T-Cell Responses Induced by an Intradermal BNT162b2 mRNA Vaccine Booster Following Primary Vaccination with Inactivated SARS-CoV-2 Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Procedures
2.2. Sample Processing
2.3. Flow Cytometry Analysis
2.4. Statistical Analysis
3. Results
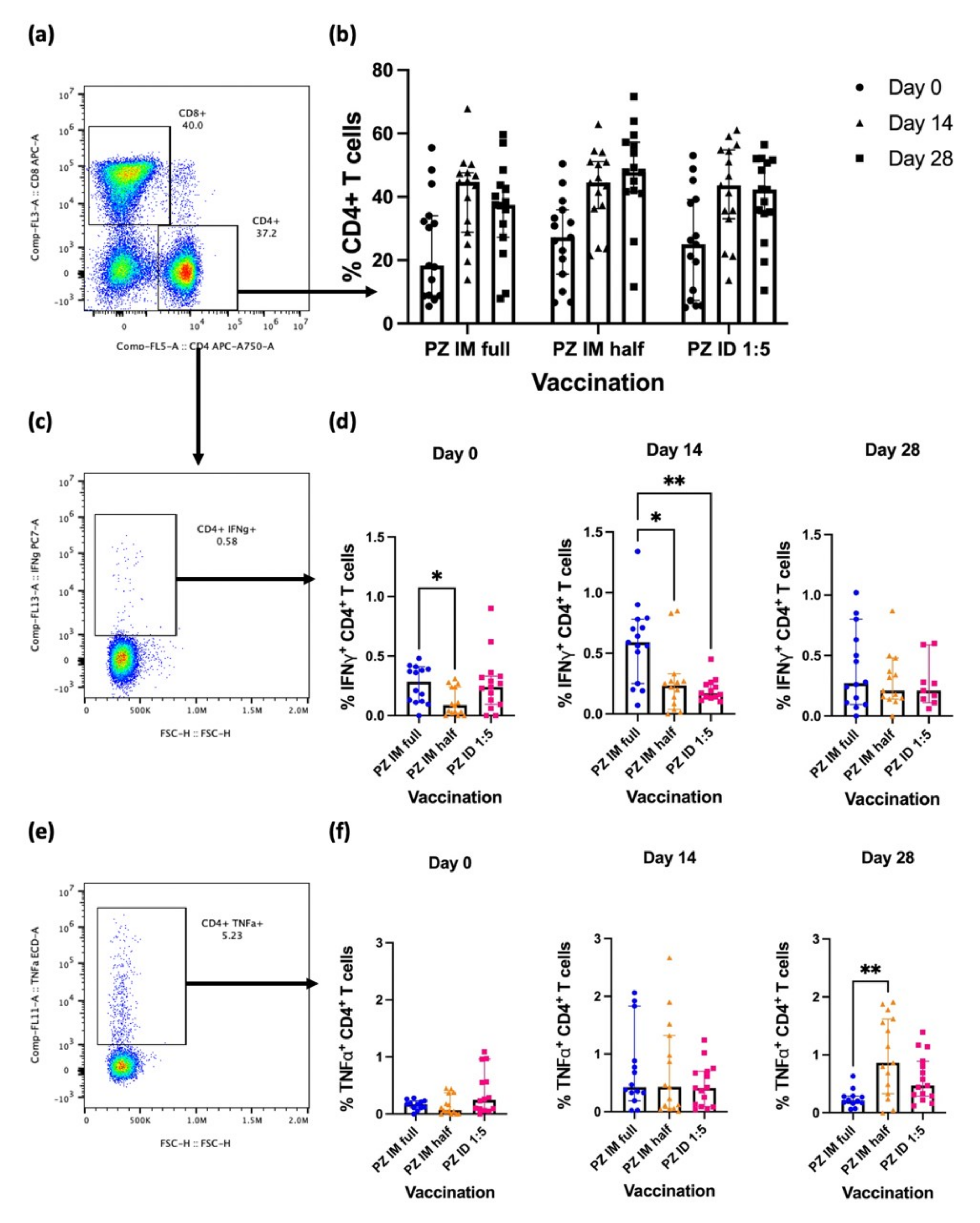
3.1. Effector CD4+ T-Cell Responses after the Booster
3.2. Effector CD8+ T-Cell Response after the Booster
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 21 June 2022).
- World Health Organization. Timeline: WHO’s COVID-19 Response. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline#event-19 (accessed on 21 June 2022).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/?mapFilter=cases (accessed on 1 July 2022).
- Bittihn, P.; Hupe, L.; Isensee, J.; Golestanian, R. Local measures enable COVID-19 containment with fewer restrictions due to cooperative effects. EClinicalMedicine 2021, 32, 100718. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.P.; Das, S.S.; Yadav, S.; Khan, W.; Afzal, M.; Alarifi, A.; Kenawy, E.R.; Ansari, M.T.; Hasnain, M.S.; Nayak, A.K. Global impacts of pre- and post-COVID-19 pandemic: Focus on socio-economic consequences. Sens. Int. 2020, 1, 100042. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Dejnirattisai, W.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Nutalai, R.; et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 2021, 184, 2348–2361. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Shaw, R.H.; Supasa, P.; Liu, C.; Stuart, A.S.; Pollard, A.J.; Liu, X.; Lambe, T.; Crook, D.; Stuart, D.I.; et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet 2022, 399, 234–236. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484. [Google Scholar] [CrossRef]
- Jantarabenjakul, W.; Sodsai, P.; Chantasrisawad, N.; Jitsatja, A.; Ninwattana, S.; Thippamom, N.; Ruenjaiman, V.; Tan, C.W.; Pradit, R.; Sophonphan, J.; et al. Dynamics of neutralizing antibody and T-cell responses to SARS-CoV-2 and variants of concern after primary immunization with coronavac and booster with BNT162b2 or ChAdOx1 in health care workers. Vaccines 2022, 10, 639. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.Ş.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Hitchings, M.D.T.; Ranzani, O.T.; Torres, M.S.S.; de Oliveira, S.B.; Almiron, M.; Said, R.; Borg, R.; Schulz, W.L.; de Oliveira, R.D.; da Silva, P.V.; et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: A test-negative case-control study. Lancet Reg. Health Am. 2021, 1, 100025. [Google Scholar] [CrossRef]
- Kontopoulou, K.; Nakas, C.T.; Papazisis, G. Significant increase in antibody titers after the 3rd Booster dose of the Pfizer-BioNTech mRNA COVID-19 vaccine in healthcare workers in Greece. Vaccines 2022, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- Intapiboon, P.; Seepathomnarong, P.; Ongarj, J.; Surasombatpattana, S.; Uppanisakorn, S.; Mahasirimongkol, S.; Sawaengdee, W.; Phumiamorn, S.; Sapsutthipas, S.; Sangsupawanich, P.; et al. Immunogenicity and safety of an intradermal BNT162b2 mRNA vaccine booster after two doses of inactivated SARS-CoV-2 vaccine in healthy population. Vaccines 2021, 9, 1375. [Google Scholar] [CrossRef]
- Pinpathomrat, N.; Intapiboon, P.; Seepathomnarong, P.; Ongarj, J.; Sophonmanee, R.; Hengprakop, J.; Surasombatpattana, S.; Uppanisakorn, S.; Mahasirimongkol, S.; Sawaengdee, W.; et al. Immunogenicity and safety of an intradermal ChAdOx1 nCoV-19 boost in a healthy population. NPJ Vaccines 2022, 7, 52. [Google Scholar] [CrossRef]
- O’Hara, G.A.; Duncan, C.J.; Ewer, K.J.; Collins, K.A.; Elias, S.C.; Halstead, F.D.; Goodman, A.L.; Edwards, N.J.; Reyes-Sandoval, A.; Bird, P.; et al. Clinical assessment of a recombinant simian adenovirus ChAd63: A potent new vaccine vector. J. Infect. Dis. 2012, 205, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Tawinprai, K.; Siripongboonsitti, T.; Porntharukchareon, T.; Wittayasak, K.; Thonwirak, N.; Soonklang, K.; Sornsamdang, G.; Auewarakul, C.; Mahanonda, N. Immunogenicity and safety of an intradermal fractional third dose of ChAdOx1 nCoV-19/AZD1222 vaccine compared with those of a standard intramuscular third dose in volunteers who previously received two doses of CoronaVac: A randomized controlled trial. Vaccine 2022, 40, 1761–1767. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- López-Camacho, C.; Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Boyd, M.; Badamchi-Zadeh, A.; Wallace, Z.R.; Doig, J.; Velazquez, R.S.; Neto, R.D.L.; et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat. Commun. 2018, 9, 2441. [Google Scholar] [CrossRef] [PubMed]
- Pinpathomrat, N.; Bull, N.; Pasricha, J.; Harrington-Kandt, R.; McShane, H.; Stylianou, E. Using an effective TB vaccination regimen to identify immune responses associated with protection in the murine model. Vaccine 2021, 39, 1452–1462. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Jordan, S.C.; Shin, B.H.; Gadsden, T.M.; Chu, M.; Petrosyan, A.; Le, C.N.; Zabner, R.; Oft, J.; Pedraza, I.; Cheng, S.; et al. T cell immune responses to SARS-CoV-2 and variants of concern (Alpha and Delta) in infected and vaccinated individuals. Cell Mol. Immunol. 2021, 18, 2554–2556. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.J.; Khoury, D.S.; Reynaldi, A.; Juno, J.A.; Wheatley, A.K.; Stadler, E.; John Wherry, E.; Triccas, J.; Sasson, S.C.; Cromer, D.; et al. Disentangling the relative importance of T cell responses in COVID-19: Leading actors or supporting cast? Nat. Rev. Immunol. 2022, 22, 387–397. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Garliss, C.C.; Blankson, J.N. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HcoV-NL63. J. Clin. Investig. 2021, 131, e149335. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, G.; Wang, Y.; Zhang, Q.; Ren, L.; Gu, X.; Huang, T.; Zhong, J.; Wang, Y.; Wang, X.; et al. SARS-CoV-2-specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: A longitudinal cohort study. Lancet Microbe 2022, 3, e348–e356. [Google Scholar] [CrossRef]
- Roozen, G.V.T.; Prins, M.L.M.; van Binnendijk, R.S.; den Hartog, G.; Kuiper, V.P.; Prins, C.; Janse, J.J.; Kruithof, A.C.; Feltkamp, M.C.W.; Kuijer, M.; et al. Tolerability, safety and immunogenicity of intradermal delivery of a fractional dose mRNA-1273 SARS-CoV-2 vaccine in healthy adults as a dose sparing strategy. medRxiv (In press). 2021. [Google Scholar] [CrossRef]
- Singhatiraj, E.; Pongpirul, K.; Jongkaewwattana, A.; Hirankarn, N. Intradermal ChAdOx1 vaccine following two CoronaVac Shots: A case report. Vaccines 2021, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Mobarak, A.M.; Miguel, E.; Abaluck, J.; Ahuja, A.; Alsan, M.; Banerjee, A.; Breza, E.; Chandrasekhar, A.G.; Duflo, E.; Dzansi, J.; et al. End COVID-19 in low-and middle-income countries. Science 2022, 375, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Tameris, M.D.; Hatherill, M.; Landry, B.S.; Scriba, T.J.; Snowden, M.A.; Lockhart, S.; Shea, J.E.; McClain, J.B.; Hussey, G.D.; Hanekom, W.A.; et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet 2013, 381, 1021–1028. [Google Scholar] [CrossRef]
- Dockrell, H.M.; Smith, S.G. What Have We Learnt about BCG Vaccination in the Last 20 Years? Front. Immunol. 2017, 8, 1134. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sophonmanee, R.; Ongarj, J.; Seeyankem, B.; Seepathomnarong, P.; Intapiboon, P.; Surasombatpattana, S.; Uppanisakorn, S.; Sangsupawanich, P.; Chusri, S.; Pinpathomrat, N. T-Cell Responses Induced by an Intradermal BNT162b2 mRNA Vaccine Booster Following Primary Vaccination with Inactivated SARS-CoV-2 Vaccine. Vaccines 2022, 10, 1494. https://doi.org/10.3390/vaccines10091494
Sophonmanee R, Ongarj J, Seeyankem B, Seepathomnarong P, Intapiboon P, Surasombatpattana S, Uppanisakorn S, Sangsupawanich P, Chusri S, Pinpathomrat N. T-Cell Responses Induced by an Intradermal BNT162b2 mRNA Vaccine Booster Following Primary Vaccination with Inactivated SARS-CoV-2 Vaccine. Vaccines. 2022; 10(9):1494. https://doi.org/10.3390/vaccines10091494
Chicago/Turabian StyleSophonmanee, Ratchanon, Jomkwan Ongarj, Bunya Seeyankem, Purilap Seepathomnarong, Porntip Intapiboon, Smonrapat Surasombatpattana, Supattra Uppanisakorn, Pasuree Sangsupawanich, Sarunyou Chusri, and Nawamin Pinpathomrat. 2022. "T-Cell Responses Induced by an Intradermal BNT162b2 mRNA Vaccine Booster Following Primary Vaccination with Inactivated SARS-CoV-2 Vaccine" Vaccines 10, no. 9: 1494. https://doi.org/10.3390/vaccines10091494







