Microneedle Delivery of an Adjuvanted Microparticulate Vaccine Induces High Antibody Levels in Mice Vaccinated against Coronavirus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Formulation of Vaccine Microparticles (MP)
2.2.2. Percentage Recovery Yield and Characterization of MP
2.2.3. Percentage Encapsulation Efficiency (% EE)
2.2.4. In Vitro Immunogenicity Assessment of Vaccine and Adjuvant MP
2.2.5. In Vitro Cytotoxicity Assessment of Vaccine and Adjuvant MP
2.2.6. Determining the Expression of Antigen-Presenting Molecules and Their Co-Stimulatory Molecules
2.2.7. Preparation and Characterization of Vaccine-Loaded Quick Dissolving MNs
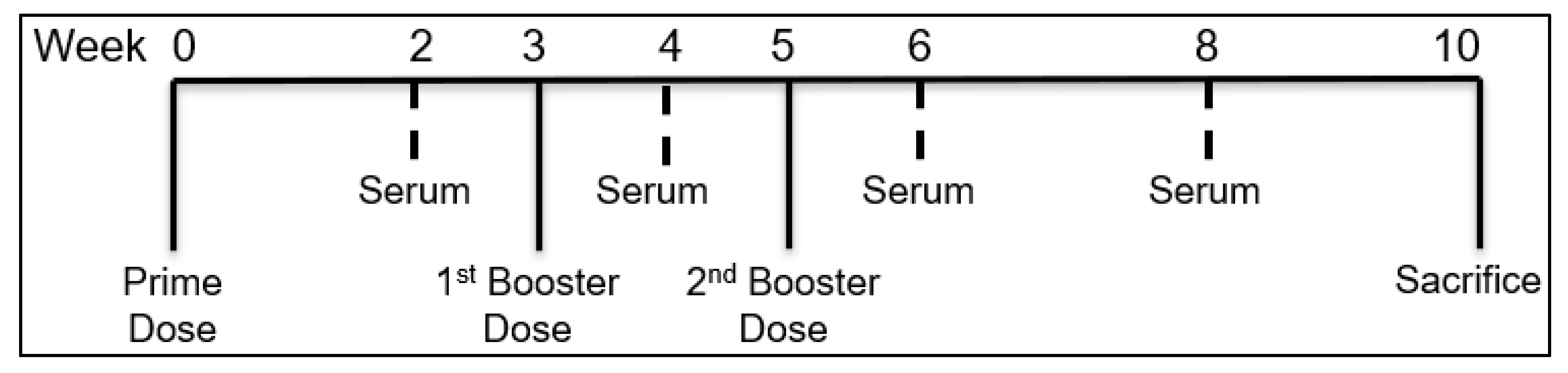
2.2.8. In Vivo Immunization Using MN
2.2.9. Assessment of Serum Antibody Levels following MN Vaccination
2.2.10. Statistical Analysis
3. Results
3.1. Percentage Recovery Yield and Characterization of MP
3.2. Percentage Encapsulation Efficiency (% EE)
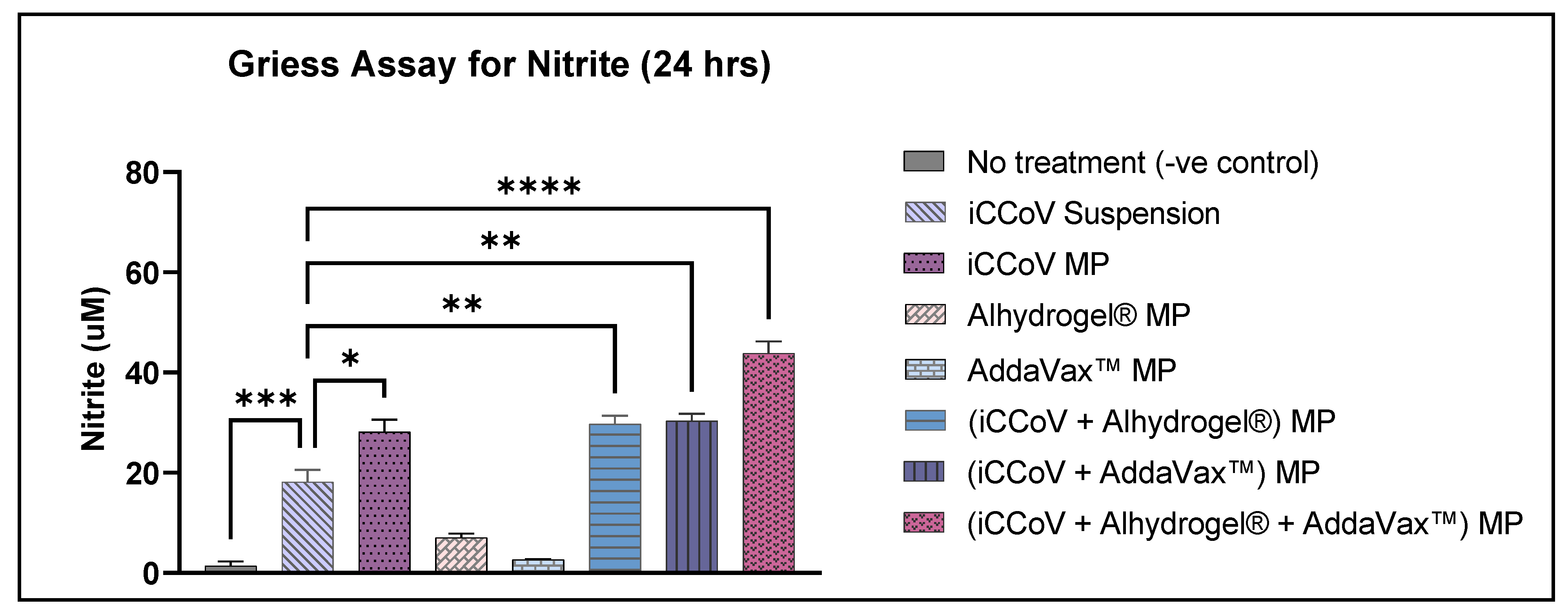
3.3. In Vitro Immunogenicity of Vaccine and Adjuvant MP
3.4. In Vitro Cytotoxicity of Vaccine and Adjuvant MP
3.5. Expression of Antigen-Presenting Molecules and Their Co-Stimulatory Molecules
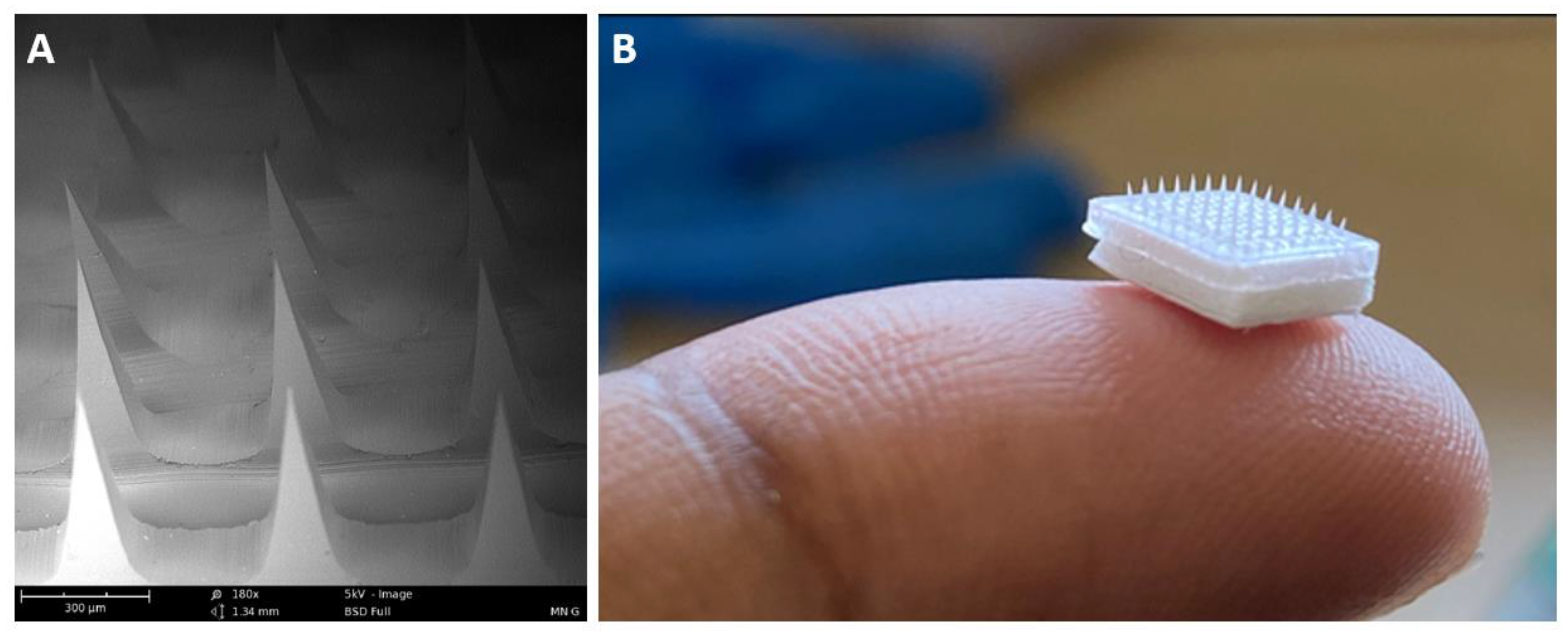
3.6. Characterization of MN
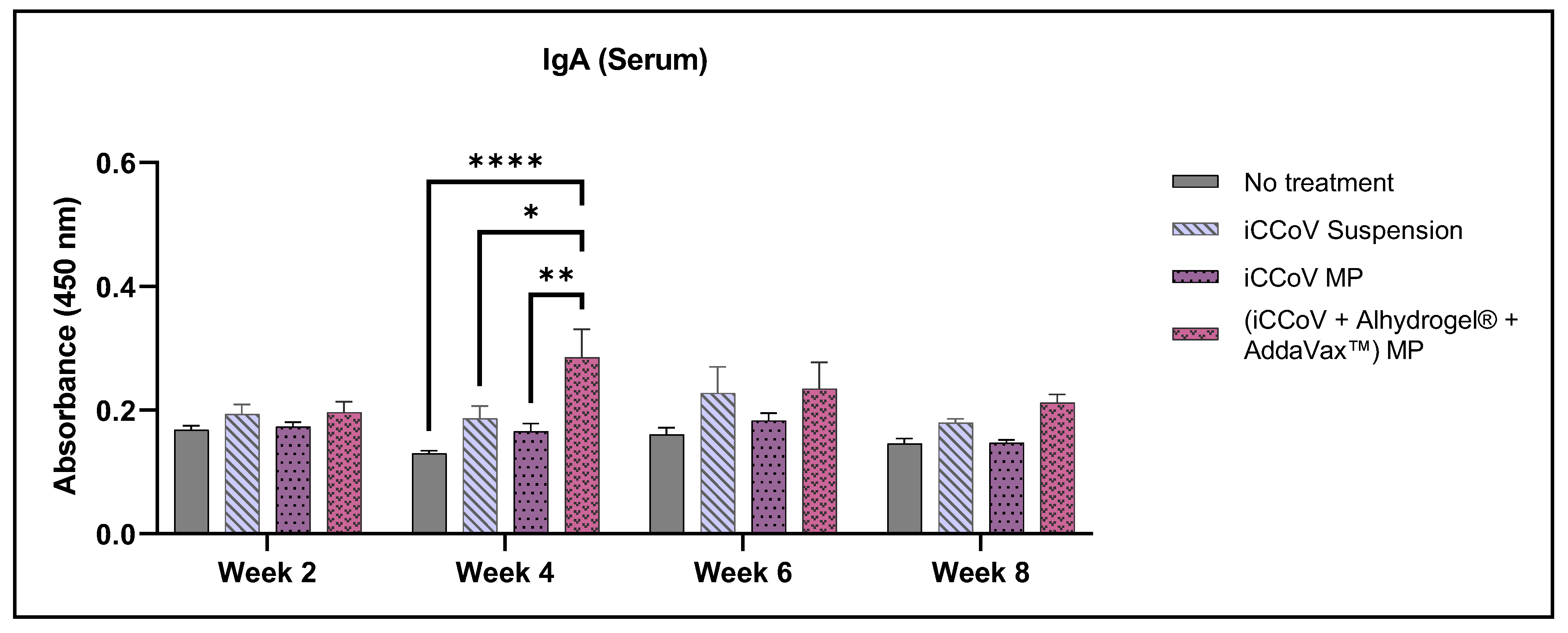
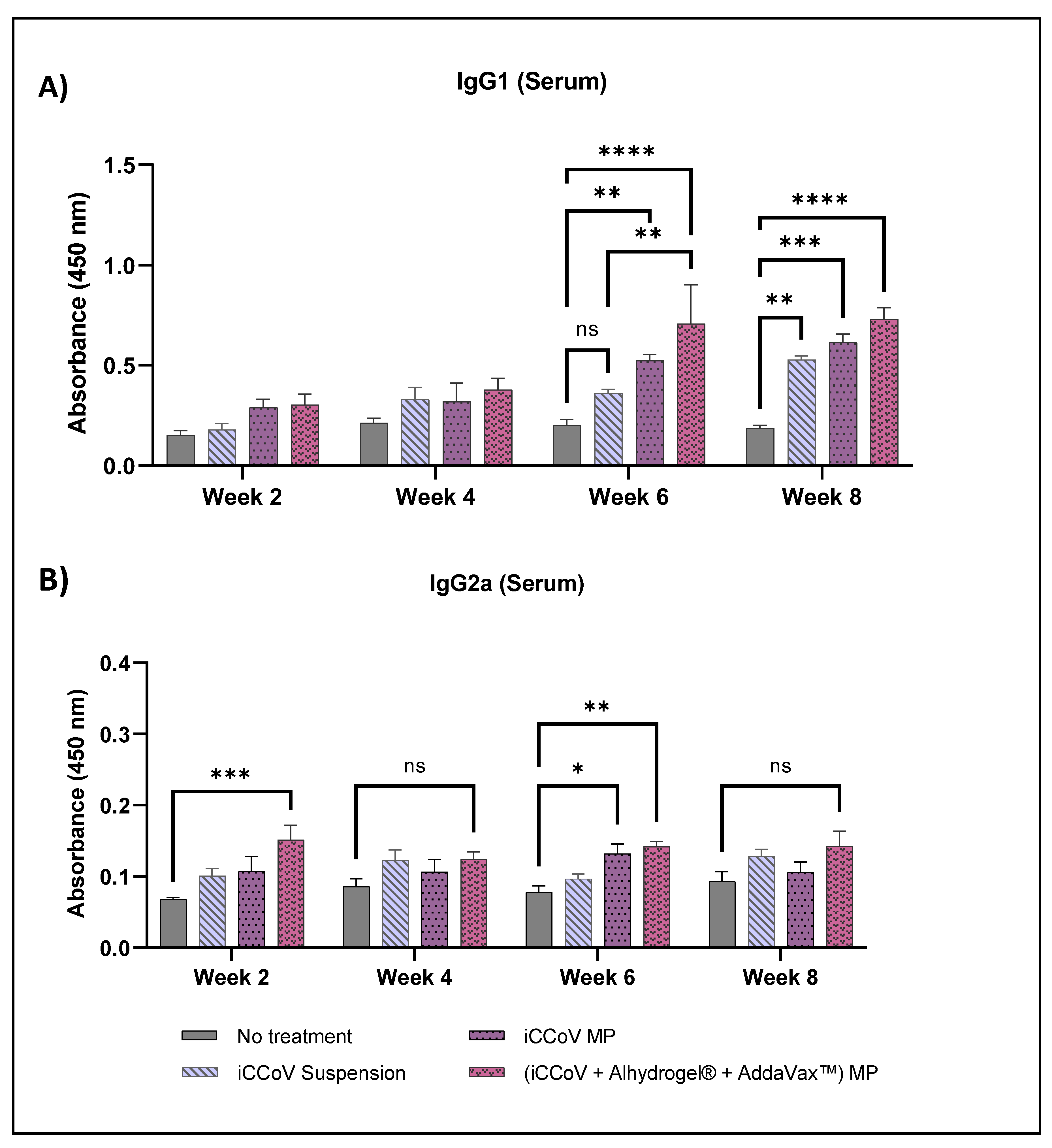
3.7. Assessment of Serum Antibody Levels Using ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haake, C.; Cook, S.; Pusterla, N.; Murphy, B. Coronavirus Infections in Companion Animals: Virology, Epidemiology, Clinical and Pathologic Features. Viruses 2020, 12, 1023. [Google Scholar] [CrossRef]
- Buonavoglia, C.; Decaro, N.; Martella, V.; Elia, G.; Campolo, M.; Desario, C.; Castagnaro, M.; Tempesta, M. Canine Coronavirus Highly Pathogenic for Dogs. Emerg. Infect. Dis. 2006, 12, 492–494. [Google Scholar] [CrossRef]
- Szczepanski, A.; Owczarek, K.; Bzowska, M.; Gula, K.; Drebot, I.; Ochman, M.; Maksym, B.; Rajfur, Z.; Mitchell, J.A.; Pyrc, K. Canine Respiratory Coronavirus, Bovine Coronavirus, and Human Coronavirus OC43: Receptors and Attachment Factors. Viruses 2019, 11, 328. [Google Scholar] [CrossRef]
- Sharun, K.; Sircar, S.; Malik, Y.S.; Singh, R.K.; Dhama, K. How Close Is SARS-CoV-2 to Canine and Feline Coronaviruses? J. Small Anim. Pract. 2020, 61, 523–526. [Google Scholar] [CrossRef]
- Priestnall, S.L. Canine Respiratory Coronavirus: A Naturally Occurring Model of COVID-19? Vet. Pathol. 2020, 57, 467–471. [Google Scholar] [CrossRef]
- WebMD. Canine Coronavirus: Is It a Threat to Humans? Available online: https://www.webmd.com/lung/canine-coronavirus (accessed on 17 June 2022).
- Decaro, N.; Balboni, A.; Bertolotti, L.; Martino, P.A.; Mazzei, M.; Mira, F.; Pagnini, U. SARS-CoV-2 Infection in Dogs and Cats: Facts and Speculations. Front. Vet. Sci. 2021, 8, 619207. [Google Scholar]
- Gala, R.P.; Zaman, R.U.; D’Souza, M.J.; Zughaier, S.M. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines 2018, 6, 60. [Google Scholar] [CrossRef]
- Lü, J.-M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current Advances in Research and Clinical Applications of PLGA-Based Nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef]
- Silva, A.L.; Soema, P.C.; Slütter, B.; Ossendorp, F.; Jiskoot, W. PLGA Particulate Delivery Systems for Subunit Vaccines: Linking Particle Properties to Immunogenicity. Hum. Vaccin. Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Gala, R.P.; Popescu, C.; Knipp, G.T.; McCain, R.R.; Ubale, R.V.; Addo, R.; Bhowmik, T.; Kulczar, C.D.; D’Souza, M.J. Physicochemical and Preclinical Evaluation of a Novel Buccal Measles Vaccine. AAPS PharmSciTech 2017, 18, 283–292. [Google Scholar] [CrossRef]
- Carcaboso, A.M.; Hernández, R.M.; Igartua, M.; Rosas, J.E.; Patarroyo, M.E.; Pedraz, J.L. Enhancing Immunogenicity and Reducing Dose of Microparticulated Synthetic Vaccines: Single Intradermal Administration. Pharm. Res. 2004, 21, 121–126. [Google Scholar] [CrossRef]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-Microparticles as Immune Adjuvants: Correlating Particle Sizes and the Resultant Immune Responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef]
- Ben-Akiva, E.; Witte, S.E.; Meyer, R.A.; Rhodes, K.R.; Green, J.J. Polymeric Micro- and Nanoparticles for Immune Modulation. Biomater. Sci. 2018, 7, 14–30. [Google Scholar] [CrossRef]
- Snapper, C.M. Distinct Immunologic Properties of Soluble Versus Particulate Antigens. Front. Immunol. 2018, 9, 598. [Google Scholar] [CrossRef]
- Waeckerle-Men, Y.; Allmen, E.U.; Gander, B.; Scandella, E.; Schlosser, E.; Schmidtke, G.; Merkle, H.P.; Groettrup, M. Encapsulation of Proteins and Peptides into Biodegradable Poly(D,L-Lactide-Co-Glycolide) Microspheres Prolongs and Enhances Antigen Presentation by Human Dendritic Cells. Vaccine 2006, 24, 1847–1857. [Google Scholar] [CrossRef]
- InvivoGen. Alhydrogel® adjuvant 2%. Available online: https://www.invivogen.com/alhydrogel (accessed on 1 July 2022).
- Bansal, A.; Gamal, W.; Wu, X.; Yang, Y.; Olson, V.; D’Souza, M.J. Evaluation of an Adjuvanted Hydrogel-Based PDNA Nanoparticulate Vaccine for Rabies Prevention and Immunocontraception. Nanomedicine 2019, 21, 102049. [Google Scholar] [CrossRef]
- Hotez, P.J.; Corry, D.B.; Strych, U.; Bottazzi, M.E. COVID-19 Vaccines: Neutralizing Antibodies and the Alum Advantage. Nat. Rev. Immunol. 2020, 20, 399–400. [Google Scholar] [CrossRef]
- Nanishi, E.; Borriello, F.; O’Meara, T.R.; McGrath, M.E.; Saito, Y.; Haupt, R.E.; Seo, H.-S.; van Haren, S.D.; Cavazzoni, C.B.; Brook, B.; et al. An Aluminum Hydroxide:CpG Adjuvant Enhances Protection Elicited by a SARS-CoV-2 Receptor Binding Domain Vaccine in Aged Mice. Sci. Transl. Med. 2021, 14, eabj5305. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, H.; Yang, Y.; Zhang, W.; Shi, L.; et al. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 589833. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in Aluminum Hydroxide-Based Adjuvant Research and Its Mechanism. Hum. Vaccin. Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Brewer, J.M. (How) Do Aluminium Adjuvants Work? Immunol. Lett. 2006, 102, 10–15. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Kazmin, D.; Napolitani, G.; Clutterbuck, E.A.; Pulendran, B.; Siegrist, C.-A.; Pollard, A.J. AS03- and MF59-Adjuvanted Influenza Vaccines in Children. Front. Immunol. 2017, 8, 1760. [Google Scholar]
- Nian, X.; Zhang, J.; Deng, T.; Liu, J.; Gong, Z.; Lv, C.; Yao, L.; Li, J.; Huang, S.; Yang, X. AddaVax Formulated with PolyI:C as a Potential Adjuvant of MDCK-Based Influenza Vaccine Enhances Local, Cellular, and Antibody Protective Immune Response in Mice. AAPS PharmSciTech 2021, 22, 270. [Google Scholar] [CrossRef]
- Hernandez-Davies, J.E.; Felgner, J.; Strohmeier, S.; Pone, E.J.; Jain, A.; Jan, S.; Nakajima, R.; Jasinskas, A.; Strahsburger, E.; Krammer, F.; et al. Administration of Multivalent Influenza Virus Recombinant Hemagglutinin Vaccine in Combination-Adjuvant Elicits Broad Reactivity Beyond the Vaccine Components. Front. Immunol. 2021, 12, 2695. [Google Scholar]
- Adjuvants and Vaccines|Vaccine Safety|CDC. Available online: https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html (accessed on 1 July 2022).
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans Cells—Programmed by the Epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef]
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’Souza, M.J.; Zughaier, S.M. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef]
- Chen, D.; Bowersock, T.; Weeratna, R.; Yeoh, T. Current Opportunities and Challenges in Intradermal Vaccination. Ther. Deliv. 2015, 6, 1101–1108. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Prausnitz, M.R. Enabling Skin Vaccination Using New Delivery Technologies. Drug Deliv. Transl. Res. 2011, 1, 7–12. [Google Scholar] [CrossRef]
- Koutsonanos, D.G.; Esser, E.S.; McMaster, S.R.; Kalluri, P.; Lee, J.W.; Prausnitz, M.R.; Skountzou, I.; Denning, T.L.; Kohlmeier, J.E.; Compans, R.W. Enhanced immune responses by skin vaccination with influenza subunit vaccine in young hosts. Vaccine 2015, 33, 4675–4682. [Google Scholar] [CrossRef]
- Munz, C. Antigen Processing for MHC Class II Presentation via Autophagy. Front. Immunol. 2012, 3, 9. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef]
- Braz Gomes, K.; D’Souza, B.; Vijayanand, S.; Menon, I.; D’Souza, M.J. A Dual-Delivery Platform for Vaccination Using Antigen-Loaded Nanoparticles in Dissolving Microneedles. Int. J. Pharm. 2022, 613, 121393. [Google Scholar] [CrossRef]
- Joshi, D.; Chbib, C.; Uddin, M.N.; D’Souza, M.J. Evaluation of Microparticulate (S)-4,5-Dihydroxy-2,3-Pentanedione (DPD) as a Potential Vaccine Adjuvant. AAPS J. 2021, 23, 84. [Google Scholar] [CrossRef]
- Menon, I.; Moo Kang, S.; D’Souza, M. Nanoparticle Formulation of the Fusion Protein Virus like Particles of Respiratory Syncytial Virus Stimulates Enhanced in Vitro Antigen Presentation and Autophagy. Int. J. Pharm. 2022, 623, 121919. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, A.; Veenstra, J.; Ozog, D.M.; Mi, Q.-S. The Roles of Skin Langerhans Cells in Immune Tolerance and Cancer Immunity. Vaccines 2022, 10, 1380. [Google Scholar] [CrossRef]
- Useful Numbers for Cell Culture | Thermo Fisher Scientific-US. Available online: https://www.thermofisher.com/us/en/home/references/gibco-cell-culture-basics/cell-culture-protocols/cell-culture-useful-numbers.html (accessed on 20 June 2022).
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Gomes, K.B.; Allotey-Babington, G.L.; D’Sa, S.; Kang, S.-M.; D’Souza, M.J. Dendritic Cell Activation by a Micro Particulate Based System Containing the Influenza Matrix-2 Protein Virus-like Particle (M2e VLP). Int. J. Pharm. 2022, 622, 121667. [Google Scholar] [CrossRef]
- Kale, A.; Joshi, D.; Menon, I.; Bagwe, P.; Patil, S.; Vijayanand, S.; Braz Gomes, K.; D’Souza, M. Novel Microparticulate Zika Vaccine Induces a Significant Immune Response in a Preclinical Murine Model after Intramuscular Administration. Int. J. Pharm. 2022, 624, 121975. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Chapter 3-Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric Oxide and the Immune Response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Tripathi, P. Nitric Oxide and Immune Response. Indian J. Biochem. Biophys. 2007, 44, 310–319. [Google Scholar]
- Uehara, E.U.; Shida, B.d.S.; de Brito, C.A. Role of Nitric Oxide in Immune Responses against Viruses: Beyond Microbicidal Activity. Inflamm. Res. 2015, 64, 845–852. [Google Scholar] [CrossRef]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.S.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric Oxide and Redox Mechanisms in the Immune Response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Shen, H.; Ackerman, A.L.; Cody, V.; Giodini, A.; Hinson, E.R.; Cresswell, P.; Edelson, R.L.; Saltzman, W.M.; Hanlon, D.J. Enhanced and Prolonged Cross-Presentation Following Endosomal Escape of Exogenous Antigens Encapsulated in Biodegradable Nanoparticles. Immunology 2006, 117, 78–88. [Google Scholar] [CrossRef]
- T-Cell Activation | British Society for Immunology. Available online: https://www.immunology.org/public-information/bitesized-immunology/systems-and-processes/t-cell-activation (accessed on 7 August 2022).
- Tai, Y.; Wang, Q.; Korner, H.; Zhang, L.; Wei, W. Molecular Mechanisms of T Cells Activation by Dendritic Cells in Autoimmune Diseases. Front. Pharmacol. 2018, 9, 642. [Google Scholar]
- Woolard, S.N.; Kumaraguru, U. Viral Vaccines and CTL Response. J. Biomed. Biotechnol. 2010, 2010, 141657. [Google Scholar] [CrossRef]
- West, H.C.; Bennett, C.L. Redefining the Role of Langerhans Cells As Immune Regulators within the Skin. Front. Immunol. 2018, 8, 1941. [Google Scholar]
- Almahboub, S.A.; Algaissi, A.; Alfaleh, M.A.; ElAssouli, M.-Z.; Hashem, A.M. Evaluation of Neutralizing Antibodies Against Highly Pathogenic Coronaviruses: A Detailed Protocol for a Rapid Evaluation of Neutralizing Antibodies Using Vesicular Stomatitis Virus Pseudovirus-Based Assay. Front. Microbiol. 2020, 11, 2020. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of Vaccine Immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA Dominates the Early Neutralizing Antibody Response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Chao, Y.X.; Rötzschke, O.; Tan, E.-K. The Role of IgA in COVID-19. Brain Behav. Immun. 2020, 87, 182–183. [Google Scholar] [CrossRef]
- Trincado, V.; Gala, R.P.; Morales, J.O. Buccal and Sublingual Vaccines: A Review on Oral Mucosal Immunization and Delivery Systems. Vaccines 2021, 9, 1177. [Google Scholar] [CrossRef]
- Corthesy, B. Multi-Faceted Functions of Secretory IgA at Mucosal Surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 3221. [Google Scholar] [CrossRef]
- Rosenthal, K.S.; Zimmerman, D.H. Vaccines: All Things Considered. Clin. Vaccine Immunol. 2006, 13, 821–829. [Google Scholar] [CrossRef] [Green Version]


| Treatment Groups for In Vivo Study (n = 4) | |||
|---|---|---|---|
| No. | Group | Description | Dose of Antigen/Adjuvant |
| 1 | Naive | No treatment | N/A |
| 2 | iCCoV suspension | Inactivated CCoV antigen suspension in MN | 20 μg antigen suspension |
| 3 | iCCoV MP | Inactivated CCoV PLGA MP in MN | MP equivalent to 20 μg antigen |
| 4 | (iCCoV + Alhydrogel® + AddaVax™) MP | (Inactivated CCoV PLGA MP + Alhydrogel® PLGA MP + AddaVax™ PLGA MP) in MN | MP equivalent to (20 μg iCCoV + 30 μg Alhydrogel® + 5 μg AddaVax™) |
| S.No | Parameter | Mean ± Standard Deviation (SD) | ||
|---|---|---|---|---|
| iCCoV MP | Alhydrogel® MP | AddaVax™ MP | ||
| 1. | Product yield (%) | 91 ± 5 | 93 ± 5 | 90.5 ± 5 |
| 2. | Particle size (nm) | 809.2 ± 209.8 | 1573 ± 278.6 | 1274 ± 259.1 |
| 3. | Zeta potential (mV) | −15.4 ± 2.31 | 12 ± 0.252 | −12.5 ±2.65 |
| 4. | PDI | 0.70 ± 0.036 | 0.954 ± 0.04 | 0.896 ± 0.069 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayanand, S.; Patil, S.; Joshi, D.; Menon, I.; Braz Gomes, K.; Kale, A.; Bagwe, P.; Yacoub, S.; Uddin, M.N.; D’Souza, M.J. Microneedle Delivery of an Adjuvanted Microparticulate Vaccine Induces High Antibody Levels in Mice Vaccinated against Coronavirus. Vaccines 2022, 10, 1491. https://doi.org/10.3390/vaccines10091491
Vijayanand S, Patil S, Joshi D, Menon I, Braz Gomes K, Kale A, Bagwe P, Yacoub S, Uddin MN, D’Souza MJ. Microneedle Delivery of an Adjuvanted Microparticulate Vaccine Induces High Antibody Levels in Mice Vaccinated against Coronavirus. Vaccines. 2022; 10(9):1491. https://doi.org/10.3390/vaccines10091491
Chicago/Turabian StyleVijayanand, Sharon, Smital Patil, Devyani Joshi, Ipshita Menon, Keegan Braz Gomes, Akanksha Kale, Priyal Bagwe, Shadi Yacoub, Mohammad N. Uddin, and Martin J. D’Souza. 2022. "Microneedle Delivery of an Adjuvanted Microparticulate Vaccine Induces High Antibody Levels in Mice Vaccinated against Coronavirus" Vaccines 10, no. 9: 1491. https://doi.org/10.3390/vaccines10091491
APA StyleVijayanand, S., Patil, S., Joshi, D., Menon, I., Braz Gomes, K., Kale, A., Bagwe, P., Yacoub, S., Uddin, M. N., & D’Souza, M. J. (2022). Microneedle Delivery of an Adjuvanted Microparticulate Vaccine Induces High Antibody Levels in Mice Vaccinated against Coronavirus. Vaccines, 10(9), 1491. https://doi.org/10.3390/vaccines10091491