Comparison of the Immune Responses to COVID-19 Vaccines in Bangladeshi Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participant Recruitment
2.2. Data and Specimen Collection
2.3. Specimen Processing
2.4. Assessment of SARS-CoV-2-Specific Antibodies
2.5. Pseudovirus Neutralization Assay (PNA)
2.6. Flow Cytometry of PBMC
2.7. ELISPOT Assay
2.8. Statistical Analysis Plan
3. Results
3.1. Participants
3.2. Vaccine-Specific Antibody Response
3.3. Neutralizing Antibodies
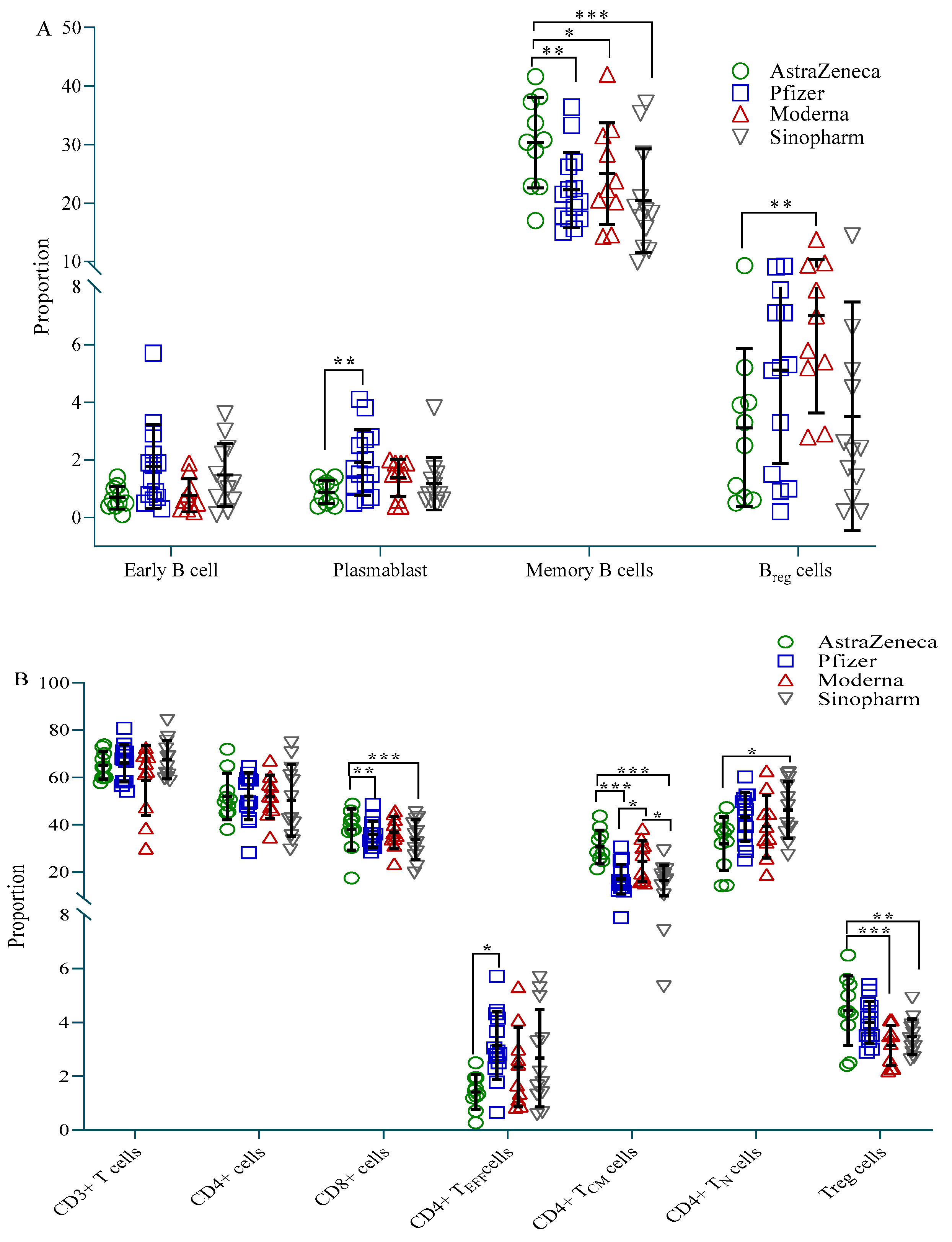
3.4. Immune Cell Profile
3.5. T Cell Function
3.6. Correlation between S-Specific IgG Antibodies, NT50 and Cell Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. The need for broadly protective COVID-19 vaccines: Beyond S-only approaches. Vaccine 2021, 39, 4239–4241. [Google Scholar] [CrossRef] [PubMed]
- Addetia, A.; Crawford, K.H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020, 58, e02107-20. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Klausner, J.D. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect. Dis. 2022, 22, 12–14. [Google Scholar] [CrossRef]
- Sherina, N.; Piralla, A.; Du, L.; Wan, H.; Kumagai-Braesch, M.; Andrell, J.; Braesch-Andersen, S.; Cassaniti, I.; Percivalle, E.; Sarasini, A.; et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med 2021, 2, 281–295.e4. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Bao, L.; Wang, L.; Cao, L.; Chi, H.; Hu, Y.; Li, Q.; Zhou, Y.; Jiang, Y.; et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature 2022, 603, 919–925. [Google Scholar] [CrossRef]
- Tracking Coronavirus Vaccinations around the World. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 20 August 2022).
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernan, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Gonzalez, S.; Olszevicki, S.; Salazar, M.; Calabria, A.; Regairaz, L.; Marin, L.; Campos, P.; Varela, T.; Martinez, V.V.G.; Ceriani, L.; et al. Effectiveness of the first component of Gam-COVID-Vac (Sputnik V) on reduction of SARS-CoV-2 confirmed infections, hospitalisations and mortality in patients aged 60-79: A retrospective cohort study in Argentina. EClinicalMedicine 2021, 40, 101126. [Google Scholar] [CrossRef]
- Kaura, A.; Trickey, A.; Shah, A.S.V.; Benedetto, U.; Glampson, B.; Mulla, A.; Mercuri, L.; Gautama, S.; Costelloe, C.E.; Goodman, I.; et al. Comparing the longer-term effectiveness of a single dose of the Pfizer-BioNTech and Oxford-AstraZeneca COVID-19 vaccines across the age spectrum. EClinicalMedicine 2022, 46, 101344. [Google Scholar] [CrossRef]
- Laing, E.D.; Weiss, C.D.; Samuels, E.C.; Coggins, S.A.; Wang, W.; Wang, R.; Vassell, R.; Sterling, S.L.; Tso, M.S.; Conner, T.; et al. Durability of Antibody Response and Frequency of SARS-CoV-2 Infection 6 Months after COVID-19 Vaccination in Healthcare Workers. Emerg. Infect. Dis. 2022, 28, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Pernat, C.A.; Gavralidis, A.; St Denis, K.J.; Lam, E.C.; Spring, L.M.; Isakoff, S.J.; Farmer, J.R.; Zubiri, L.; Hobbs, G.S.; et al. Immunogenicity and Reactogenicity of SARS-CoV-2 Vaccines in Patients with Cancer: The CANVAX Cohort Study. J. Clin. Oncol. 2022, 40, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Scobie, H.M.; Johnson, A.G.; Suthar, A.B.; Severson, R.; Alden, N.B.; Balter, S.; Bertolino, D.; Blythe, D.; Brady, S.; Cadwell, B.; et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status—13 U.S. Jurisdictions, April 4-July 17, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1284–1290. [Google Scholar] [CrossRef]
- Sritipsukho, P.; Khawcharoenporn, T.; Siribumrungwong, B.; Damronglerd, P.; Suwantarat, N.; Satdhabudha, A.; Chaiyakulsil, C.; Sinlapamongkolkul, P.; Tangsathapornpong, A.; Bunjoungmanee, P.; et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: A test-negative case-control study. Emerg. Microbes Infect. 2022, 11, 585–592. [Google Scholar] [CrossRef]
- Yu, X.; Qi, X.; Cao, Y.; Li, P.; Lu, L.; Wang, P.; Feng, Y.; Yang, J.; Wei, H.; Guo, L.; et al. Three doses of an inactivation-based COVID-19 vaccine induces cross-neutralizing immunity against the SARS-CoV-2 Omicron variant. Emerg. Microbes Infect. 2022, 11, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ju, B.; Zhou, X.; Cheng, L.; Wang, H.; Liao, X.; Wang, M.; Wei, L.; Song, S.; Zhou, B.; et al. The SARS-CoV-2 inactivated vaccine enhances the broad neutralization against variants in individuals recovered from COVID-19 up to one year. Emerg. Microbes Infect. 2022, 11, 753–756. [Google Scholar]
- Barouch, D.H.; Stephenson, K.E.; Sadoff, J.; Yu, J.; Chang, A.; Gebre, M.; McMahan, K.; Liu, J.; Chandrashekar, A.; Patel, S.; et al. Durable Humoral and Cellular Immune Responses 8 Months after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 385, 951–953. [Google Scholar] [CrossRef]
- Byazrova, M.G.; Kulemzin, S.V.; Astakhova, E.A.; Belovezhets, T.N.; Efimov, G.A.; Chikaev, A.N.; Kolotygin, I.O.; Gorchakov, A.A.; Taranin, A.V.; Filatov, A.V. Memory B Cells Induced by Sputnik V Vaccination Produce SARS-CoV-2 Neutralizing Antibodies Upon Ex Vivo Restimulation. Front. Immunol. 2022, 13, 840707. [Google Scholar] [CrossRef]
- Jeewandara, C.; Aberathna, I.S.; Danasekara, S.; Gomes, L.; Fernando, S.; Guruge, D.; Ranasinghe, T.; Gunasekera, B.; Kamaladasa, A.; Kuruppu, H.; et al. Comparison of the Immunogenicity of five COVID-19 vaccines in Sri Lanka. Immunology 2022. [Google Scholar] [CrossRef]
- Kageyama, T.; Ikeda, K.; Tanaka, S.; Taniguchi, T.; Igari, H.; Onouchi, Y.; Kaneda, A.; Matsushita, K.; Hanaoka, H.; Nakada, T.A.; et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin. Microbiol. Infect. 2021, 27, 1861.e1–1861.e5. [Google Scholar] [CrossRef]
- Nikolova, M.; Todorova, Y.; Emilova, R.; Trifonova, I.; Gladnishka, T.; Petrova-Yancheva, N.; Chervenyakova, T.; Dragusheva, E.; Popov, G.; Christova, I. Induction of humoral and cellular immune responses to COVID-19 mRNA and vector vaccines: A prospective cohort study in Bulgarian healthcare workers. J. Med. Virol. 2022, 94, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadeq, D.W.; Shurrab, F.M.; Ismail, A.; Amanullah, F.H.; Thomas, S.; Aldewik, N.; Yassine, H.M.; Abdul Rahim, H.F.; Abu-Raddad, L.; Nasrallah, G.K. Comparison of antibody immune responses between BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in naive and previously infected individuals. J. Travel Med. 2021, 28, taab190. [Google Scholar] [CrossRef] [PubMed]
- Dashdorj, N.J.; Wirz, O.F.; Roltgen, K.; Haraguchi, E.; Buzzanco, A.S.; 3rd Sibai, M.; Wang, H.; Miller, J.A.; Solis, D.; Sahoo, M.K.; et al. Direct comparison of antibody responses to four SARS-CoV-2 vaccines in Mongolia. Cell Host Microbe 2021, 29, 1738–1743.e1734. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Miyakawa, K.; Ohtake, N.; Go, H.; Yamaoka, Y.; Yajima, S.; Shimada, T.; Goto, A.; Nakajima, H.; Ryo, A. Antibody titers against the Alpha, Beta, Gamma, and Delta variants of SARS-CoV-2 induced by BNT162b2 vaccination measured using automated chemiluminescent enzyme immunoassay. J. Infect. Chemother. 2022, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination with BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Valyi-Nagy, I.; Matula, Z.; Gonczi, M.; Tasnady, S.; Beko, G.; Reti, M.; Ajzner, E.; Uher, F. Comparison of antibody and T cell responses elicited by BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) vaccines against SARS-CoV-2 in healthy adult humans. Geroscience 2021, 43, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Kagina, B.M.; Dochez, C. COVID-19 vaccine-induced immunity: Head-to-head comparison of mRNA (BNT162b2) versus inactivated (CoronaVac) vaccines. Respirology 2022, 27, 260–261. [Google Scholar] [CrossRef]
- Kang, Y.M.; Minn, D.; Lim, J.; Lee, K.D.; Jo, D.H.; Choe, K.W.; Kim, M.J.; Kim, J.M.; Kim, K.N. Comparison of Antibody Response Elicited by ChAdOx1 and BNT162b2 COVID-19 Vaccine. J. Korean Med. Sci. 2021, 36, e311. [Google Scholar] [CrossRef]
- Mok, C.K.P.; Cohen, C.A.; Cheng, S.M.S.; Chen, C.; Kwok, K.O.; Yiu, K.; Chan, T.O.; Bull, M.; Ling, K.C.; Dai, Z.; et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology 2022, 27, 301–310. [Google Scholar] [CrossRef]
- Muecksch, F.; Wise, H.; Batchelor, B.; Squires, M.; Semple, E.; Richardson, C.; McGuire, J.; Clearly, S.; Furrie, E.; Greig, N.; et al. Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients. J. Infect. Dis. 2021, 223, 389–398. [Google Scholar] [CrossRef]
- Buchholz, U.J.; Bukreyev, A.; Yang, L.; Lamirande, E.W.; Murphy, B.R.; Subbarao, K.; Collins, P.L. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. USA 2004, 101, 9804–9809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvatori, G.; Luberto, L.; Maffei, M.; Aurisicchio, L.; Roscilli, G.; Palombo, F.; Marra, E. SARS-CoV-2 SPIKE PROTEIN: An optimal immunological target for vaccines. J. Transl. Med. 2020, 18, 222. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Garbuglia, A.R.; Minosse, C.; Del Porto, P. mRNA- and Adenovirus-Based Vaccines against SARS-CoV-2 in HIV-Positive People. Viruses 2022, 14, 748. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Tormo, N.; Navalpotro, D.; Martinez-Serrano, M.; Moreno, M.; Grosson, F.; Tur, I.; Guna, M.R.; Soriano, P.; Tornero, A.; Gimeno, C. Commercial Interferon-gamma release assay to assess the immune response to first and second doses of mRNA vaccine in previously COVID-19 infected versus uninfected individuals. Diagn. Microbiol. Infect. Dis. 2022, 102, 115573. [Google Scholar] [CrossRef]
- Fan, Y.J.; Chan, K.H.; Hung, I.F. Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3. Vaccines 2021, 9, 989. [Google Scholar] [CrossRef]
- Ali, H.; Alahmad, B.; Al-Shammari, A.A.; Alterki, A.; Hammad, M.; Cherian, P.; Alkhairi, I.; Sindhu, S.; Thanaraj, T.A.; Mohammad, A.; et al. Previous COVID-19 Infection and Antibody Levels After Vaccination. Front. Public Health 2021, 9, 778243. [Google Scholar] [CrossRef]
- Bates, T.A.; McBride, S.K.; Leier, H.C.; Guzman, G.; Lyski, Z.L.; Schoen, D.; Winders, B.; Lee, J.Y.; Lee, D.X.; Messer, W.B.; et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci. Immunol. 2022, 7, eabn8014. [Google Scholar] [CrossRef]
- Roltgen, K.; Boyd, S.D. Antibody and B cell responses to SARS-CoV-2 infection and vaccination. Cell Host Microbe 2021, 29, 1063–1075. [Google Scholar] [CrossRef]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.D.; Bassett, J.; Millar, J.B.; Grinshtein, N.; Yang, T.C.; Parsons, R.; Evelegh, C.; Wan, Y.; Parks, R.J.; Bramson, J.L. Persistence of transgene expression influences CD8+ T-cell expansion and maintenance following immunization with recombinant adenovirus. J. Virol. 2009, 83, 12027–12036. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, N.; Fitzgerald, J.C.; Reyes-Sandoval, A.; Harris-McCoy, K.C.; Hensley, S.E.; Zhou, D.; Lin, S.W.; Bian, A.; Xiang, Z.Q.; Iparraguirre, A.; et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: Implications for their use as vaccines. Blood 2007, 110, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Goulding, J.; Abboud, G.; Tahiliani, V.; Desai, P.; Hutchinson, T.E.; Salek-Ardakani, S. CD8 T cells use IFN-gamma to protect against the lethal effects of a respiratory poxvirus infection. J. Immunol. 2014, 192, 5415–5425. [Google Scholar] [CrossRef]
- Kang, S.; Brown, H.M.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw. 2018, 18, e33. [Google Scholar] [CrossRef]
- Ruby, J.; Ramshaw, I. The antiviral activity of immune CD8+ T cells is dependent on interferon-gamma. Lymphokine Cytokine Res. 1991, 10, 353–358. [Google Scholar]
- Anghelina, D.; Zhao, J.; Trandem, K.; Perlman, S. Role of regulatory T cells in coronavirus-induced acute encephalitis. Virology 2009, 385, 358–367. [Google Scholar] [CrossRef]
- Karkhah, A.; Javanian, M.; Ebrahimpour, S. The role of regulatory T cells in immunopathogenesis and immunotherapy of viral infections. Infect. Genet. Evol. 2018, 59, 32–37. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Cervantes-Diaz, R.; Sosa-Hernandez, V.A.; Romero-Ramirez, S.; Torres-Ruiz, J.; Perez-Fragoso, A.; Meza-Sanchez, D.E.; Gomez-Martin, D.; Maravillas-Montero, J.L. Circulating B10 regulatory cells are decreased in severe and critical COVID-19. J. Leukoc. Biol. 2022, 112, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.; Naderi, N. Toward an understanding of regulatory T cells in COVID-19: A systematic review. J. Med. Virol. 2021, 93, 4167–4181. [Google Scholar] [CrossRef] [PubMed]
- Dowling, M.R.; Kan, A.; Heinzel, S.; Marchingo, J.M.; Hodgkin, P.D.; Hawkins, E.D. Regulatory T Cells Suppress Effector T Cell Proliferation by Limiting Division Destiny. Front. Immunol. 2018, 9, 2461. [Google Scholar] [CrossRef] [PubMed]



| Symptoms | After 1st Vaccination | After 2nd Vaccination | ||
|---|---|---|---|---|
| β-Coff (95% CI) | p-Value | β-Coff (95% CI) | p-Value | |
| Fever | 2.69 (1.86, 3.80) | <0.001 | 2.88 (2.04, 4.07) | <0.001 |
| Tiredness | 1.91 (1.23, 2.98) | 0.004 | 1.86 (1.17, 2.95) | 0.008 |
| Muscle pain | 1.70 (1.17, 2.45) | 0.006 | 1.29 (0.91, 1.82) | 0.149 |
| Headache | 2.04 (1.20, 3.47) | 0.009 | 2.75 (1.62, 4.79) | <0.001 |
| Whole body pain | 1.17 (0.85, 1.62) | 0.332 | 1.27 (0.91, 1.78) | 0.159 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarker, P.; Akhtar, E.; Kuddusi, R.U.; Alam, M.M.; Haq, M.A.; Hosen, M.B.; Chanda, B.C.; Haque, F.; Alam, M.; Razzaque, A.; et al. Comparison of the Immune Responses to COVID-19 Vaccines in Bangladeshi Population. Vaccines 2022, 10, 1498. https://doi.org/10.3390/vaccines10091498
Sarker P, Akhtar E, Kuddusi RU, Alam MM, Haq MA, Hosen MB, Chanda BC, Haque F, Alam M, Razzaque A, et al. Comparison of the Immune Responses to COVID-19 Vaccines in Bangladeshi Population. Vaccines. 2022; 10(9):1498. https://doi.org/10.3390/vaccines10091498
Chicago/Turabian StyleSarker, Protim, Evana Akhtar, Rakib Ullah Kuddusi, Mohammed Mamun Alam, Md. Ahsanul Haq, Md. Biplob Hosen, Bikash Chandra Chanda, Farjana Haque, Muntasir Alam, Abdur Razzaque, and et al. 2022. "Comparison of the Immune Responses to COVID-19 Vaccines in Bangladeshi Population" Vaccines 10, no. 9: 1498. https://doi.org/10.3390/vaccines10091498
APA StyleSarker, P., Akhtar, E., Kuddusi, R. U., Alam, M. M., Haq, M. A., Hosen, M. B., Chanda, B. C., Haque, F., Alam, M., Razzaque, A., Rahman, M., Ahmed, F., Kibria, M. G., Islam, M. Z., Ahmed, S., & Raqib, R. (2022). Comparison of the Immune Responses to COVID-19 Vaccines in Bangladeshi Population. Vaccines, 10(9), 1498. https://doi.org/10.3390/vaccines10091498







