The Roles of Skin Langerhans Cells in Immune Tolerance and Cancer Immunity
Abstract
:1. Introduction
2. Langerhans Cells in Ultraviolet Radiation-Induced Immune Suppression
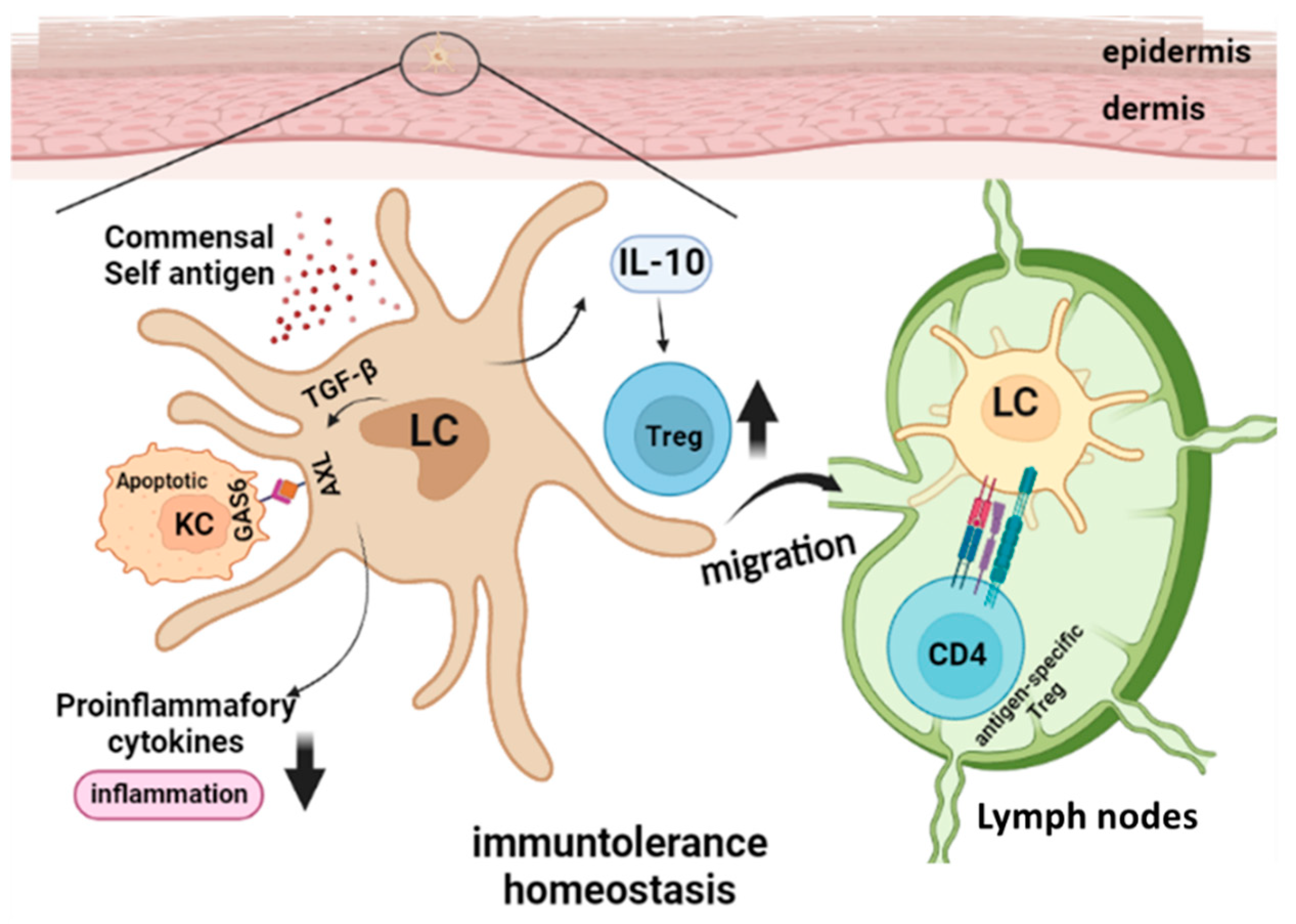
3. Langerhans Cells in Steady State Skin Immune Homeostasis
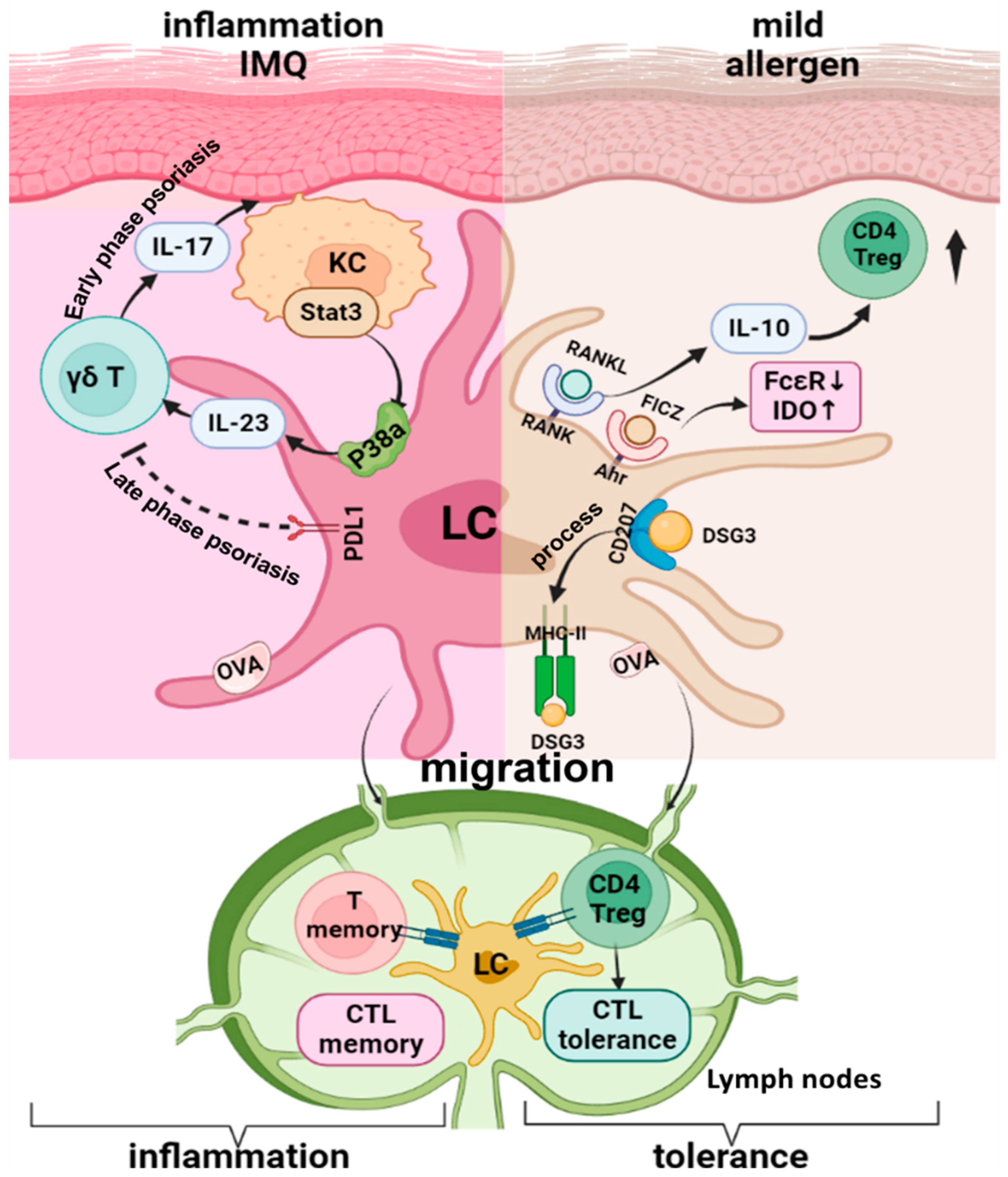
4. Langerhans Cells in Skin Treg Differentiation and Inflammatory Disease Models
5. Langerhans Cells in Skin Cancer Development and Progression
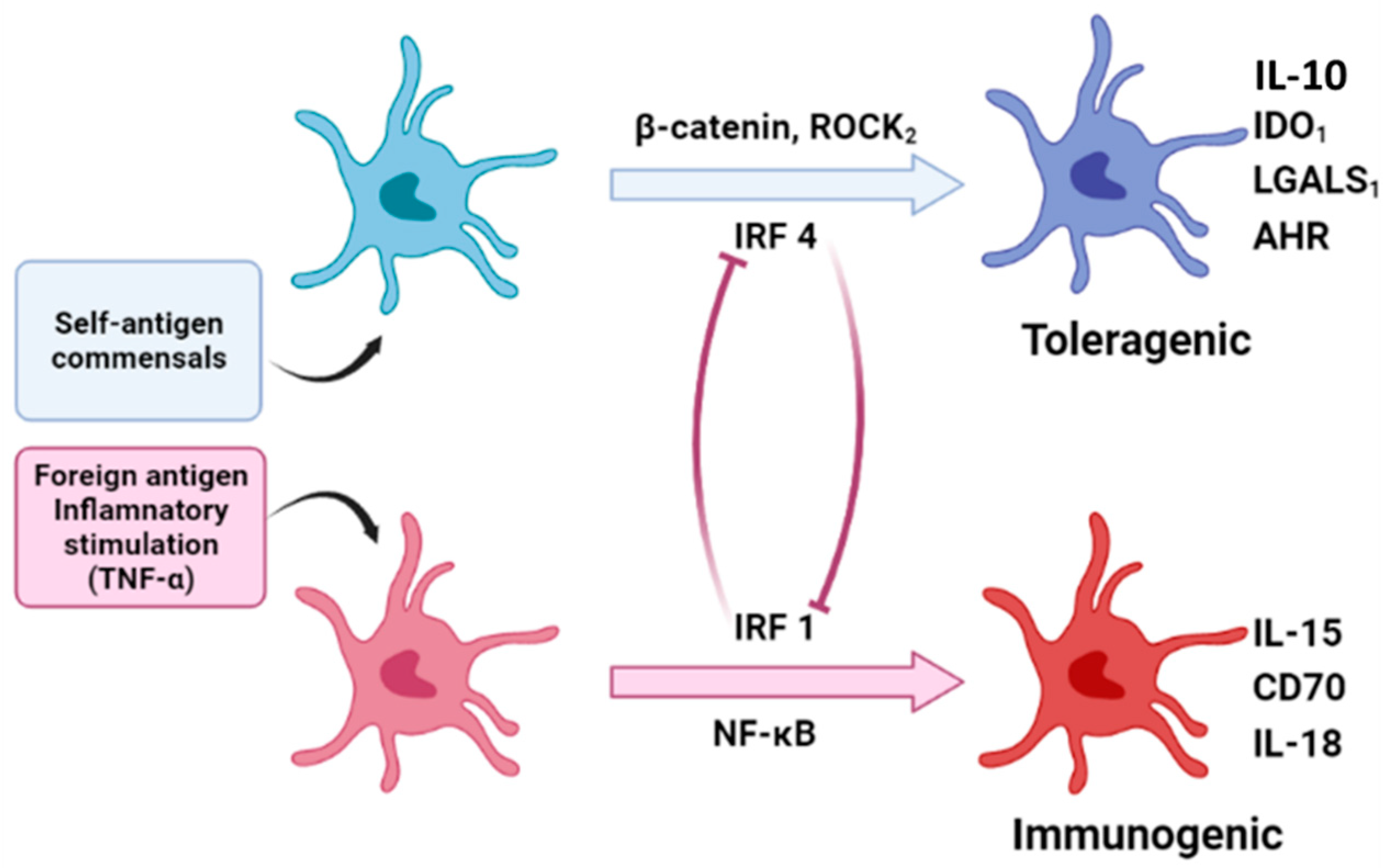
6. Potential Mechanisms of Langerhans Cells in Priming Treg Responses Versus Effector T Cell Responses
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mass, E.; Ballesteros, I.; Farlik, M.; Halbritter, F.; Günther, P.; Crozet, L.; Jacome-Galarza, C.E.; Händler, K.; Klughammer, J.; Kobayashi, Y.; et al. Specification of tissue-resident macrophages during organogenesis. Science 2016, 353, aaf4238. [Google Scholar] [CrossRef] [PubMed]
- Lovy, J.; Savidant, G.P.; Speare, D.J.; Wright, G.M. Langerin/CD207 positive dendritic-like cells in the haemopoietic tissues of salmonids. Fish Shellfish Immunol. 2009, 27, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Wang, Y.; Greter, M.; See, P.; Teo, P.; Malleret, B.; Leboeuf, M.; Low, D.; Oller, G.; Almeida, F.; et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012, 209, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Tacke, F.; Angeli, V.; Bogunovic, M.; Loubeau, M.; Dai, X.; Stanley, E.R.; Randolph, G.J.; Merad, M. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006, 7, 265–273. [Google Scholar] [CrossRef]
- Assefa, Z.; Van Laethem, A.; Garmyn, M.; Agostinis, P. Ultraviolet radiation-induced apoptosis in keratinocytes: On the role of cytosolic factors. Biochim. Biophys. Acta 2005, 1755, 90–106. [Google Scholar] [CrossRef]
- Jans, J.; Garinis, G.A.; Schul, W.; van Oudenaren, A.; Moorhouse, M.; Smid, M.; Sert, Y.-G.; van der Velde, A.; Rijksen, Y.; de Gruijl, F.R.; et al. Differential Role of Basal Keratinocytes in UV-Induced Immunosuppression and Skin Cancer. Mol. Cell. Biol. 2006, 26, 8515–8526. [Google Scholar] [CrossRef]
- Cao, C.; Wan, Y. Parameters of protection against ultraviolet radiation-induced skin cell damage. J. Cell. Physiol. 2009, 220, 277–284. [Google Scholar] [CrossRef]
- Schwarz, A.; Noordegraaf, M.; Maeda, A.; Torii, K.; Clausen, B.E.; Schwarz, T. Langerhans Cells Are Required for UVR-Induced Immunosuppression. J. Investig. Dermatol. 2010, 130, 1419–1427. [Google Scholar] [CrossRef]
- Wang, L.; Jameson, S.C.; Hogquist, K.A. Epidermal Langerhans Cells Are Not Required for UV-Induced Immunosuppression. J. Immunol. 2009, 183, 5548–5553. [Google Scholar] [CrossRef]
- Otsuka, M.; Egawa, G.; Kabashima, K. Uncovering the Mysteries of Langerhans Cells, Inflammatory Dendritic Epidermal Cells, and Monocyte-Derived Langerhans Cell-Like Cells in the Epidermis. Front. Immunol. 2018, 9, 1768. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Fukunaga, A.; Washio, K.; Taguchi, K.; Oda, Y.; Ogura, K.; Nishigori, C. Anti-Inflammatory Role of Langerhans Cells and Apoptotic Keratinocytes in Ultraviolet-B-Induced Cutaneous Inflammation. J. Immunol. 2017, 199, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Fadok, A.V.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.-L.N.; Malcolm, K.C.; Kotaru, C.; Tilstra, J.A.; Westcott, J.Y.; Fadok, V.A.; Wenzel, S.E. Defective Apoptotic Cell Phagocytosis Attenuates Prostaglandin E2 and 15-Hydroxyeicosatetraenoic Acid in Severe Asthma Alveolar Macrophages. Am. J. Respir. Crit. Care Med. 2005, 172, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.-L.N.; Fadok, V.A.; Henson, P.M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Investig. 2002, 109, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Loser, K.; Mehling, A.; Loeser, S.; Apelt, J.; Kuhn, A.; Grabbe, S.; Schwarz, T.; Penninger, J.M.; Beissert, S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat. Med. 2006, 12, 1372–1379. [Google Scholar] [CrossRef]
- Yoshiki, R.; Kabashima, K.; Sakabe, J.-I.; Sugita, K.; Bito, T.; Nakamura, M.; Malissen, B.; Tokura, Y. The Mandatory Role of IL-10–Producing and OX40 Ligand-Expressing Mature Langerhans Cells in Local UVB-Induced Immunosuppression. J. Immunol. 2010, 184, 5670–5677. [Google Scholar] [CrossRef]
- Nakahashi-Oda, C.; Udayanga, K.G.S.; Nakamura, Y.; Nakazawa, Y.; Totsuka, N.; Miki, H.; Iino, S.; Tahara-Hanaoka, S.; Honda, S.-I.; Shibuya, K.; et al. Apoptotic epithelial cells control the abundance of Treg cells at barrier surfaces. Nat. Immunol. 2016, 17, 441–450. [Google Scholar] [CrossRef]
- Lewis, J.M.; Bürgler, C.D.; Freudzon, M.; Golubets, K.; Gibson, J.F.; Filler, R.B.; Girardi, M. Langerhans Cells Facilitate UVB-Induced Epidermal Carcinogenesis. J. Investig. Dermatol. 2015, 135, 2824–2833. [Google Scholar] [CrossRef]
- Lewis, J.M.; Monico, P.F.; Mirza, F.N.; Xu, S.; Yumeen, S.; Turban, J.L.; Galan, A.; Girardi, M. Chronic UV radiation–induced RORγt+ IL-22–producing lymphoid cells are associated with mutant KC clonal expansion. Proc. Natl. Acad. Sci. USA 2021, 118, e2016963118. [Google Scholar] [CrossRef]
- West, H.C.; Bennett, C.L. Redefining the Role of Langerhans Cells as Immune Regulators within the Skin. Front. Immunol. 2017, 8, 1941. [Google Scholar] [CrossRef]
- Ohyagi, H.; Onai, N.; Sato, T.; Yotsumoto, S.; Liu, J.; Akiba, H.; Yagita, H.; Atarashi, K.; Honda, K.; Roers, A.; et al. Monocyte-Derived Dendritic Cells Perform Hemophagocytosis to Fine-Tune Excessive Immune Responses. Immunity 2013, 39, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, G.-H.; Yu, Q.; Xu, Y.; Cvetkovski, S.; Wang, X.; Parajuli, N.; Udo-Inyang, I.; Kaplan, D.; Zhou, L.; et al. Smad2/4 Signaling Pathway Is Critical for Epidermal Langerhans Cell Repopulation Under Inflammatory Condition but Not Required for Their Homeostasis at Steady State. Front. Immunol. 2020, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-P.; Shi, Y.; Cui, Z.-Z.; Jiang, H.H.; Li, L.; Wang, X.-F.; Zhou, L.; Mi, Q.-S. TGFβ/Smad3 Signal Pathway Is Not Required for Epidermal Langerhans Cell Development. J. Investig. Dermatol. 2012, 132, 2106–2109. [Google Scholar] [CrossRef]
- Kaplan, D.H. Ontogeny and function of murine epidermal Langerhans cells. Nat. Immunol. 2017, 18, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Lemke, G. Homeostatic Regulation of the Immune System by Receptor Tyrosine Kinases of the Tyro 3 Family. Science 2001, 293, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.; Zagórska, A.; Jurkin, J.; Yasmin, N.; Köffel, R.; Richter, S.; Gesslbauer, B.; Lemke, G.; Strobl, H. Identification of Axl as a downstream effector of TGF-β1 during Langerhans cell differentiation and epidermal homeostasis. J. Exp. Med. 2012, 209, 2033–2047. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.N.; Šošić, D.; Rothlin, C.V.; Kelly, E.; Lemke, G.; Olson, E.N.; Ivashkiv, L.B. Twist mediates suppression of inflammation by type I IFNs and Axl. J. Exp. Med. 2006, 203, 1891–1901. [Google Scholar] [CrossRef]
- Rothlin, C.V.; Lemke, G. TAM receptor signaling and autoimmune disease. Curr. Opin. Immunol. 2010, 22, 740–746. [Google Scholar] [CrossRef]
- Seneschal, J.; Clark, R.A.; Gehad, A.; Baecher-Allan, C.M.; Kupper, T.S. Human Epidermal Langerhans Cells Maintain Immune Homeostasis in Skin by Activating Skin Resident Regulatory T Cells. Immunity 2012, 36, 873–884. [Google Scholar] [CrossRef]
- Ghigo, C.; Mondor, I.; Jorquera, A.; Nowak, J.; Wienert, S.; Zahner, S.P.; Clausen, B.; Luche, H.; Malissen, B.; Klauschen, F.; et al. Multicolor fate mapping of Langerhans cell homeostasis. J. Exp. Med. 2013, 210, 1657–1664. [Google Scholar] [CrossRef]
- Van der Aar, A.M.; Picavet, D.I.; Muller, F.J.; de Boer, L.; van Capel, T.M.; Zaat, S.A.; Bos, J.D.; Janssen, H.; George, T.C.; Kapsenberg, M.L.; et al. Langerhans Cells Favor Skin Flora Tolerance through Limited Presentation of Bacterial Antigens and Induction of Regulatory T Cells. J. Investig. Dermatol. 2013, 133, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Bouteau, A.; Cardenas, J.; Uthra, B.; Wang, Y.; Smitherman, C.; Gu, J.; Igyártó, B.Z. Brief communication: Long-term absence of Langerhans cells alters the gene expression profile of keratinocytes and dendritic epidermal T cells. PLoS ONE 2020, 15, e0223397. [Google Scholar] [CrossRef] [PubMed]
- Denning, T.L.; Wang, Y.-C.; Patel, S.R.; Williams, I.R.; Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17–producing T cell responses. Nat. Immunol. 2007, 8, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Müller, W.; Sparwasser, T.; Förster, R.; Pabst, O. Intestinal Tolerance Requires Gut Homing and Expansion of FoxP3+ Regulatory T Cells in the Lamina Propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef]
- Soroosh, P.; Doherty, T.; Duan, W.; Mehta, A.K.; Choi, H.; Adams, Y.F.; Mikulski, Z.; Khorram, N.; Rosenthal, P.; Broide, D.H.; et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J. Exp. Med. 2013, 210, 775–788. [Google Scholar] [CrossRef]
- Coleman, M.M.; Ruane, D.; Moran, B.; Dunne, P.J.; Keane, J.; Mills, K.H.G. Alveolar Macrophages Contribute to Respiratory Tolerance by Inducing FoxP3 Expression in Naive T Cells. Am. J. Respir. Cell Mol. Biol. 2013, 48, 773–780. [Google Scholar] [CrossRef]
- Erhardt, A.; Biburger, M.; Papadopoulos, T.; Tiegs, G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology 2007, 45, 475–485. [Google Scholar] [CrossRef]
- Breous, E.; Somanathan, S.; Vandenberghe, L.H.; Wilson, J.M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 2009, 50, 612–621. [Google Scholar] [CrossRef]
- Heymann, F.; Peusquens, J.; Ludwig-Portugall, I.; Kohlhepp, M.; Ergen, C.; Niemietz, P.; Martin, C.; van Rooijen, N.; Ochando, J.C.; Randolph, G.J.; et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015, 62, 279–291. [Google Scholar] [CrossRef]
- Ebner, F.; Brandt, C.; Thiele, P.; Richter, D.; Schliesser, U.; Siffrin, V.; Schueler, J.; Stubbe, T.; Ellinghaus, A.; Meisel, C.; et al. Microglial Activation Milieu Controls Regulatory T Cell Responses. J. Immunol. 2013, 191, 5594–5602. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Lopes, J.E.; Chong, M.; Ivanov, I.I.; Min, R.; Victora, G.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 2008, 453, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Marie, J.; Letterio, J.J.; Gavin, M.; Rudensky, A.Y. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 2005, 201, 1061–1067. [Google Scholar] [CrossRef]
- Hill, J.A.; Hall, J.A.; Sun, C.-M.; Cai, Q.; Ghyselinck, N.; Chambon, P.; Belkaid, Y.; Mathis, D.; Benoist, C. Retinoic Acid Enhances Foxp3 Induction Indirectly by Relieving Inhibition from CD4+CD44hi Cells. Immunity 2008, 29, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Idoyaga, J.; Fiorese, C.; Zbytnuik, L.; Lubkin, A.; Miller, J.; Malissen, B.; Mucida, D.; Merad, M.; Steinman, R.M. Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Investig. 2013, 123, 844–854. [Google Scholar] [CrossRef]
- Wu, C.; Rauch, U.; Korpos, E.; Song, J.; Loser, K.; Crocker, P.R.; Sorokin, L.M. Sialoadhesin-Positive Macrophages Bind Regulatory T Cells, Negatively Controlling Their Expansion and Autoimmune Disease Progression. J. Immunol. 2009, 182, 6508–6516. [Google Scholar] [CrossRef]
- Kaplan, D.H.; Igyártó, B.Z.; Gaspari, A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012, 12, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Van Rijn, E.; Jung, S.; Inaba, K.; Steinman, R.M.; Kapsenberg, M.L.; Clausen, B.E. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 2005, 169, 569–576. [Google Scholar] [CrossRef]
- Kissenpfennig, A.; Henri, S.; Dubois, B.; Laplace-Builhé, C.; Perrin, P.; Romani, N.; Tripp, C.H.; Douillard, P.; Leserman, L.; Kaiserlian, D.; et al. Dynamics and Function of Langerhans Cells In Vivo: Dermal Dendritic Cells Colonize Lymph Node AreasDistinct from Slower Migrating Langerhans Cells. Immunity 2005, 22, 643–654. [Google Scholar] [CrossRef]
- Bennett, C.; Noordegraaf, M.; Martina, C.A.E.; Clausen, B. Langerhans Cells Are Required for Efficient Presentation of Topically Applied Hapten to T Cells. J. Immunol. 2007, 179, 6830–6835. [Google Scholar] [CrossRef]
- Noordegraaf, M.; Flacher, V.; Stoitzner, P.; Clausen, B.E. Functional Redundancy of Langerhans Cells and Langerin+ Dermal Dendritic Cells in Contact Hypersensitivity. J. Investig. Dermatol. 2010, 130, 2752–2759. [Google Scholar] [CrossRef]
- Kaplan, D.H.; Jenison, M.C.; Saeland, S.; Shlomchik, W.D.; Shlomchik, M.J. Epidermal Langerhans Cell-Deficient Mice Develop Enhanced Contact Hypersensitivity. Immunity 2005, 23, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Zahner, S.P.; Kel, J.M.; Martina, C.A.E.; Brouwers-Haspels, I.; Van Roon, M.A.; Clausen, B.E. Conditional Deletion of TGF-βR1 Using Langerin-Cre Mice Results in Langerhans Cell Deficiency and Reduced Contact Hypersensitivity. J. Immunol. 2011, 187, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Shklovskaya, E.; O’Sullivan, B.J.; Ng, L.G.; Roediger, B.; Thomas, R.; Weninger, W.; de St Groth, B.F. Langerhans cells are precommitted to immune tolerance induction. Proc. Natl. Acad. Sci. USA 2011, 108, 18049–18054. [Google Scholar] [CrossRef] [PubMed]
- Gomez De Agüero, M.; Vocanson, M.; Hacini-Rachinel, F.; Taillardet, M.; Sparwasser, T.; Kissenpfennig, A.; Malissen, B.; Kaiserlian, D.; Dubois, B. Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8+ T cells and activating Foxp3+ regulatory T cells. J. Clin. Investig. 2012, 122, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Igyarto, B.Z.; Jenison, M.C.; Dudda, J.C.; Roers, A.; Muller, W.; Koni, P.; Campbell, D.J.; Shlomchik, M.J.; Kaplan, D.H. Langerhans Cells Suppress Contact Hypersensitivity Responses Via Cognate CD4 Interaction and Langerhans Cell-Derived IL-10. J. Immunol. 2009, 183, 5085–5093. [Google Scholar] [CrossRef]
- Yoshiki, R.; Kabashima, K.; Sugita, K.; Atarashi, K.; Shimauchi, T.; Tokura, Y. IL-10-Producing Langerhans Cells and Regulatory T Cells Are Responsible for Depressed Contact Hypersensitivity in Grafted Skin. J. Investig. Dermatol. 2009, 129, 705–713. [Google Scholar] [CrossRef]
- Kim, J.H.; Hu, Y.; Yongqing, T.; Kim, J.; Hughes, V.A.; Le Nours, J.; Marquez, E.A.; Purcell, A.; Wan, Q.; Sugita, M.; et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat. Immunol. 2016, 17, 1159–1166. [Google Scholar] [CrossRef]
- Bursch, L.S.; Wang, L.; Igyarto, B.; Kissenpfennig, A.; Malissen, B.; Kaplan, D.H.; Hogquist, K. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 2007, 204, 3147–3156. [Google Scholar] [CrossRef]
- Koch, S.; Stroisch, T.J.; Vorac, J.; Herrmann, N.; Leib, N.; Schnautz, S.; Kirins, H.; Förster, I.; Weighardt, H.; Bieber, T. AhR mediates an anti-inflammatory feedback mechanism in human Langerhans cells involving Fcε RI and IDO. Allergy 2017, 72, 1686–1693. [Google Scholar] [CrossRef]
- Kitashima, D.Y.; Kobayashi, T.; Woodring, T.; Idouchi, K.; Doebel, T.; Voisin, B.; Adachi, T.; Ouchi, T.; Takahashi, H.; Nishifuji, K.; et al. Langerhans Cells Prevent Autoimmunity via Expansion of Keratinocyte Antigen-Specific Regulatory T Cells. eBioMedicine 2017, 27, 293–303. [Google Scholar] [CrossRef]
- Strandt, H.; Pinheiro, D.F.; Kaplan, D.H.; Wirth, D.; Gratz, I.K.; Hammerl, P.; Thalhamer, J.; Stoecklinger, A. Neoantigen Expression in Steady-State Langerhans Cells Induces CTL Tolerance. J. Immunol. 2017, 199, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Dioszeghy, V.; Mondoulet, L.; Laoubi, L.; Dhelft, V.; Plaquet, C.; Bouzereau, A.; Dupont, C.; Sampson, H. Antigen Uptake by Langerhans Cells Is Required for the Induction of Regulatory T Cells and the Acquisition of Tolerance During Epicutaneous Immunotherapy in OVA-Sensitized Mice. Front. Immunol. 2018, 9, 1951. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, S.; Liu, X.; Wen, H.; Li, W.; Yao, X. Langerhans cells mediate the skin-induced tolerance to ovalbumin via Langerin in a murine model. Allergy 2019, 74, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Nümm, T.J.; Koch, S.; Herrmann, N.; Leib, N.; Bieber, T.; Stroisch, T.J. Langerhans and inflammatory dendritic epidermal cells in atopic dermatitis are tolerized toward TLR2 activation. Allergy 2018, 73, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Hulsebosch, H.J.; Krieg, S.R.; Bakker, P.M.; Cormane, R.H. Immunocompetent cells in psoriasis. Arch. Dermatol. Res. 1983, 275, 181–189. [Google Scholar] [CrossRef]
- Glitzner, E.; Korosec, A.; Brunner, P.; Drobits, B.; Amberg, N.; Schonthaler, H.B.; Kopp, T.; Wagner, E.F.; Stingl, G.; Holcmann, M.; et al. Specific roles for dendritic cell subsets during initiation and progression of psoriasis. EMBO Mol. Med. 2014, 6, 1312–1327. [Google Scholar] [CrossRef]
- Baker, B.S.; Swain, A.F.; Griffiths, E.C.; Leonard, J.N.; Fry, L.; Valdimarsson, H. Epidermal T lymphocytes and dendritic cells in chronic plaque psoriasis: The effects of PUVA treatment. Clin. Exp. Immunol. 1985, 61, 526–534. [Google Scholar]
- Komine, M.; Karakawa, M.; Takekoshi, T.; Sakurai, N.; Minatani, Y.; Mitsui, H.; Tada, Y.; Saeki, H.; Asahina, A.; Tamaki, K. Early Inflammatory Changes in the “Perilesional Skin” of Psoriatic Plaques: Is there Interaction between Dendritic Cells and Keratinocytes? J. Investig. Dermatol. 2007, 127, 1915–1922. [Google Scholar] [CrossRef]
- Czernielewski, J.; Juhlin, L.; Shroot, S.; Brun, P. Langerhans’ cells in patients with psoriasis: Effect of treatment with PUVA, PUVA bath, etretinate and anthralin. Acta Derm.-Venereol. 1985, 65, 97–101. [Google Scholar]
- Martini, E.; Wikén, M.; Cheuk, S.H.; Sérézal, I.G.; Baharom, F.; Ståhle, M.; Smed-Sörensen, A.; Eidsmo, L. Dynamic Changes in Resident and Infiltrating Epidermal Dendritic Cells in Active and Resolved Psoriasis. J. Investig. Dermatol. 2017, 137, 865–873. [Google Scholar] [CrossRef]
- Nakajima, K.; Kataoka, S.; Sato, K.; Takaishi, M.; Yamamoto, M.; Nakajima, H.; Sano, S. Stat3 activation in epidermal keratinocytes induces Langerhans cell activation to form an essential circuit for psoriasis via IL-23 production. J. Dermatol. Sci. 2019, 93, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhao, W.; Li, H.; Xiao, S.; Hu, R.; Han, M.; Liu, H.; Liu, Y.; Otsu, K.; Liu, X.; et al. p38α signaling in Langerhans cells promotes the development of IL-17–producing T cells and psoriasiform skin inflammation. Sci. Signal. 2018, 11, eaao1685. [Google Scholar] [CrossRef] [PubMed]
- Yoshiki, R.; Kabashima, K.; Honda, T.; Nakamizo, S.; Sawada, Y.; Sugita, K.; Yoshioka, H.; Ohmori, S.; Malissen, B.; Tokura, Y.; et al. IL-23 from Langerhans Cells Is Required for the Development of Imiquimod-Induced Psoriasis-Like Dermatitis by Induction of IL-17A-Producing γδ T Cells. J. Investig. Dermatol. 2014, 134, 1912–1921. [Google Scholar] [CrossRef]
- Xiao, C.; Zhu, Z.; Sun, S.; Gao, J.; Fu, M.; Liu, Y.; Wang, G.; Yao, X.; Li, W. Activation of Langerhans cells promotes the inflammation in imiquimod-induced psoriasis-like dermatitis. J. Dermatol. Sci. 2016, 85, 170–177. [Google Scholar] [CrossRef]
- Eaton, L.; Mellody, K.; Pilkington, S.; Dearman, R.; Kimber, I.; Griffiths, C. Impaired Langerhans cell migration in psoriasis is due to an altered keratinocyte phenotype induced by interleukin-17. Br. J. Dermatol. 2017, 178, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Terhorst, D.; Chelbi, R.; Wohn, C.; Malosse, C.; Tamoutounour, S.; Jorquera, A.; Bajenoff, M.; Dalod, M.; Malissen, B.; Henri, S. Dynamics and Transcriptomics of Skin Dendritic Cells and Macrophages in an Imiquimod-Induced, Biphasic Mouse Model of Psoriasis. J. Immunol. 2015, 195, 4953–4961. [Google Scholar] [CrossRef]
- Tanaka, R.; Ichimura, Y.; Kubota, N.; Konishi, R.; Nakamura, Y.; Mizuno, S.; Takahashi, S.; Fujimoto, M.; Nomura, T.; Okiyama, N. The role of programmed cell death ligand-1 on Langerhans cells in the regulation of psoriasis. J. Investig. Dermatol. 2022. [Google Scholar] [CrossRef]
- Park, S. Building vs. Rebuilding Epidermis: Comparison Embryonic Development and Adult Wound Repair. Front. Cell Dev. Biol. 2022, 9, 796080. [Google Scholar] [CrossRef]
- Lewis, J.; Filler, R.; Smith, D.A.; Golubets, K.; Girardi, M. The contribution of Langerhans cells to cutaneous malignancy. Trends Immunol. 2010, 31, 460–466. [Google Scholar] [CrossRef]
- Collins, L.; Asfour, L.; Stephany, M.; Lear, J.; Stasko, T. Management of Non-melanoma Skin Cancer in Transplant Recipients. Clin. Oncol. 2019, 31, 779–788. [Google Scholar] [CrossRef]
- Martincorena, I.; Campbell, P.J. Somatic mutation in cancer and normal cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Martincorena, I.; Roshan, A.; Gerstung, M.; Ellis, P.; Van Loo, P.; McLaren, S.; Wedge, D.C.; Fullam, A.; Alexandrov, L.B.; Tubio, J.M.; et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015, 348, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Flacher, V.; Sparber, F.; Tripp, C.H.; Romani, N.; Stoitzner, P. Targeting of epidermal Langerhans cells with antigenic proteins: Attempts to harness their properties for immunotherapy. Cancer Immunol. Immunother. 2008, 58, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Brocks, T.; Fedorchenko, O.; Schliermann, N.; Stein, A.; Moll, U.M.; Seegobin, S.; Dewor, M.; Hallek, M.; Marquardt, Y.; Fietkau, K.; et al. Macrophage migration inhibitory factor protects from nonmelanoma epidermal tumors by regulating the number of antigen-presenting cells in skin. FASEB J. 2016, 31, 526–543. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Duncan, F.J.; Keiser, T.; Shin, S.; Kusewitt, D.F.; Oberyszyn, T.; Satoskar, A.R.; VanBuskirk, A.M. Macrophage migration inhibitory factor (MIF) plays a critical role in pathogenesis of ultraviolet-B (UVB) -induced nonmelanoma skin cancer (NMSC). FASEB J. 2009, 23, 720–730. [Google Scholar] [CrossRef]
- Honda, A.; Abe, R.; Yoshihisa, Y.; Makino, T.; Matsunaga, K.; Nishihira, J.; Shimizu, H.; Shimizu, T. Deficient deletion of apoptotic cells by macrophage migration inhibitory factor (MIF) overexpression accelerates photocarcinogenesis. Carcinogenesis 2009, 30, 1597–1605. [Google Scholar] [CrossRef]
- Bifulco, C.; McDaniel, K.; Leng, L.; Bucala, R. Tumor Growth-Promoting Properties of Macrophage Migration Inhibitory Factor. Curr. Pharm. Des. 2008, 14, 3790–3801. [Google Scholar] [CrossRef]
- Zhang, S.; Ramsay, E.S.; Mock, B.A. Cdkn2a, the cyclin-dependent kinase inhibitor encoding p16 INK4a and p19 ARF, is a candidate for the plasmacytoma susceptibility locus, Pctr1. Proc. Natl. Acad. Sci. USA 1998, 95, 2429–2434. [Google Scholar] [CrossRef]
- Abel, E.L.; Angel, J.M.; Kiguchi, K.; DiGiovanni, J. Multi-stage chemical carcinogenesis in mouse skin: Fundamentals and applications. Nat. Protoc. 2009, 4, 1350–1362. [Google Scholar] [CrossRef]
- Mastrangelo, G.; Fadda, E.; Marzia, V. Polycyclic aromatic hydrocarbons and cancer in man. Environ. Health Perspect. 1996, 104, 1166–1170. [Google Scholar] [CrossRef]
- Strid, J.; Roberts, S.J.; Filler, R.B.; Lewis, J.M.; Kwong, B.Y.; Schpero, W.; Kaplan, D.H.; Hayday, A.; Girardi, M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 2008, 9, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Modi, B.G.; Neustadter, J.; Binda, E.; Lewis, J.; Filler, R.B.; Roberts, S.J.; Kwong, B.Y.; Reddy, S.; Overton, J.D.; Galan, A.; et al. Langerhans Cells Facilitate Epithelial DNA Damage and Squamous Cell Carcinoma. Science 2012, 335, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.M.; Bürgler, C.D.; Fraser, J.A.; Liao, H.; Golubets, K.; Kucher, C.L.; Zhao, P.Y.; Filler, R.B.; Tigelaar, R.E.; Girardi, M. Mechanisms of Chemical Cooperative Carcinogenesis by Epidermal Langerhans Cells. J. Investig. Dermatol. 2015, 135, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Athar, M.; Tubesing, K.A.; Rothaupt, D.; Xu, H.; Mukhtar, H. Susceptibility to the biological effects of polyaromatic hydrocarbons is influenced by genes of the major histocompatibility complex. Proc. Natl. Acad. Sci. USA 1998, 95, 14915–14919. [Google Scholar] [CrossRef]
- Maraee, A.; Farag, A.G.A.; Gadallah, M.M.; Abdou, A.G.; Antar, A.G.F. Tumour-infiltrating Langerhans cells in non-melanoma skin cancer, a clinical and immunohistochemical study. Ecancermedicalscience 2020, 14, 1045. [Google Scholar] [CrossRef]
- Pogorzelska-Dyrbuś, J.; Szepietowski, J.C. Density of Langerhans Cells in Nonmelanoma Skin Cancers: A Systematic Review. Mediat. Inflamm. 2020, 2020, 8745863. [Google Scholar] [CrossRef]
- Klechevsky, E.; Morita, R.; Liu, M.; Cao, Y.; Coquery, S.; Thompson-Snipes, L.; Briere, F.; Chaussabel, D.; Zurawski, G.; Palucka, A.K.; et al. Functional Specializations of Human Epidermal Langerhans Cells and CD14+ Dermal Dendritic Cells. Immunity 2008, 29, 497–510. [Google Scholar] [CrossRef]
- Fujita, H.; Suárez-Fariñas, M.; Mitsui, H.; Gonzalez, J.; Bluth, M.J.; Zhang, S.; Felsen, D.; Krueger, J.G.; Carucci, J.A. Langerhans Cells from Human Cutaneous Squamous Cell Carcinoma Induce Strong Type 1 Immunity. J. Investig. Dermatol. 2012, 132, 1645–1655. [Google Scholar] [CrossRef]
- Banchereau, J.; Thompson-Snipes, L.; Zurawski, S.; Blanck, J.-P.; Cao, Y.; Clayton, S.; Gorvel, J.-P.; Zurawski, G.; Klechevsky, E. The differential production of cytokines by human Langerhans cells and dermal CD14+ DCs controls CTL priming. Blood 2012, 119, 5742–5749. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; Berard, F.; Essert, G.; Chalouni, C.; Pulendran, B.; Davoust, J.; Bridges, G.; Palucka, A.K.; Banchereau, J. Interleukin 15 Skews Monocyte Differentiation into Dendritic Cells with Features of Langerhans Cells. J. Exp. Med. 2001, 194, 1013–1020. [Google Scholar] [CrossRef]
- Dubsky, P.; Saito, H.; Leogier, M.; Dantin, C.; Connolly, J.E.; Banchereau, J.; Palucka, A.K. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur. J. Immunol. 2007, 37, 1678–1690. [Google Scholar] [CrossRef] [PubMed]
- Penel-Sotirakis, K.; Simonazzi, E.; Péguet-Navarro, J.; Rozières, A. Differential Capacity of Human Skin Dendritic Cells to Polarize CD4+T Cells into IL-17, IL-21 and IL-22 Producing Cells. PLoS ONE 2012, 7, e45680. [Google Scholar] [CrossRef] [PubMed]
- Furio, L.; Briotet, I.; Journeaux, A.; Billard, H.; Péguet-Navarro, J. Human Langerhans Cells Are More Efficient Than CD14−CD1c+ Dermal Dendritic Cells at Priming Naive CD4+ T Cells. J. Investig. Dermatol. 2010, 130, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Dearman, R.; Cumberbatch, M.; Kimber, I. Cytokines and Langerhans cell mobilisation in mouse and man. Cytokine 2005, 32, 67–70. [Google Scholar] [CrossRef]
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–399. [Google Scholar]
- Bennett, C.L.; Fallah-Arani, F.; Conlan, T.; Trouillet, C.; Goold, H.; Chorro, L.; Flutter, B.; Means, T.K.; Geissmann, F.; Chakraverty, R. Langerhans cells regulate cutaneous injury by licensing CD8 effector cells recruited to the skin. Blood 2011, 117, 7063–7069. [Google Scholar] [CrossRef]
- Denniston, A.K.; Kottoor, S.H.; Khan, I.J.; Oswal, K.; Williams, G.P.; Abbott, J.; Wallace, G.R.; Salmon, M.; Rauz, S.; Murray, P.I.; et al. Endogenous Cortisol and TGF-β in Human Aqueous Humor Contribute to Ocular Immune Privilege by Regulating Dendritic Cell Function. J. Immunol. 2011, 186, 305–311. [Google Scholar] [CrossRef]
- Polak, M.E.; Borthwick, N.J.; Gabriel, F.G.; Johnson, P.; Higgins, B.; Hurren, J.; McCormick, D.; Jager, M.J.; Cree, A.I. Mechanisms of local immunosuppression in cutaneous melanoma. Br. J. Cancer 2007, 96, 1879–1887. [Google Scholar] [CrossRef]
- Ochiel, D.O.; Ochsenbauer, C.; Kappes, J.C.; Ghosh, M.; Fahey, J.V.; Wira, C.R. Uterine Epithelial Cell Regulation of DC-SIGN Expression Inhibits Transmitted/Founder HIV-1 Trans Infection by Immature Dendritic Cells. PLoS ONE 2010, 5, e14306. [Google Scholar] [CrossRef]
- Esebanmen, G.E.; Langridge, W.H.R. The role of TGF-beta signaling in dendritic cell tolerance. Immunol. Res. 2017, 65, 987–994. [Google Scholar] [CrossRef]
- Bain, C.C.; Montgomery, J.; Scott, C.; Kel, J.M.; Girard-Madoux, M.J.H.; Martens, L.; Zangerle-Murray, T.F.P.; Ober-Blöbaum, J.; Lindenbergh-Kortleve, D.; Samsom, J.N.; et al. TGFβR signalling controls CD103+CD11b+ dendritic cell development in the intestine. Nat. Commun. 2017, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Laouar, Y.; Town, T.; Jeng, D.; Tran, E.; Wan, Y.; Kuchroo, V.K.; Flavell, R.A. TGF-β signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2008, 105, 10865–10870. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, N.; Konradi, S.; Eisenwort, G.; Schichl, Y.M.; Seyerl, M.; Bauer, T.; Stöckl, J.; Strobl, H. β-Catenin Promotes the Differentiation of Epidermal Langerhans Dendritic Cells. J. Investig. Dermatol. 2013, 133, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, T.A.; Letterio, J.J.; Farr, A.G.; Udey, M.C. A Role for Endogenous Transforming Growth Factor β1 in Langerhans Cell Biology: The Skin of Transforming Growth Factor β1 Null Mice Is Devoid of Epidermal Langerhans Cells. J. Exp. Med. 1996, 184, 2417–2422. [Google Scholar] [CrossRef]
- Mohammed, J.; Beura, L.K.; Bobr, A.; Astry, B.; Chicoine, B.; Kashem, S.W.; Welty, N.E.; Igyártó, B.Z.; Wijeyesinghe, S.; Thompson, E.A.; et al. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β. Nat. Immunol. 2016, 17, 414–421. [Google Scholar] [CrossRef]
- Bobr, A.; Igyarto, B.Z.; Haley, K.M.; Li, M.O.; Flavell, R.A.; Kaplan, D.H. Autocrine/paracrine TGF-β1 inhibits Langerhans cell migration. Proc. Natl. Acad. Sci. USA 2012, 109, 10492–10497. [Google Scholar] [CrossRef]
- Polak, M.E.; Singh, H. Tolerogenic and immunogenic states of Langerhans cells are orchestrated by epidermal signals acting on a core maturation gene module. BioEssays 2021, 43, 2000182. [Google Scholar] [CrossRef]
- Biswas, P.S.; Gupta, S.; Chang, E.; Song, L.; Stirzaker, R.A.; Liao, J.K.; Bhagat, G.; Pernis, A.B. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J. Clin. Investig. 2010, 120, 3280–3295. [Google Scholar] [CrossRef]
- Vogel, C.F.; Goth, S.R.; Dong, B.; Pessah, I.N.; Matsumura, F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2008, 375, 331–335. [Google Scholar] [CrossRef]
- Sirvent, S.; Vallejo, A.F.; Davies, J.; Clayton, K.; Wu, Z.; Woo, J.; Riddell, J.; Chaudhri, V.K.; Stumpf, P.; Nazlamova, L.A.; et al. Genomic programming of IRF4-expressing human Langerhans cells. Nat. Commun. 2020, 11, 313. [Google Scholar] [CrossRef]
- Zucchini, C.; Manara, M.C.; Pinca, R.S.; De Sanctis, P.; Guerzoni, C.; Sciandra, M.; Lollini, P.-L.; Cenacchi, G.; Picci, P.; Valvassori, L.; et al. CD99 suppresses osteosarcoma cell migration through inhibition of ROCK2 activity. Oncogene 2014, 33, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Kimura, A.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 19961–19966. [Google Scholar] [CrossRef]
- Gerbal-Chaloin, S.; Dumé, A.-S.; Briolotti, P.; Klieber, S.; Raulet, E.; Duret, C.; Fabre, J.-M.; Ramos, J.; Maurel, P.; Daujat-Chavanieu, M. The WNT/β-Catenin Pathway Is a Transcriptional Regulator of CYP2E1, CYP1A2, and Aryl Hydrocarbon Receptor Gene Expression in Primary Human Hepatocytes. Mol. Pharmacol. 2014, 86, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.; Lin, S.H.; Clausen, B.E.; Lee, C.H. Selective AhR knockout in langerin-expressing cells abates Langerhans cells and polarizes Th2/Tr1 in epicutaneous protein sensitization. Proc. Natl. Acad. Sci. USA 2020, 117, 12980–12990. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Harden, J.L.; Anderson, C.D.; Egilmez, N.K. Tolerogenic Phenotype of IFN-γ-Induced IDO+ Dendritic Cells Is Maintained via an Autocrine IDO-Kynurenine/AhR-IDO Loop. J. Immunol. 2016, 197, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, R.; De La Cruz Diaz, J.S.; Rumpret, M.; Fuchsberger, F.F.; van Teijlingen, N.H.; Hanske, J.; Rademacher, C.; Geijtenbeek, T.B.H.; van Strijp, J.A.G.; Weidenmaier, C.; et al. Langerhans Cells Sense Staphylococcus aureus Wall Teichoic Acid through Langerin To Induce Inflammatory Responses. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Flacher, V.; Bouschbacher, M.; Verronèse, E.; Massacrier, C.; Sisirak, V.; Berthier-Vergnes, O.; de Saint-Vis, B.; Caux, C.; Dezutter-Dambuyant, C.; Lebecque, S.; et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J. Immunol. 2006, 177, 7959–7967. [Google Scholar] [CrossRef]
- Campos Carrascosa, L.; Klein, M.; Kitagawa, Y.; Lückel, C.; Marini, F.; König, A.; Guralnik, A.; Raifer, H.; Hagner-Benes, S.; Rädler, D.; et al. Reciprocal regulation of the Il9 locus by counteracting activities of transcription factors IRF1 and IRF4. Nat. Commun. 2017, 8, 15366. [Google Scholar] [CrossRef]
- Polak, M.E.; Ung, C.Y.; Masapust, J.; Freeman, T.C.; Ardern-Jones, M.R. Petri Net computational modelling of Langerhans cell Interferon Regulatory Factor Network predicts their role in T cell activation. Sci. Rep. 2017, 7, 668. [Google Scholar] [CrossRef]
- Sjöstrand, M.; Johansson, A.; Aqrawi, L.; Olsson, T.; Wahren-Herlenius, M.; Espinosa, A. The Expression of BAFF Is Controlled by IRF Transcription Factors. J. Immunol. 2016, 196, 91–96. [Google Scholar] [CrossRef]
- Davies, J.; Vallejo, A.F.; Sirvent, S.; Porter, G.; Clayton, K.; Qumbelo, Y.; Stumpf, P.; West, J.; Gray, C.M.; Chigorimbo-Murefu, N.T.L.; et al. An IRF1-IRF4 Toggle-Switch Controls Tolerogenic and Immunogenic Transcriptional Programming in Human Langerhans Cells. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
| Model Type | Observed LC Function | LC Location | Reference |
|---|---|---|---|
| UVR, Mu-Langerin-DTR | migration and induction of antigen-specific Treg post UVR | LN | [8] Schwarz A et al. |
| UVR, Mu-Langerin-DTR, Ag-specific CD8T transfer | Dermal Langerin+ DC but not LC reduce CD8 T expansion post UVR | LN | [9] Wang L, et al. |
| UVR, Mu-Langerin-DTR | phagocyte apoptotic KC, anti-inflammation post UVB | Skin | [11] Hatakeyama M et al. |
| UVR, K14-RANKLTg | RANK (LC)-RANKL (KC) interaction enhance Tregfunction | LN, Skin | [15] Loser K et al. [16] Yoshiki R et al. |
| UVR, Mu-Langerin-DTR | OX40L (LC)- OX40 (T) interaction induce T reg | LN, Skin | [16] Yoshiki R et al. |
| CD300a−/− mice | CD300a (LC)-PC (apoptotic KC) interaction inhibit Treg. | Skin | [17] Nakahashi-Oda C et al. |
| UVR, hu-Langerin DTA | promote p53 mutant KC clonal islands expansion | Skin | [18] Lewis JM et al. |
| UVR, hu-Langerin DTA | facilitate ILC3 shift enhance mutant KC growth upon UV | Skin | [19] Lewis JM et al. |
| Model Type | Tumor Model | Skin Tumor Development | LC or DC Phenotype | Reference |
|---|---|---|---|---|
| Mouse MIF KO | Chemical-DMBA-TPA | decreased tumor | decreased skin LC & other immmune cells | [84] 2017 |
| Mouse MIF KO (BALB/C) | UV-induced | Increased tumor | N/A | [85] 2009 |
| Mouse MIF Tg | UV-induced | Decreased tumor | N/A | [86] 2009 |
| Mouse LC deletion | Chemical-DMBA-TPA | Decreased tumor | N/A | [91], 2008 |
| Mouse LC deletion | Chemical-DMBA-TPA | Decreased tumor | LC matablize DMBA, induce Hras mutation | [92], 2012 |
| Mouse LC deletion | Chemical-DMBA-TPA | Decreased tumor | LC originated CYB1P1 required for KC DNA damage | [93], 2015 |
| Human | BCC, SCC | N/A | Decreased LC in BCC/SCC than HC; increased LC in BCC than SCC | [95] 2020 |
| Human | Summary of 30 nonmalanoma skin cancer studies | N/A | Increased LC in BCC than in SCC | [96] 2020 |
| Human | SCC | N/A | SCC LC better inducer of CD4/CD8 T activation than paratumoral LC | [98] 2012 |
| Human | in vitro monocyte differentiation | N/A | IL-15 skew monocytes into LC | [100] 2001 |
| Human | in vitro monocyte differentiation | N/A | LC-like IL15-DC are better inducer of tumor-specific CTL than IL4-DC | [101] 2007 |
| Human | non-tumor skin | N/A | IL-15 by LC induced CD8 T effector function | [99] 2012 |
| Human | in vitro T cell differentiation | N/A | LC are better inducer of CD4 T activation than CD1c+ DDC | [103] 2010 |
| Human | in vitro T cell differentiation | N/A | LC are better than DDC in inducing CD4 T IL21, IL22 production. | [102] 2012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Jiang, A.; Veenstra, J.; Ozog, D.M.; Mi, Q.-S. The Roles of Skin Langerhans Cells in Immune Tolerance and Cancer Immunity. Vaccines 2022, 10, 1380. https://doi.org/10.3390/vaccines10091380
Zhou L, Jiang A, Veenstra J, Ozog DM, Mi Q-S. The Roles of Skin Langerhans Cells in Immune Tolerance and Cancer Immunity. Vaccines. 2022; 10(9):1380. https://doi.org/10.3390/vaccines10091380
Chicago/Turabian StyleZhou, Li, Aimin Jiang, Jesse Veenstra, David M. Ozog, and Qing-Sheng Mi. 2022. "The Roles of Skin Langerhans Cells in Immune Tolerance and Cancer Immunity" Vaccines 10, no. 9: 1380. https://doi.org/10.3390/vaccines10091380
APA StyleZhou, L., Jiang, A., Veenstra, J., Ozog, D. M., & Mi, Q.-S. (2022). The Roles of Skin Langerhans Cells in Immune Tolerance and Cancer Immunity. Vaccines, 10(9), 1380. https://doi.org/10.3390/vaccines10091380






