIVe 6 m-64 y + HD-QIV ≥ 65 y | CAD 90,670 | CAD 53,355 | CAD 149,047 | |
| QIVc 6 m-64 y + aTIV ≥ 65 y | CAD 2764 | CAD 891 | CAD 5449 | |
| Only Three Seasons Egg-Adapted with VE | QIVe 6 m-64 y + aTIV ≥ 65 y | Dominant strategy | Dominant strategy | Dominant strategy |
| QIVe 6 m-64 y + HD-QIV ≥ 65 y | CAD 72,879 | CAD 50,288 | CAD 128,852 | |
| QIVc 6 m-64 y + aTIV ≥ 65 y | CAD 7770 | CAD 3650 | CAD 17,079 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.H.; Roy, B. Modelling the Economic Impact of lnfluenza Vaccine Programs with the Cell-Based Quadrivalent Influenza Vaccine and Adjuvanted Trivalent Influenza Vaccine in Canada. Vaccines 2022, 10, 1257. https://doi.org/10.3390/vaccines10081257
Nguyen VH, Roy B. Modelling the Economic Impact of lnfluenza Vaccine Programs with the Cell-Based Quadrivalent Influenza Vaccine and Adjuvanted Trivalent Influenza Vaccine in Canada. Vaccines. 2022; 10(8):1257. https://doi.org/10.3390/vaccines10081257
Chicago/Turabian StyleNguyen, Van Hung, and Bertrand Roy. 2022. "Modelling the Economic Impact of lnfluenza Vaccine Programs with the Cell-Based Quadrivalent Influenza Vaccine and Adjuvanted Trivalent Influenza Vaccine in Canada" Vaccines 10, no. 8: 1257. https://doi.org/10.3390/vaccines10081257
APA StyleNguyen, V. H., & Roy, B. (2022). Modelling the Economic Impact of lnfluenza Vaccine Programs with the Cell-Based Quadrivalent Influenza Vaccine and Adjuvanted Trivalent Influenza Vaccine in Canada. Vaccines, 10(8), 1257. https://doi.org/10.3390/vaccines10081257





