RNA Nanovaccine Protects against White Spot Syndrome Virus in Shrimp
Abstract
1. Introduction
2. Materials and Methods
2.1. dsRNA Synthesis
2.2. Polyanhydride and Nanoparticle Synthesis
2.3. Release Kinetics of dsRNA from Polyanhydride Nanoparticles
2.4. Stability of dsRNA Released from Polyanhydride Nanoparticles
2.5. Shrimp Rearing
2.6. Nanovaccine Safety
2.7. Histopathology
2.8. Biodistribution
2.9. Virus Stock Preparation
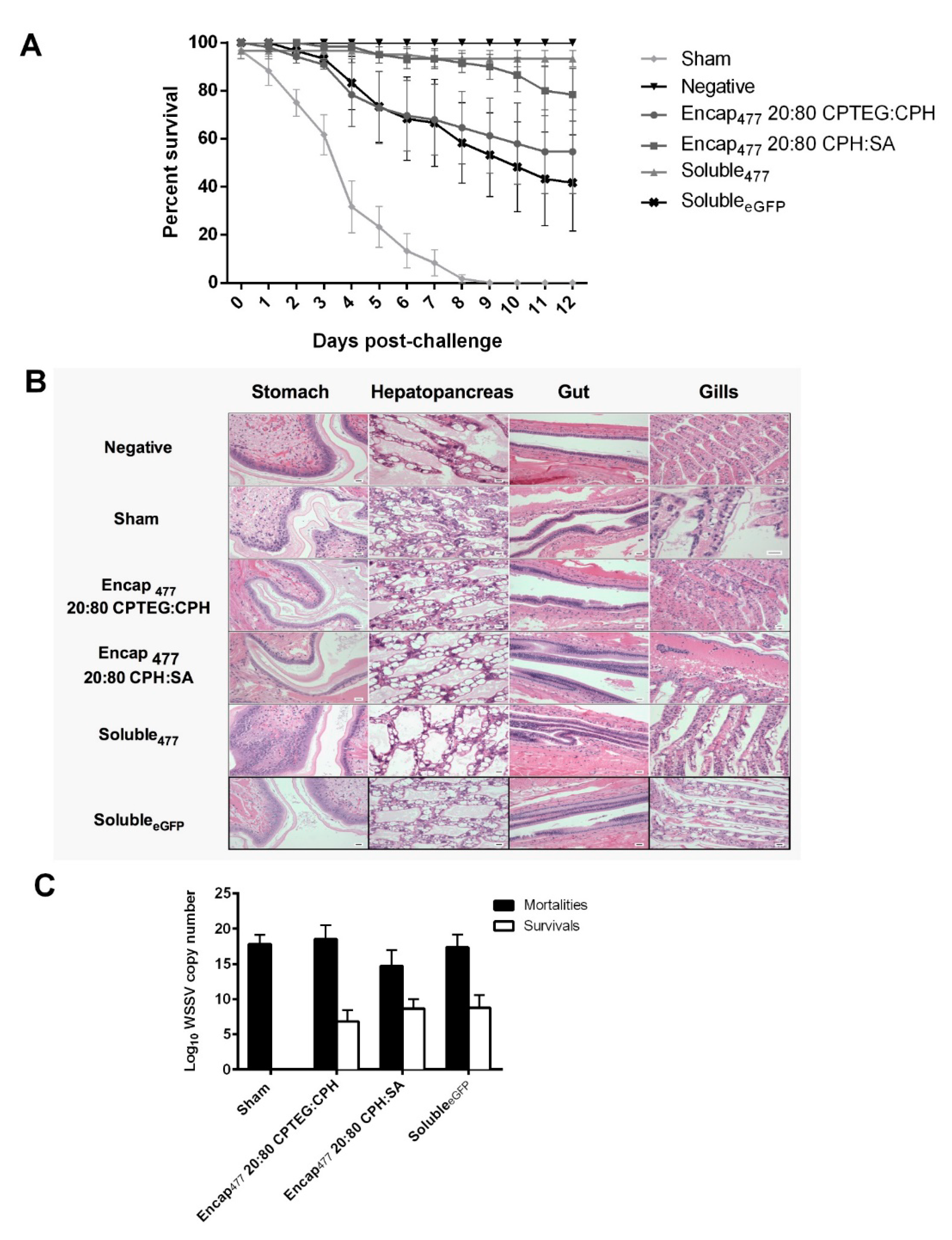
2.10. Bioassays for Survivability
2.11. Quantitative Real Time PCR
2.12. Statistical Analysis
2.13. Principal Component Analysis (PCA)
3. Results
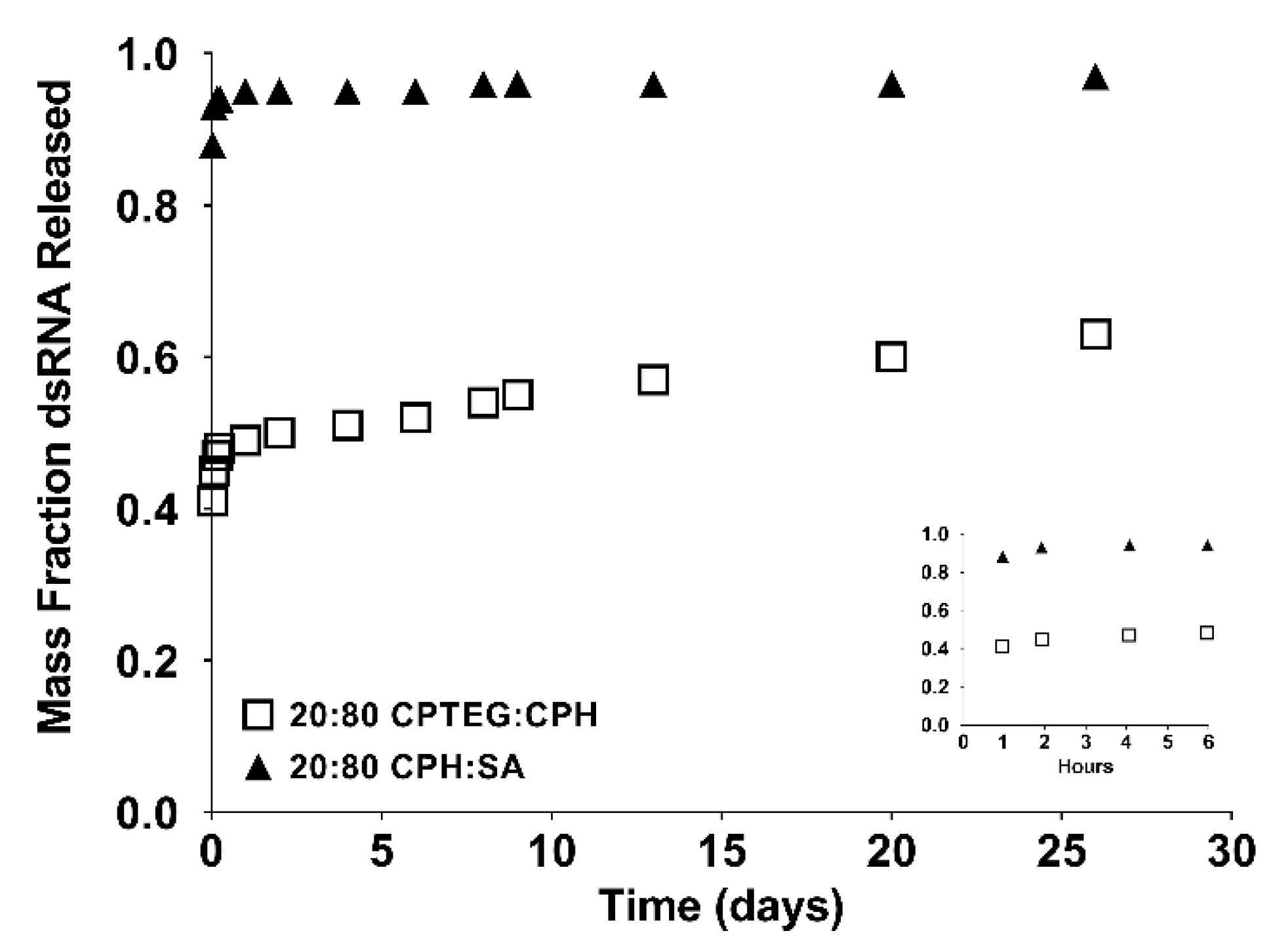
3.1. Polyanhydride Nanoparticles Provide Sustained Released of dsRNA
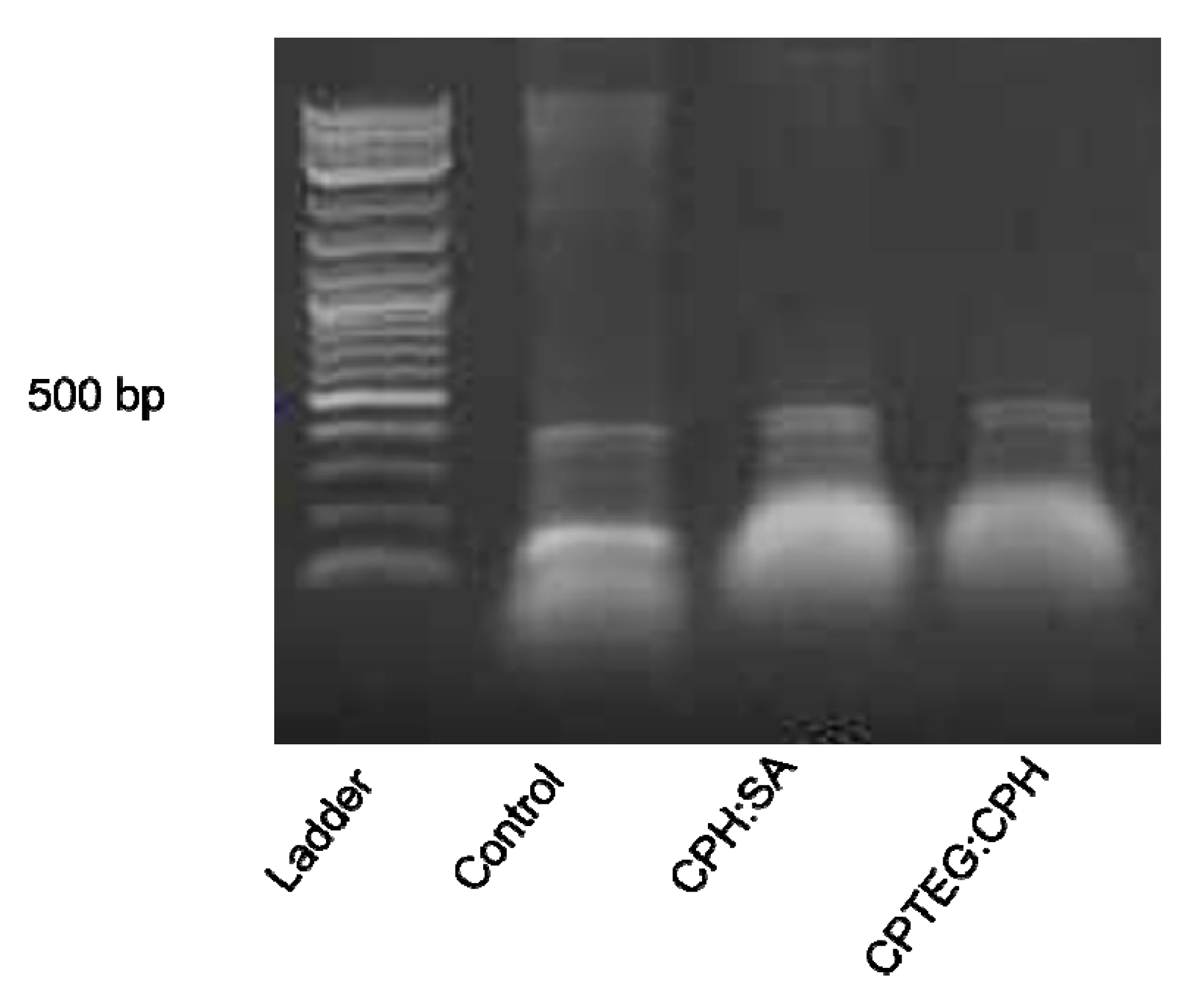
3.2. dsRNA Released from Polyanhydride Nanovaccines Is Stable
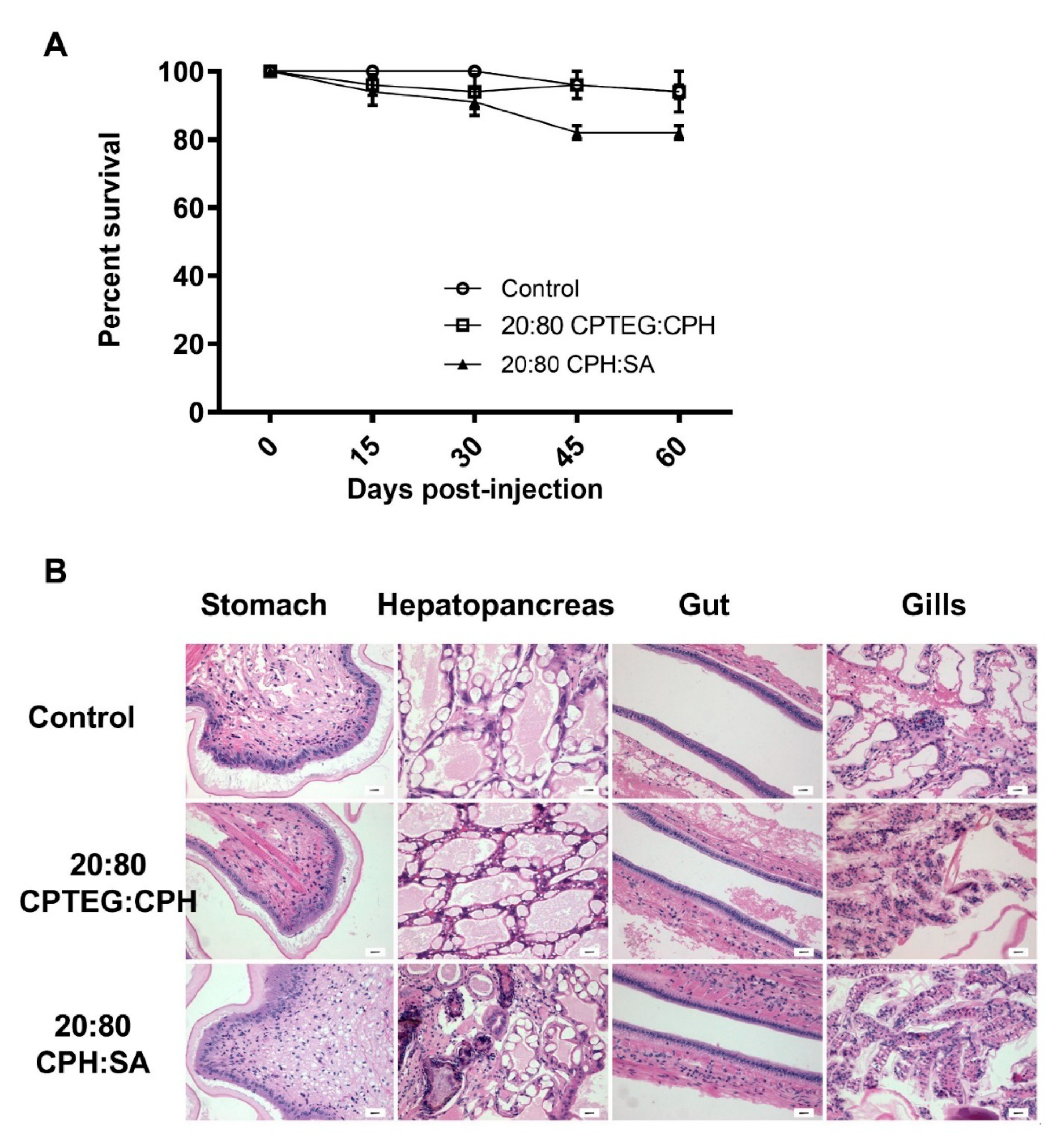
3.3. Polyanhydride Nanovaccines Do Not Induce Adverse Effects In Vivo
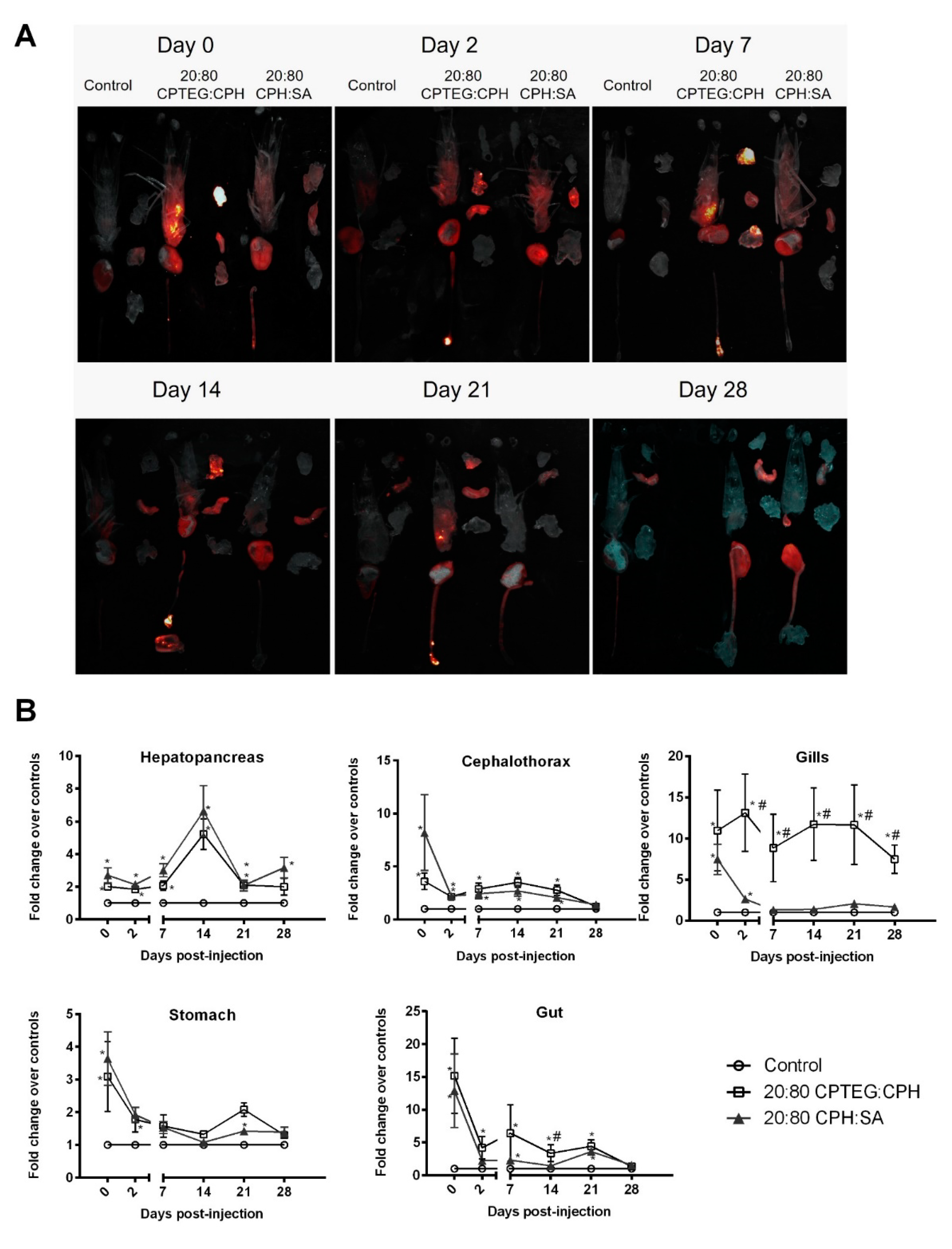
3.4. Polyanhydride Nanoparticles Exhibit Chemistry-Dependent Spatiotemporal Distribution In Vivo
3.5. Double-Stranded RNA-Based Nanovaccines Provide Protection against WSSV
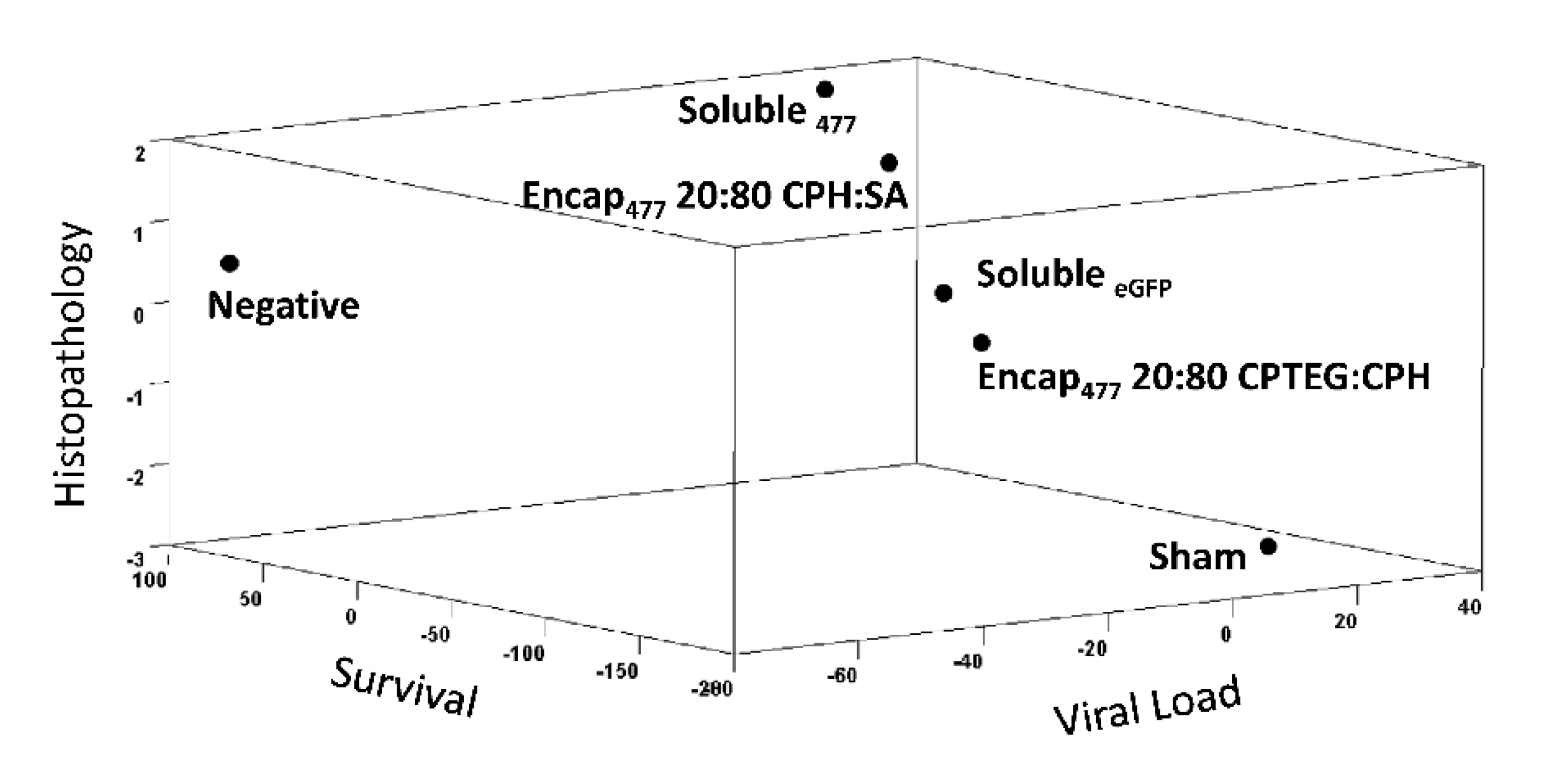
3.6. Informatics Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stentiford, G.; Neil, D.; Peeler, E.; Shields, J.; Small, H.; Flegel, T.; Vlak, J.; Jones, B.; Morado, F.; Moss, S.; et al. Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. J. Invertebr. Pathol. 2012, 110, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Verma, A.K.; Singh, S.P.; Awasthi, A. Immunostimulants for shrimp aquaculture: Paving pathway towards shrimp sustainability. Environ. Sci. Pollut. Res. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization of the United Nations Fisheries and Aquaculture Department, FishStat Plus-Fishery Statistical Software. 2009. Available online: https://www.fao.org/fishery/en/statistics/software/fishstatj/en (accessed on 16 August 2022).
- Seibert, C.H.; Pinto, A.R. Challenges in shrimp aquaculture due to viral diseases: Distribution and biology of the five major penaeid viruses and interventions to avoid viral incidence and dispersion. Braz. J. Microbiol. 2012, 43, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Bartholomay, L.C.; Loy, D.S.; Loy, J.D.; Harris, D. Nucleic-acid based antivirals: Augmenting RNA interference to ‘vaccinate’ Litopenaeus vannamei. J. Invertebr. Pathol. 2012, 110, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, C.; Hu, S.; Wu, Q.; Li, A. Recent progress in the development of white spot syndrome virus vaccines for protecting shrimp against viral infection. Arch. Virol. 2017, 162, 2923–2936. [Google Scholar] [CrossRef] [PubMed]
- Le Linh, H.; Thu, N.P.A.; Dung, T.T.X.; Van Hau, N.; Nghia, N.H.; Thao, D.T.P. Yeast cell surface displaying VP28 antigen and its potential application for shrimp farming. Appl. Microbiol. Biotechnol. 2021, 105, 6345–6354. [Google Scholar] [CrossRef]
- Mejía-Ruíz, C.H.; Vega-Peña, S.; Alvarez-Ruiz, P.; Escobedo-Bonilla, C.M. Double-stranded RNA against white spot syndrome virus (WSSV) vp28 or vp26 reduced susceptibility of Litopenaeus vannamei to WSSV, and survivors exhibited decreased susceptibility in subsequent re-infections. J. Invertebr. Pathol. 2011, 107, 65–68. [Google Scholar] [CrossRef]
- Weerachatyanukul, W.; Chotwiwatthanakun, C.; Jariyapong, P. Dual VP28 and VP37 dsRNA encapsulation in IHHNV virus-like particles enhances shrimp protection against white spot syndrome virus. Fish Shellfish Immunol. 2021, 113, 89–95. [Google Scholar] [CrossRef]
- Narasimhan, B.; Ross, K.A.; Loyd, H.; Wu, W.; Huntimer, L.; Ahmed, S.; Sambol, A.; Broderick, S.; Flickinger, Z.; Rajan, K.; et al. Hemagglutinin-based polyanhydride nanovaccines against H5N1 influenza elicit protective virus neutralizing titers and cell-mediated immunity. Int. J. Nanomed. 2015, 10, 229–243. [Google Scholar] [CrossRef]
- Huntimer, L.; Welder, J.H.W.; Ross, K.; Carrillo-Conde, B.; Pruisner, L.; Wang, C.; Narasimhan, B.; Wannemuehler, M.J.; Ramer-Tait, A.E. Single immunization with a suboptimal antigen dose encapsulated into polyanhydride microparticles promotes high titer and avid antibody responses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101B, 91–98. [Google Scholar] [CrossRef]
- Binnebose, A.M.; Haughney, S.L.; Martin, R.J.; Imerman, P.M.; Narasimhan, B.; Bellaire, B.H. Polyanhydride Nanoparticle Delivery Platform Dramatically Enhances Killing of Filarial Worms. PLOS Neglected Trop. Dis. 2015, 9, e0004173. [Google Scholar] [CrossRef]
- Phanse, Y.; Lueth, P.; Ramer-Tait, A.E.; Carrillo-Conde, B.R.; Wannemuehler, M.J.; Narasimhan, B.; Bellaire, B.H. Cellular Internalization Mechanisms of Polyanhydride Particles: Implications for Rational Design of Drug Delivery Vehicles. J. Biomed. Nanotechnol. 2016, 12, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Kingstad-Bakke, B.A.; Chandrasekar, S.S.; Phanse, Y.; Ross, K.A.; Hatta, M.; Suresh, M.; Kawaoka, Y.; Osorio, J.E.; Narasimhan, B.; Talaat, A.M. Effective mosaic-based nanovaccines against avian influenza in poultry. Vaccine 2019, 37, 5051–5058. [Google Scholar] [CrossRef] [PubMed]
- Kipper, M.J.; Wilson, J.H.; Wannemuehler, M.J.; Narasimhan, B. Single dose vaccine based on biodegradable polyanhydride microspheres can modulate immune response mechanism. J. Biomed. Mater. Res. Part A 2006, 76A, 798–810. [Google Scholar] [CrossRef]
- Ross, K.A.; Loyd, H.; Wu, W.; Huntimer, L.; Wannemuehler, M.J.; Carpenter, S.; Narasimhan, B. Structural and antigenic stability of H5N1 hemagglutinin trimer upon release from polyanhydride nanoparticles. J. Biomed. Mater. Res. Part A 2014, 102, 4161–4168. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Phanse, Y.; Sinha, A.; Wannemuehler, M.J.; Narasimhan, B.; Bellaire, B.H. Polymer Chemistry Influences Monocytic Uptake of Polyanhydride Nanospheres. Pharm. Res. 2009, 26, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Phanse, Y.; Carrillo-Conde, B.R.; Ramer-Tait, A.; Roychoudhury, R.; Broderick, S.; Pohl, N.; Rajan, K.; Narasimhan, B.; Wannemuehler, M.J.; Bellaire, B.H. Functionalization promotes pathogen-mimicking characteristics of polyanhydride nanoparticle adjuvants. J. Biomed. Mater. Res. Part A 2017, 105, 2762–2771. [Google Scholar] [CrossRef]
- Loy, D.S. Host-Virus Interactions in the Pacific White Shrimp, Litopenaeus vannamei. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar]
- Kipper, M.J.; Shen, E.; Determan, A.; Narasimhan, B. Design of an injectable system based on bioerodible polyanhydride microspheres for sustained drug delivery. Biomaterials 2002, 23, 4405–4412. [Google Scholar] [CrossRef]
- Torres, M.P.; Vogel, B.M.; Narasimhan, B.; Mallapragada, S.K. Synthesis and characterization of novel polyanhydrides with tailored erosion mechanisms. J. Biomed. Mater. Res. Part A 2006, 76A, 102–110. [Google Scholar] [CrossRef]
- Ulery, B.D.; Kumar, D.; Ramer-Tait, A.; Metzger, D.; Wannemuehler, M.J.; Narasimhan, B. Design of a Protective Single-Dose Intranasal Nanoparticle-Based Vaccine Platform for Respiratory Infectious Diseases. PLoS ONE 2011, 6, e17642. [Google Scholar] [CrossRef]
- Loy, J.D.; Mogler, M.A.; Loy, D.S.; Janke, B.; Kamrud, K.; Scura, E.D.; Harris, D.H.; Bartholomay, L.C. Double Stranded RNA Provides Sequence Dependent Protection Against Infectious Myonecrosis Virus in Litopenaeus vannamei. J. Gen. Virol. 2012, 93, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Paquette, C.C.H.; Phanse, Y.; Perry, J.L.; Sanchez-Vargas, I.; Airs, P.M.; Dunphy, B.M.; Xu, J.; Carlson, J.O.; Luft, J.C.; DeSimone, J.M.; et al. Biodistribution and Trafficking of Hydrogel Nanoparticles in Adult Mosquitoes. PLOS Neglected Trop. Dis. 2015, 9, e0003745. [Google Scholar] [CrossRef] [PubMed]
- Phanse, Y.; Dunphy, B.M.; Perry, J.L.; Airs, P.M.; Paquette, C.C.H.; Carlson, J.O.; Xu, J.; Luft, J.C.; DeSimone, J.M.; Beaty, B.J.; et al. Biodistribution and Toxicity Studies of PRINT Hydrogel Nanoparticles in Mosquito Larvae and Cells. PLOS Neglected Trop. Dis. 2015, 9, e0003735. [Google Scholar] [CrossRef] [PubMed]
- Durand, S.V.; Lightner, D.V. Quantitative real time PCR for the measurement of white spot syndrome virus in shrimp. J. Fish Dis. 2002, 25, 381–389. [Google Scholar] [CrossRef]
- Jolliffee, I.T. Principal Component Analysis; Springer: Secaucus, NJ, USA, 2002. [Google Scholar]
- Korenius, T.; Laurikkala, J.; Juhola, M. On principal component analysis, cosine and Euclidean measures in information retrieval. Inf. Sci. 2007, 177, 4893–4905. [Google Scholar] [CrossRef]
- Broderick, S.R.; Rajan, K. Eigenvalue decomposition of spectral features in density of states curves. Eur. Lett. 2011, 95, 57005. [Google Scholar] [CrossRef]
- Broderick, S.R.; Bryden, A.; Suram, S.K.; Rajan, K. Data mining for isotope discrimination in atom probe tomography. Ultramicroscopy 2013, 132, 121–128. [Google Scholar] [CrossRef]
- Phanse, Y.; Wu, C.-W.; Venturino, A.J.; Hansen, C.; Nelson, K.; Broderick, S.R.; Steinberg, H.; Talaat, A.M. A Protective Vaccine against Johne’s Disease in Cattle. Microorganisms 2020, 8, 1427. [Google Scholar] [CrossRef]
- Lopac, S.K.; Torres, M.P.; Wilson-Welder, J.H.; Wannemuehler, M.J.; Narasimhan, B. Effect of polymer chemistry and fabrication method on protein release and stability from polyanhydride microspheres. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 938–947. [Google Scholar] [CrossRef]
- Petersen, L.K.; Sackett, C.K.; Narasimhan, B. Novel, High Throughput Method to Study in Vitro Protein Release from Polymer Nanospheres. J. Comb. Chem. 2010, 12, 51–56. [Google Scholar] [CrossRef]
- Robalino, J.; Browdy, C.L.; Prior, S.; Metz, A.; Parnell, P.; Gross, P.; Warr, G. Induction of Antiviral Immunity by Double-Stranded RNA in a Marine Invertebrate. J. Virol. 2004, 78, 10442–10448. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kosuke, Z.; Nam, Y.K.; Kim, S.K.; Kim, K.H. Protection of shrimp (Penaeus chinensis) against white spot syndrome virus (WSSV) challenge by double-stranded RNA. Fish Shellfish Immunol. 2007, 23, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.K.; Phanse, Y.; Ramer-Tait, A.E.; Wannemuehler, M.; Narasimhan, B. Amphiphilic Polyanhydride Nanoparticles Stabilize Bacillus anthracis Protective Antigen. Mol. Pharm. 2012, 9, 874–882. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phanse, Y.; Carrillo-Conde, B.R.; Ramer-Tait, A.; Broderick, S.R.; Kong, C.S.; Rajan, K.; Flick, R.; Mandell, R.B.; Narasimhan, B.; Wannemuehler, M.J. A systems approach to designing next generation vaccines: Combining α-galactose modified antigens with nanoparticle platforms. Sci. Rep. 2014, 4, 3775. [Google Scholar] [CrossRef]
- Carrillo-Conde, B.; Schiltz, E.; Yu, J.; Minion, C.; Phillips, G.J.; Wannemuehler, M.J.; Narasimhan, B. Encapsulation into amphiphilic polyanhydride microparticles stabilizes Yersinia pestis antigens. Acta Biomater. 2010, 6, 3110–3119. [Google Scholar] [CrossRef]
- Huntimer, L.; Ramer-Tait, A.E.; Petersen, L.K.; Ross, K.A.; Walz, K.A.; Wang, C.; Hostetter, J.; Narasimhan, B.; Wannemuehler, M.J. Evaluation of Biocompatibility and Administration Site Reactogenicity of Polyanhydride-Particle-Based Platform for Vaccine Delivery. Adv. Health Mater. 2013, 2, 369–378. [Google Scholar] [CrossRef]
- Ulery, B.D.; Petersen, L.K.; Phanse, Y.; Kong, C.S.; Broderick, S.R.; Kumar, D.; Ramer-Tait, A.E.; Carrillo-Conde, B.; Rajan, K.; Wannemuehler, M.J.; et al. Rational Design of Pathogen-Mimicking Amphiphilic Materials as Nanoadjuvants. Sci. Rep. 2011, 1, 198. [Google Scholar] [CrossRef]
- Chantanachookin, C.; Boonyaratpalin, S.; Kasornchandra, J.; Direkbusarakom, S.; Ekpanithanpong, U.; Supamataya, K.; Sriurairatana, S.; Flegel, T. Histology and ultrastructure reveal a new granulosis-like virus in Penaeus monodon affected by yellow-head disease. Dis. Aquat. Org. 1993, 17, 145–157. [Google Scholar] [CrossRef]
- Tuyen, N.; Verreth, J.; Vlak, J.; de Jong, M. Horizontal transmission dynamics of White spot syndrome virus by cohabitation trials in juvenile Penaeus monodon and P. vannamei. Prev. Vet. Med. 2014, 117, 286–294. [Google Scholar] [CrossRef]
- Miguel, K.S.; Scott, J.G. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag. Sci. 2016, 72, 801–809. [Google Scholar] [CrossRef]
| Chemistry | Structure | Scanning Electron Microscopy | Diameter (Mean ± SEM) | Encapsulation Efficiency (Mean ± SEM) |
|---|---|---|---|---|
| 20:80 CPH:SA + 1% Rhodamine |  |  | 273 ± 126 nm | N/A |
| 20:80 CPTEG:CPH + 1% Rhodamine | 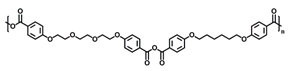 |  | 219 ± 74 nm | N/A |
| 20:80 CPH:SA + 11% dsRNA |  |  | 290 ± 160 nm | 33.8 ± 1.2% |
| 20:80 CPTEG:CPH + 11% dsRNA |  |  | 164 ± 66 nm | 74.0 ± 1.0% |
| Group | Average Initial Weight (WI) (gm) | Average Final Weight (WF) (gm) | Weight Gain (WF − WI) (gm) | % Weight Gain [(WF − WI)/WI] × 100 |
|---|---|---|---|---|
| Control | 5.01 ± 0.18 | 9.10± 0.34 (a) | 4.08 | 81.4 |
| 20:80 CPTEG:CPH | 4.80 ± 0.16 | 10.52 ± 0.21 (b) | 5.73 | 119.4 |
| 20:80 CPH:SA | 4.99 ± 0.17 | 10.17 ± 0.35 (b) | 5.18 | 103.76 |
| Parameter | Control | 20:80 CPTEG:CPH | 20:80 CPH:SA |
|---|---|---|---|
| Hepatopancreas vacuolization | 0.62 ± 0.18 | 0.85 ± 0.14 | 1.00 ± 0 |
| Enteric bacteria | 1.25 ± 0.47 | 1.33 ± 0.66 | 1.50 ± 0.28 |
| Septic hepatopancreatic necrosis | 0 | 0 | 0.50 ± 0.50 |
| Hemocytic nodules | 0.25 ± 0.16 | 0.42 ± 0.20 | 0.37 ± 0.26 |
| Hemocytic congestion | 0.12 ± 0.12 | 0.28 ±0.18 | 0.37 ± 0.18 |
| Skeletal muscle necrosis | 0.75 ± 0.31 | 0.14 ± 0.14 | 0.87 ± 0.29 |
| Lymphoid organ spheroids | 2.00 ± 0.0 | 1.75 ± 0.25 | 0.75 ± 0.25 |
| Treatment 1 | Treatment 2 | Estimate | Standard Deviation | Lower HPD | Upper HPD |
|---|---|---|---|---|---|
| Encap477 20:80 CPTEG:CPH | Sham | 251.84 | 192.17 | 5.0118 | 642.83 |
| Encap477 20:80 CPH: SA | Sham | 252.48 | 192.18 | 5.4316 | 643.55 |
| Soluble477 | Sham | 253.66 | 192.16 | 6.8842 | 644.4 |
| Negative | Sham | 930.27 | 586.33 | 97.4592 | 2135.09 |
| Group | Hepatopancreas Vacuolization | Lymphoid Organ Spheroids | Hemocytic Nodules | Skeletal Muscle Necrosis | WSSV Lesions |
|---|---|---|---|---|---|
| Negative | 1 | 0.5 ± 0.5 | 0 | 0 | 0 |
| Sham | 1.6 ± 0.33 | 0 | 0 | 0.66 ± 0.66 | 2.66 ± 1.33 |
| Encap477 20:80 CPTEG:CPH | 1.6 ± 0.33 | 1 ± 0.57 | 0.33 ± 0.33 | 0 | 0 |
| Encap477 20:80 CPH:SA | 0 | 0 | 1 ± 1 | 0 | 0 |
| Soluble477 | 1 | 0.66 ± 0.66 | 1 ± 1 | 0 | 0 |
| SolubleeGFP | 1.6 ± 0.33 | 2 ± 0.57 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phanse, Y.; Puttamreddy, S.; Loy, D.; Ramirez, J.V.; Ross, K.A.; Alvarez-Castro, I.; Mogler, M.; Broderick, S.; Rajan, K.; Narasimhan, B.; et al. RNA Nanovaccine Protects against White Spot Syndrome Virus in Shrimp. Vaccines 2022, 10, 1428. https://doi.org/10.3390/vaccines10091428
Phanse Y, Puttamreddy S, Loy D, Ramirez JV, Ross KA, Alvarez-Castro I, Mogler M, Broderick S, Rajan K, Narasimhan B, et al. RNA Nanovaccine Protects against White Spot Syndrome Virus in Shrimp. Vaccines. 2022; 10(9):1428. https://doi.org/10.3390/vaccines10091428
Chicago/Turabian StylePhanse, Yashdeep, Supraja Puttamreddy, Duan Loy, Julia Vela Ramirez, Kathleen A. Ross, Ignacio Alvarez-Castro, Mark Mogler, Scott Broderick, Krishna Rajan, Balaji Narasimhan, and et al. 2022. "RNA Nanovaccine Protects against White Spot Syndrome Virus in Shrimp" Vaccines 10, no. 9: 1428. https://doi.org/10.3390/vaccines10091428
APA StylePhanse, Y., Puttamreddy, S., Loy, D., Ramirez, J. V., Ross, K. A., Alvarez-Castro, I., Mogler, M., Broderick, S., Rajan, K., Narasimhan, B., & Bartholomay, L. C. (2022). RNA Nanovaccine Protects against White Spot Syndrome Virus in Shrimp. Vaccines, 10(9), 1428. https://doi.org/10.3390/vaccines10091428







