In-Silico Molecular Modeling Studies to Identify Novel Potential Inhibitors of HPV E6 Protein
Abstract
:1. Introduction
2. Material and Methods
2.1. Collection and Curation of the Chemical Library
2.2. Structure-Based Virtual Screening
2.3. Molecular Docking
2.4. Post Docking Analysis
2.5. In silico Pharmacokinetics (PK)/Pharmacodynamics (PD) Evaluation
2.6. Molecular Dynamics
3. Results and Discussion
3.1. Virtual Screening
3.2. Molecular Docking
3.3. In Silico Pharmacokinetics (PK)/Pharmacodynamics (PD) Evaluation
3.4. Molecular Dynamics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Carper, M.B.; Goel, S.; Zhang, A.M.; Damrauer, J.S.; Cohen, S.; Zimmerman, M.P.; Gentile, G.M.; Parag-Sharma, K.; Murphy, R.M.; Sato, K.; et al. Activation of the CREB Coactivator CRTC2 by Aberrant Mitogen Signaling Promotes Oncogenic Functions in HPV16 Positive Head and Neck Cancer. Neoplasia 2022, 29, 100799. [Google Scholar] [CrossRef] [PubMed]
- Vonsky, M.; Shabaeva, M.; Runov, A.; Lebedeva, N.; Chowdhury, S.; Palefsky, J.M.; Isaguliants, M. Carcinogenesis Associated with Human Papillomavirus Infection. Mechanisms and Potential for Immunotherapy. Biochem. Moscow 2019, 84, 782–799. [Google Scholar] [CrossRef] [PubMed]
- Tognon, M.; Tagliapietra, A.; Magagnoli, F.; Mazziotta, C.; Oton-Gonzalez, L.; Lanzillotti, C.; Vesce, F.; Contini, C.; Rotondo, J.C.; Martini, F. Investigation on Spontaneous Abortion and Human Papillomavirus Infection. Vaccines 2020, 8, 473. [Google Scholar] [CrossRef]
- Condrat, C.E.; Filip, L.; Gherghe, M.; Cretoiu, D.; Suciu, N. Maternal HPV Infection: Effects on Pregnancy Outcome. Viruses 2021, 13, 2455. [Google Scholar] [CrossRef]
- Thomas, M.; Narayan, N.; Pim, D.; Tomaić, V.; Massimi, P.; Nagasaka, K.; Kranjec, C.; Gammoh, N.; Banks, L. Human papillomaviruses, cervical cancer and cell polarity. Oncogene 2008, 27, 7018–7030. [Google Scholar] [CrossRef]
- Burk, R.D.; Chen, Z.; Van Doorslaer, K. Human Papillomaviruses: Genetic Basis of Carcinogenicity. Public Health Genom. 2009, 12, 281–290. [Google Scholar] [CrossRef]
- Oyervides-Muñoz, M.A.; Pérez-Maya, A.A.; Rodríguez-Gutiérrez, H.F.; Gómez-Macias, G.S.; Fajardo, O.R.; Trevino, V.; Barrera-Saldaña, H.A.; Garza-Rodríguez, M.L. Understanding the HPV Integration and Its Progression to Cervical Cancer. Infect. Genet. Evol. 2018, 61, 134–144. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, D. The Precision Prevention and Therapy of HPV-Related Cervical Cancer: New Concepts and Clinical Implications. Cancer Med. 2018, 7, 5217–5236. [Google Scholar] [CrossRef]
- Almeida, A.M.; Queiroz, J.; Sousa, F.; Sousa, Â. Cervical Cancer and HPV Infection: Ongoing Therapeutic Research to Counteract the Action of E6 and E7 Oncoproteins. Drug Discov. Today 2019, 24, 2044–2057. [Google Scholar] [CrossRef]
- Yeo-Teh, N.S.L.; Ito, Y.; Jha, S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int. J. Mol. Sci. 2018, 19, 1706. [Google Scholar] [CrossRef] [PubMed]
- Nominé, Y.; Masson, M.; Charbonnier, S.; Zanier, K.; Ristriani, T.; Deryckère, F.; Sibler, A.P.; Desplancq, D.; Andrew Atkinson, R.; Weiss, E.; et al. Structural and functional analysis of E6 oncoprotein: Insights in the molecular pathways of human papillomavirus-mediated pathogenesis. Mol. cell 2006, 21, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Vande Pol, S.; Podjarny, A.; et al. Structure of the E6/E6AP/p53 Complex Required for HPV-Mediated Degradation of P53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef]
- Huibregtse, J.M.; Scheffner, M.; Howley, P.M. Localization of the E6-AP Regions That Direct Human Papillomavirus E6 Binding, Association with P53, And Ubiquitination of Associated Proteins. Mol. Cell. Biol. 1993, 13, 4918–4927. [Google Scholar] [CrossRef] [PubMed]
- Zanier, K.; Charbonnier, S.; Sidi, A.O.M.O.; McEwen, A.G.; Ferrario, M.G.; Poussin-Courmontagne, P.; Cura, V.; Brimer, N.; Babah, K.O.; Ansari, T.; et al. Structural Basis for Hijacking of Cellular LxxLL Motifs by Papillomavirus E6 Oncoproteins. Science 2013, 339, 694–698. [Google Scholar] [CrossRef]
- Sailer, C.; Offensperger, F.; Julier, A.; Kammer, K.-M.; Walker-Gray, R.; Gold, M.G.; Scheffner, M.; Stengel, F. Structural Dynamics of the E6AP/UBE3A-E6-P53 Enzyme-Substrate Complex. Nat. Commun. 2018, 9, 4441. [Google Scholar] [CrossRef]
- Huibregtse, J.M.; Scheffner, M.; Howley, P.M. A Cellular Protein Mediates Association of P53 with the E6 Oncoprotein of Human Papillomavirus Types 16 or 18. EMBO J. 1991, 10, 4129–4135. [Google Scholar] [CrossRef]
- Pim, D.; Storey, A.; Thomas, M.; Massimi, P.; Banks, L. Mutational analysis of HPV-18 E6 identifies domains required for p53 degradation in vitro, abolition of p53 transactivation in vivo and immortalisation of primary BMK cells. Oncogene 1994, 9, 1869–1876. [Google Scholar]
- Patel, D.; Huang, S.M.; Baglia, L.A.; McCance, D.J. The E6 Protein of Human Papillomavirus Type 16 Binds to And Inhibits Co-Activation by CBP and p300. EMBO J. 1999, 18, 5061–5072. [Google Scholar] [CrossRef]
- Monie, A.; Hung, C.F.; Roden, R.; Wu, T.C. Cervarix™: A Vaccine for the Prevention of HPV 16, 18-Associated Cervical Cancer. Biol. Targets Ther. 2008, 2, 107. [Google Scholar]
- Shi, L.; Sings, H.L.; Bryan, J.T.; Wang, B.; Wang, Y.; Mach, H.; Kosinski, M.; Washabaugh, M.W.; Sitrin, R.; Barr, E. GARDASIL®: Prophylactic Human Papillomavirus Vaccine Development–from Bench Top to Bed-side. Clin. Pharmacol. Ther. 2007, 81, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Petrosky, E.; Bocchini, J.A., Jr.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E. Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Recommendations of the Advisory Committee on Immunization Practices. MMWR. Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. [Google Scholar] [PubMed]
- Hampson, L.; Martin-Hirsch, P.; Hampson, I.N. An Overview of Early Investigational Drugs for the Treatment of Human Papilloma Virus Infection and Associated Dysplasia. Expert Opin. Investig. Drugs 2015, 24, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and Drug-Like Compounds: The Rule-of-Five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- R.P.D. Bank. RCSB PDB: Homepage. Available online: http://www.rcsb.org/ (accessed on 19 April 2020).
- BIOVIA Discovery Studio. System Requirements for Discovery Studio 2020. Available online: https://www.3dsbiovia.com/products/collaborative-science/biovia-discovery-studio/requirements/technical-requirements-2020.html (accessed on 29 April 2020).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Téléchargements-MGLTools. Available online: http://mgltools.scripps.edu/downloads (accessed on 29 April 2020).
- PyMOL. pymol.org. Available online: https://pymol.org/2/ (accessed on 12 May 2020).
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sac-erdoti, F.D.; et al. Molecular dynamics—Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the 2006 ACM/IEEE Conference on Supercomputing-SC ’06, Tampa, FL, USA, 11–17 November 2006. [Google Scholar] [CrossRef]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of Absolute Solvation Free Energies using Molecular Dynamics Free Energy Perturbation and the OPLS Force Field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with Pyrx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar]
- zur Hausen, H. Papillomaviruses and Cancer: From Basic Studies to Clinical Application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Travé, G.; Zanier, K. HPV-Mediated Inactivation of Tumor Suppressor P53. Cell Cycle 2016, 15, 2231–2232. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug Delivery Systems, CNS Protection, and the Blood Brain Barrier. BioMed Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef]
- Mier, P.; Hurk, J.J. Lysosomal Hydrolases of the Epidermis. 2. Ester Hydrolases. Br. J. Dermatol. 1975, 93, 391–398. [Google Scholar] [CrossRef]
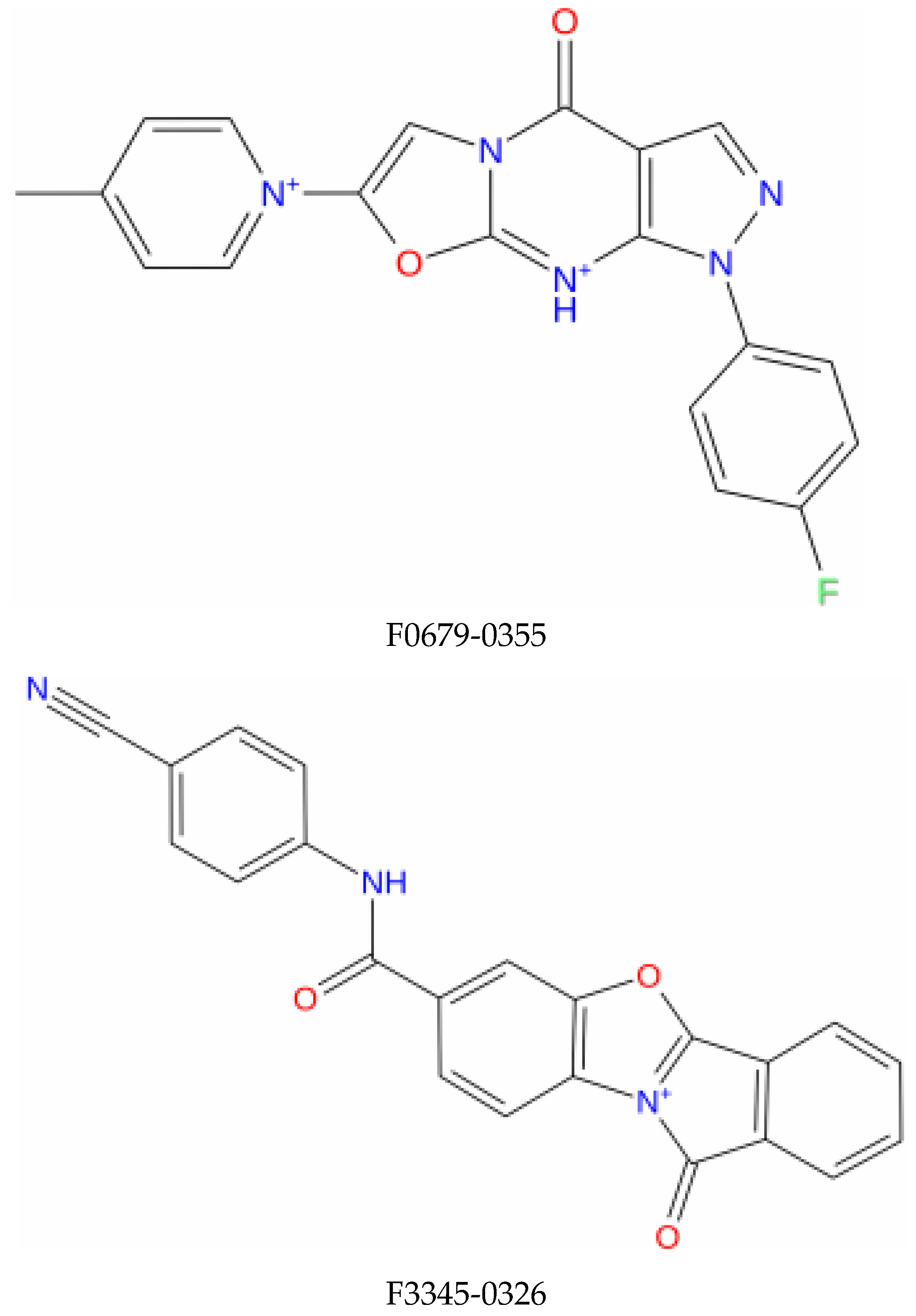
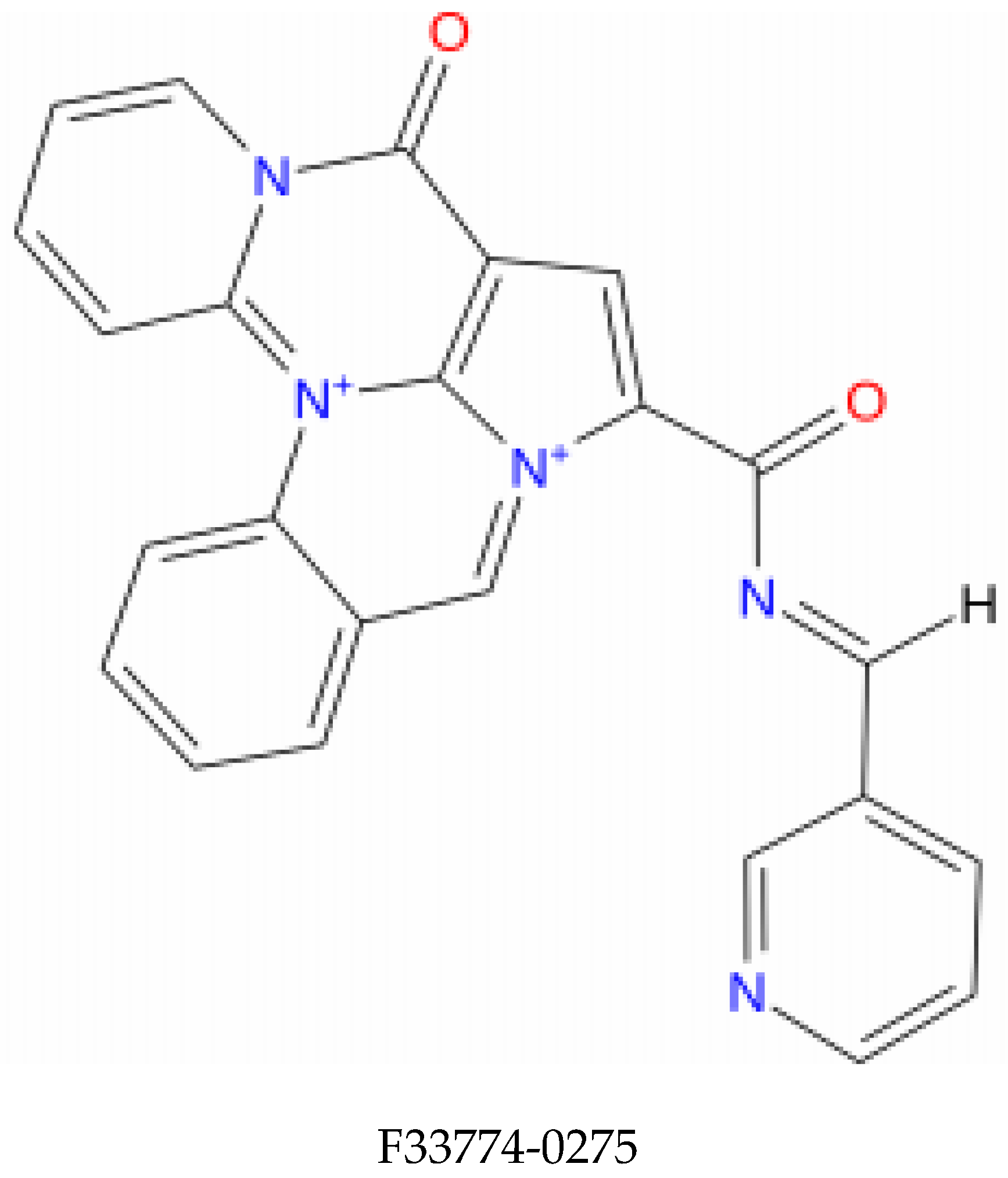
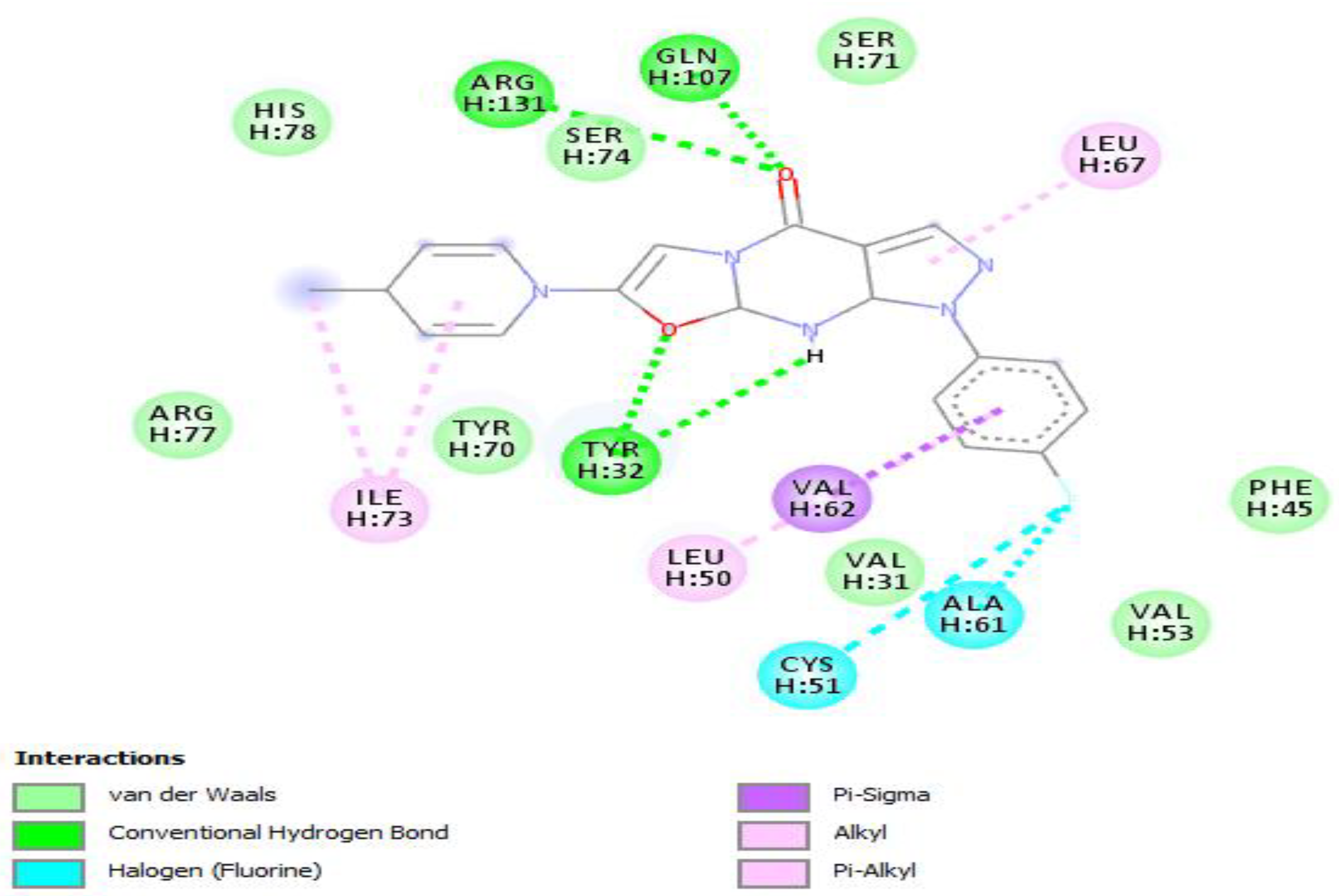

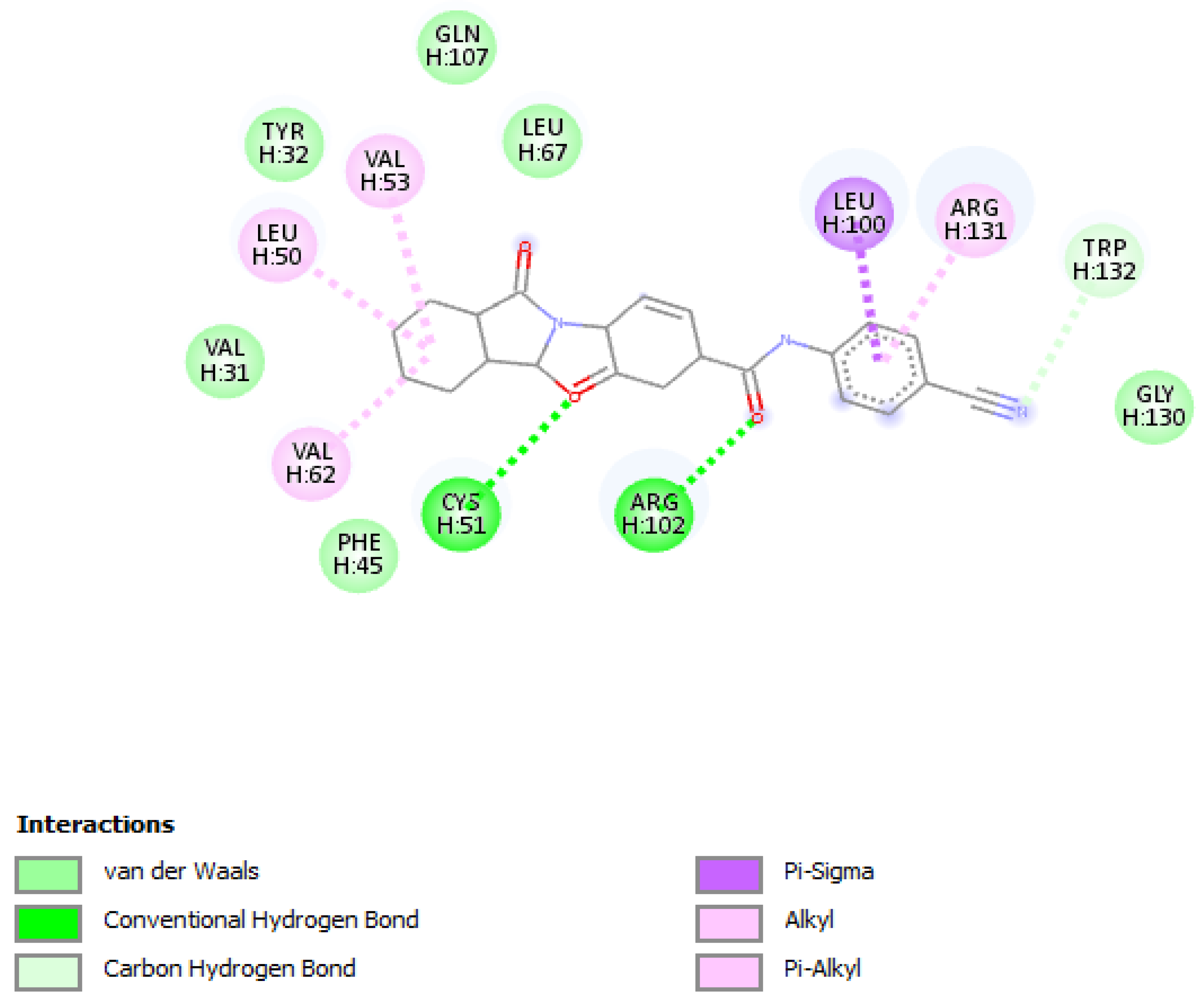



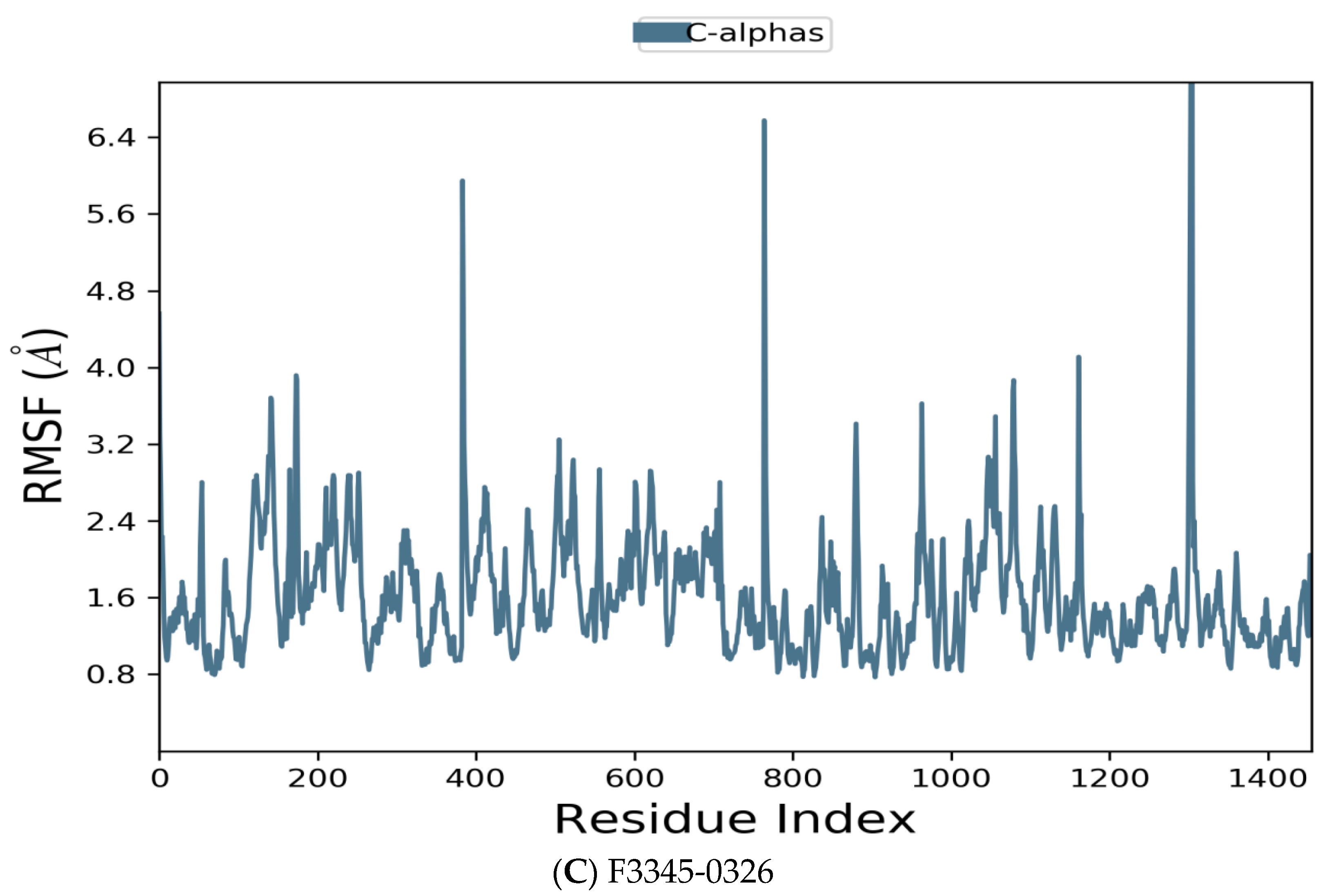

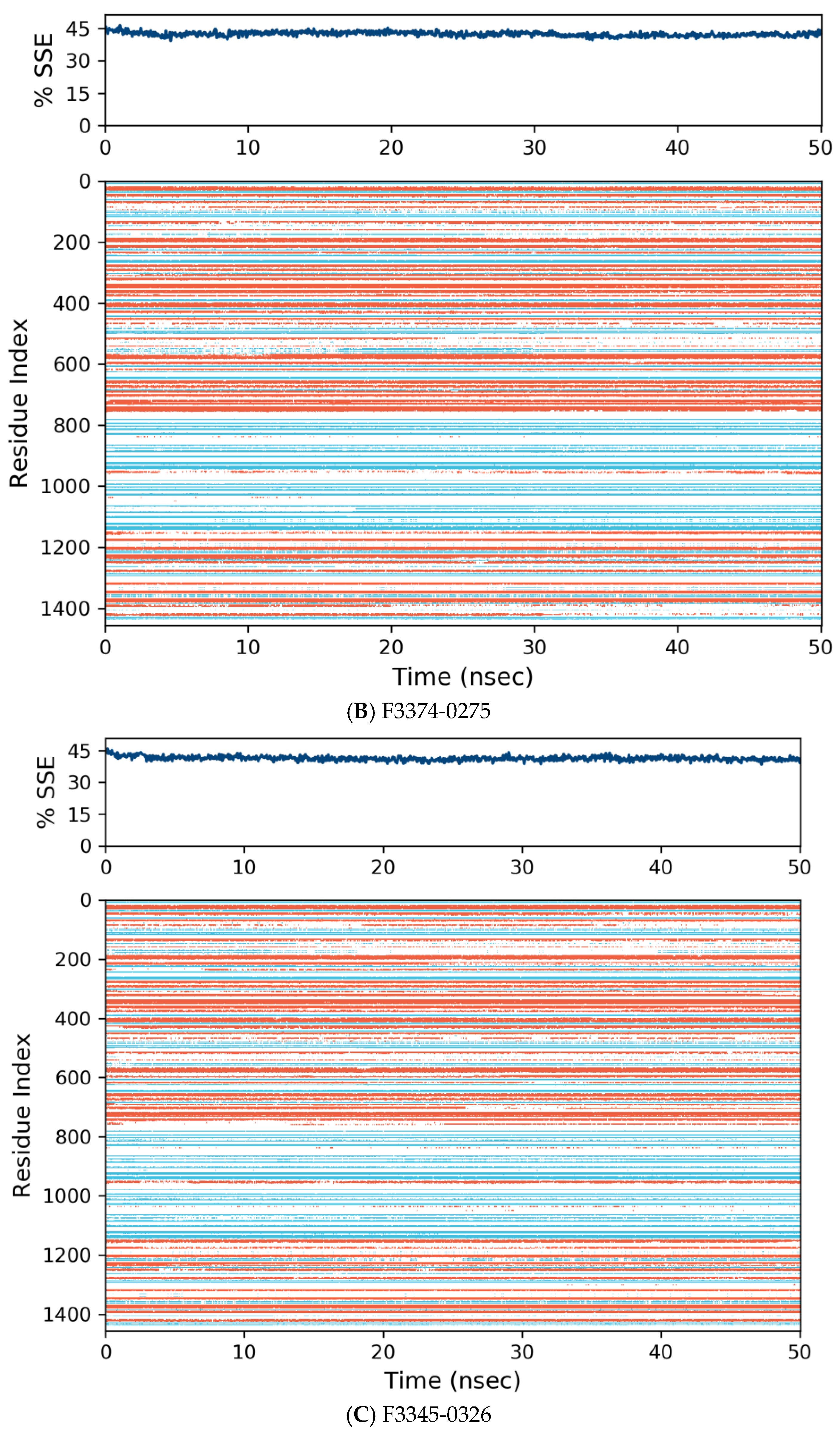
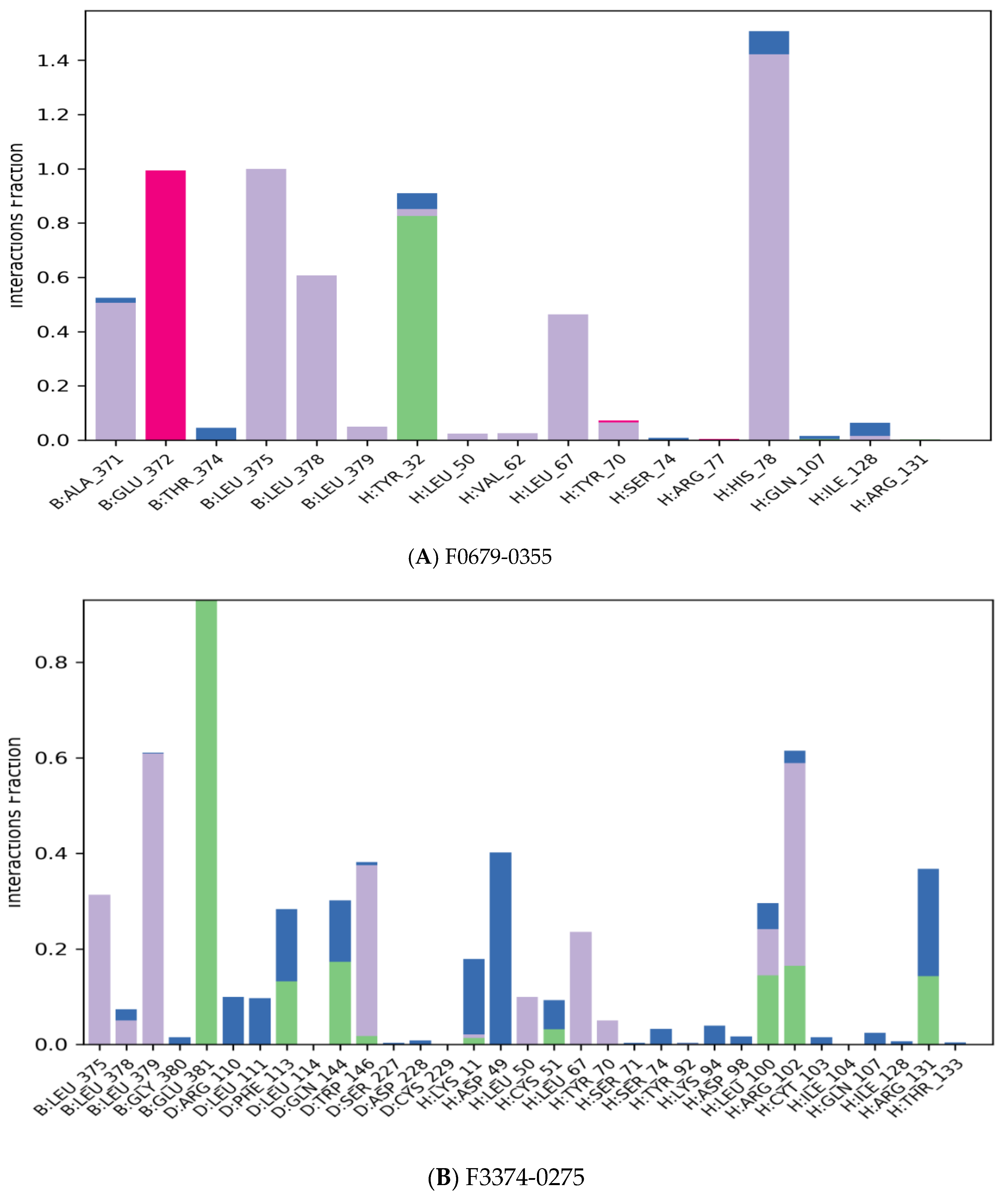
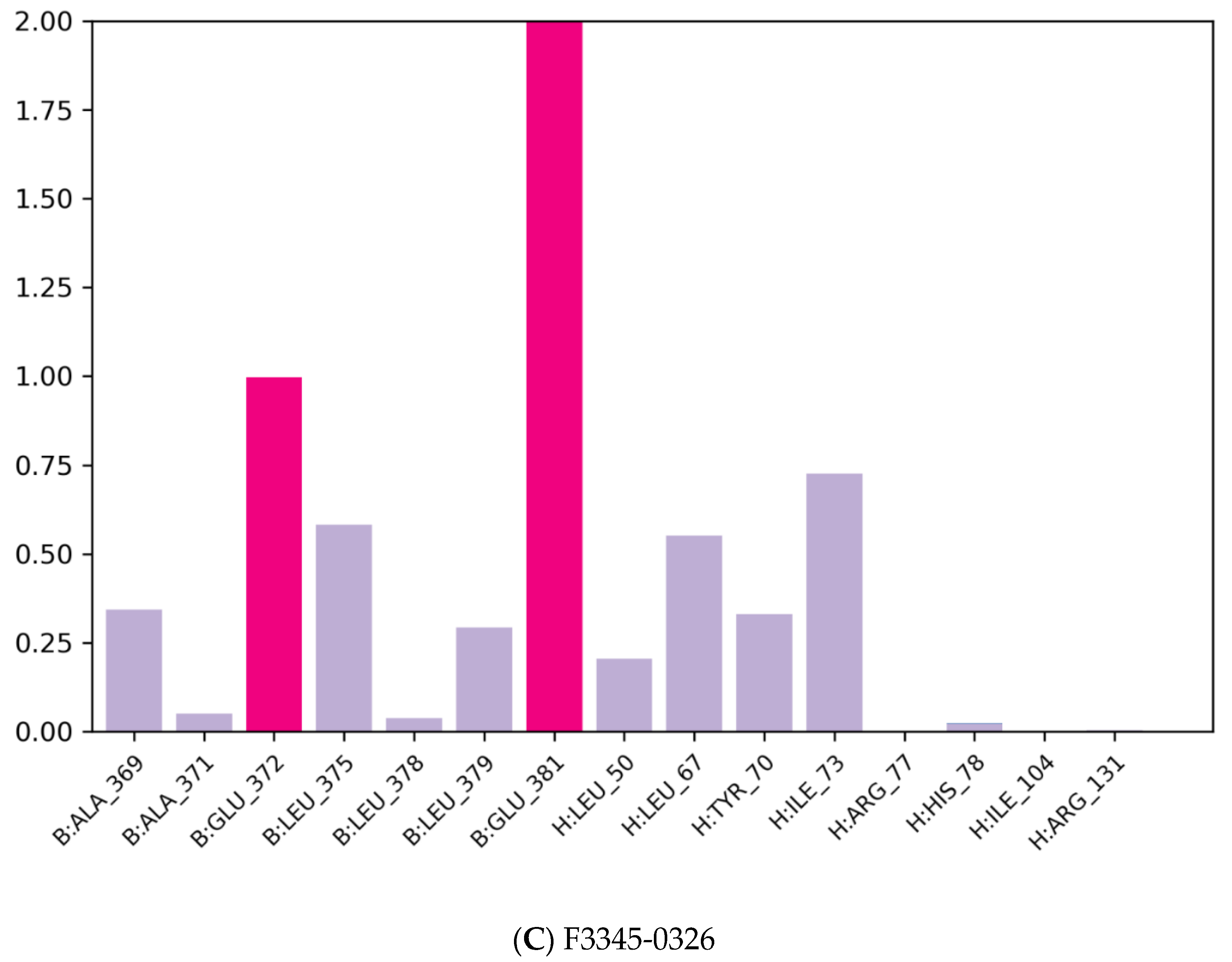
| Compound Name | Molecular Formula | PubChem CID | Hydrogen Bonds | Other Interactions | Binding Affinity |
|---|---|---|---|---|---|
| F3345-0326 | C24H19N5O2 | 4903840 | Ser(71) | Val(53), Val(62), Leu(50), Cys(51), Phe(45), Val(31), Tyr(32), Ser(74), Ile(73), Arg(129), His(78), Tyr(70), Arg(131), Leu(67), Gln(107), Arg(102) | −10.4 |
| F3374-0275 | C22H13N3O3 | 17016408 | Cys(51), Arg(102) | Gln(107), Leu(67), Tyr(32), Val(31), Val(31), Val(62), Phe(45), Val(53), Leu(50), Gln(130), Trp(132), Leu(100), Arg(131) | −10 |
| F0679-0355 | C19H20FN5O2 | 3155624 | Arg(131), Gln(107), Tyr(32) | His(78), Ser(74), Ser(71), Leu(67), Arg(77), Ile (73), Tyr(70), Leu(50), Val(62), Val(31), Cys(51), Ala(61), Val(53), Phe(45) | −9.9 |
| Compound Name | F0679-0355 | F3345-0326 | F3374-0275 |
|---|---|---|---|
| Absorption and Distribution | |||
| Blood-Brain Barrier | 0.5177 | 0.6933 | 0.9385 |
| Human Gut Absorption | 0.9953 | 0.8780 | 0.9868 |
| Caco-2 Permeability | 0.6748 | 0.7412 | 0.6142 |
| Substratglycoprotéine P | Yes | Yes | Yes |
| Inhibitor of Glycoprotein P | No | No | No |
| Metabolism | |||
| CYP450 2C9 Substrate | No | No | No |
| CYP450 2D6 Substrate | No | No | No |
| CYP450 3A4 Substrate | Yes | Yes | Yes |
| CYP450 1A2 Inhibitor | No | No | No |
| CYP450 2C9 Inhibitor | No | Yes | No |
| CYP450 2D6 Inhibitor | No | No | No |
| CYP450 2C19 Inhibitor | No | Yes | No |
| CYP3A4 Inhibitors | No | No | No |
| Excretion and Toxicity | |||
| Hepatotoxicity | No | No | No |
| Carcinogens | No | No | No |
| AMES Mutagenicity | No | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soumia, M.; Hajji, H.; El Mzibri, M.; Younes, F.Z.; Mohammed, B.; Mohamed, B.; Benaissa, M. In-Silico Molecular Modeling Studies to Identify Novel Potential Inhibitors of HPV E6 Protein. Vaccines 2022, 10, 1452. https://doi.org/10.3390/vaccines10091452
Soumia M, Hajji H, El Mzibri M, Younes FZ, Mohammed B, Mohamed B, Benaissa M. In-Silico Molecular Modeling Studies to Identify Novel Potential Inhibitors of HPV E6 Protein. Vaccines. 2022; 10(9):1452. https://doi.org/10.3390/vaccines10091452
Chicago/Turabian StyleSoumia, Moujane, Halima Hajji, Mohamed El Mzibri, Filali Zegzouti Younes, Bouachrine Mohammed, Benlyas Mohamed, and Moualij Benaissa. 2022. "In-Silico Molecular Modeling Studies to Identify Novel Potential Inhibitors of HPV E6 Protein" Vaccines 10, no. 9: 1452. https://doi.org/10.3390/vaccines10091452





