Antibody Titers and Protection against Omicron (BA.1 and BA.2) SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. SARS-CoV-2 Detection, Variant Screening, and Genome Sequencing
2.3. Neutralizing and Binding Antibodies against SARS-CoV-2 Spike Protein
2.4. Antibodies against SARS-CoV-2 Nucleocapsid Protein
2.5. Statistical Analysis
3. Results
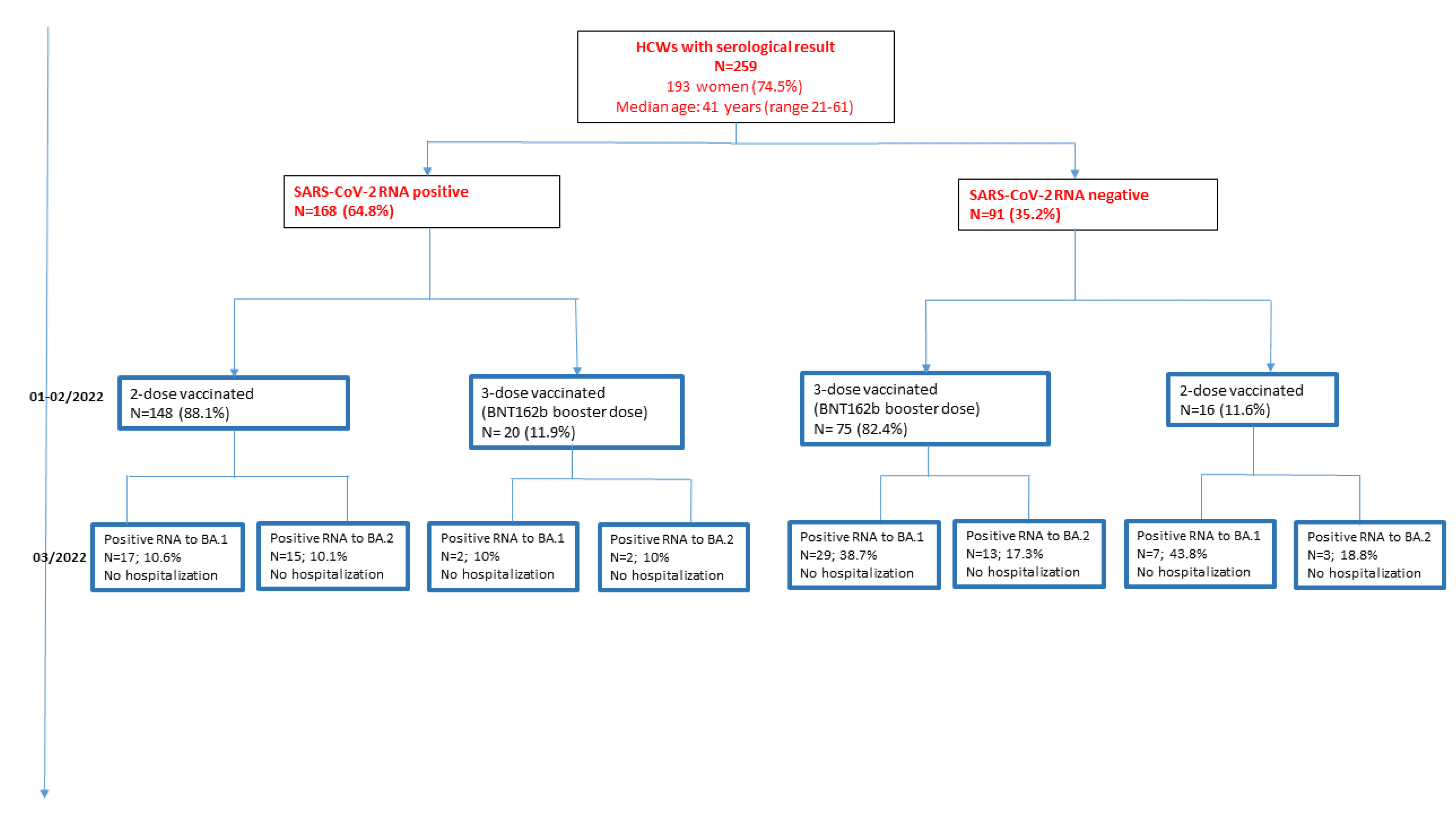
3.1. Population Characteristics and Vaccination Status
3.2. Occurrence of SARS-CoV-2 Infections
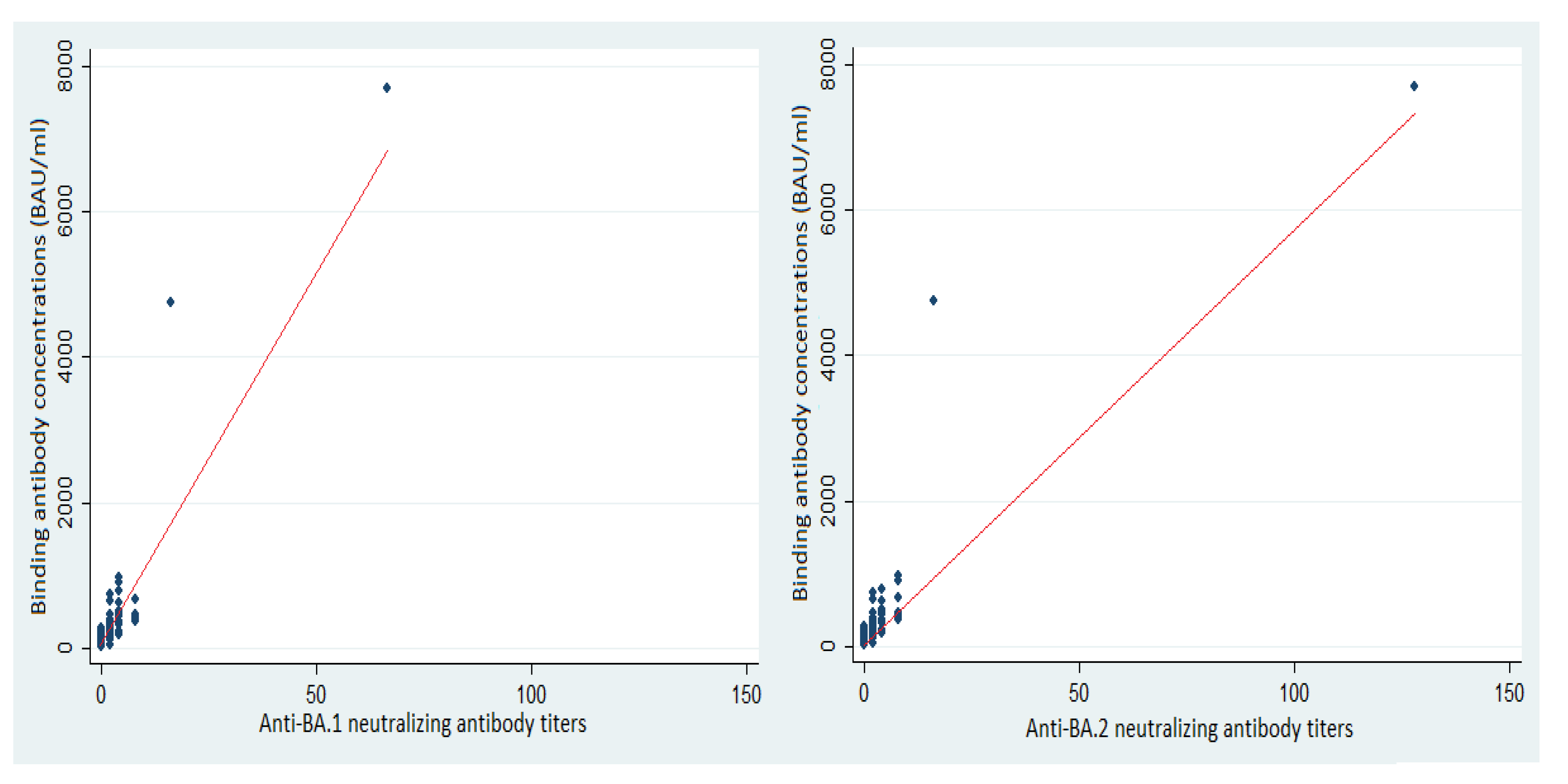
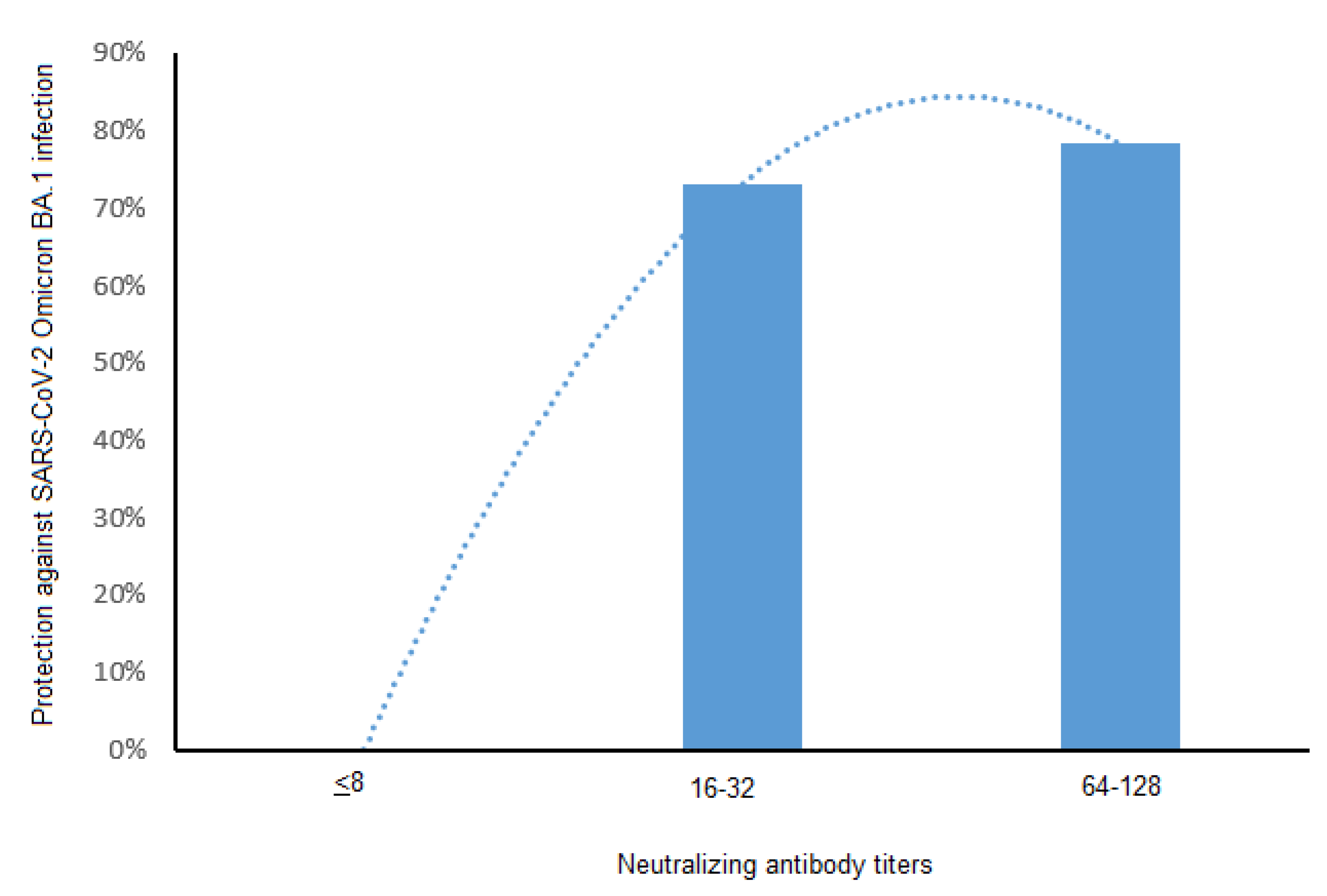
3.3. Neutralizing Antibodies and Protection against SARS-CoV-2 BA.1 and BA.2
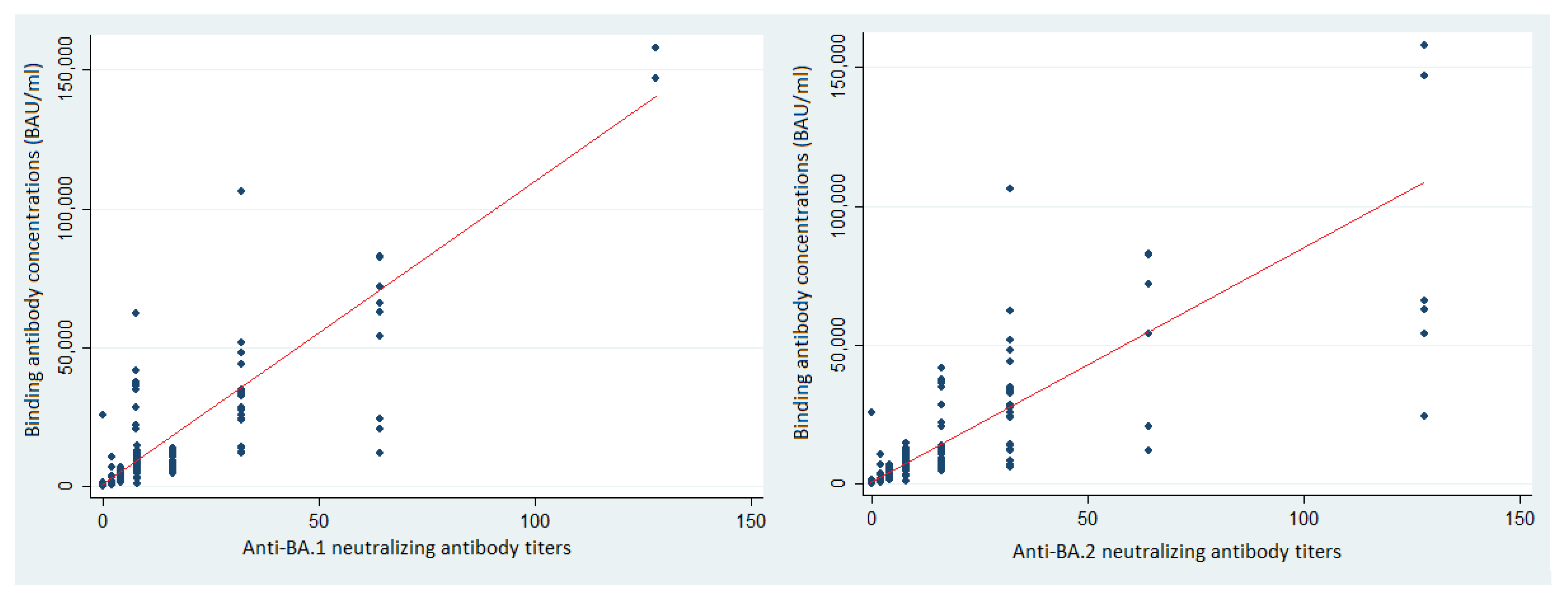
3.4. Binding Antibodies and Protection against SARS-CoV-2 BA.1 and BA.2
3.5. Antibody Concentrations and Vaccination Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bok, K.; Sitar, S.; Graham, B.S.; Mascola, J.R. Accelerated COVID-19 vaccine development: Milestones, lessons, and prospects. Immunity 2021, 54, 1636–1651. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Miédougé, M.; Abravanel, F.; Da Silva, I.; Porcheron, M.; Fillaux, J.; Diméglio, C.; Izopet, J. Evaluation of Three Quantitative Anti-SARS-CoV-2 Antibody Immunoassays. Microbiol. Spectr. 2021, 9, e0137621. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Resman Rus, K.; Korva, M.; Knap, N.; Avšič Županc, T.; Poljak, M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J. Clin. Virol. 2021, 139, 104820. [Google Scholar] [CrossRef]
- Dimeglio, C.; Herin, F.; Martin-Blondel, G.; Miedougé, M.; Izopet, J. Antibody titers and protection against a SARS-CoV-2 infection. J. Infect. 2022, 84, 248–288. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; Burgers, W.A.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef]
- Dimeglio, C.; Herin, F.; Da-Silva, I.; Gernigon, C.; Porcheron, M.; Chapuy-Regaud, S.; Izopet, J. Decreased Efficiency of Neutralizing Antibodies from Previously Infected or Vaccinated Individuals against the B.1.617.2 (Delta) SARS-CoV-2 Variant. Microbiol. Spectr. 2022, 10, e0270621. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Heavily mutated Omicron variant puts scientists on alert. Nature. 2021, 600, 21. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Azman, A.S.; Sun, R. Neutralizing antibodies against SARS-CoV-2 variants induced by natural infection or vaccination: A systematic review and pooled meta-analysis. Clin. Infect. Dis 2021, 73, 1722–1732. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2021, 602, 654–656. [Google Scholar] [CrossRef]
- Schmidt, F.; Muecksch, F.; Weisblum, Y.; da Silva, J.; Bednarski, E.; Cho, A.; Wang, Z.; Gaebler, C.; Caskey, M.; Nussenzweig, M.C.; et al. Plasma neutralization of the SARS-CoV-2 Omicron variant. N. Engl. J. Med. 2022, 386, 599–601. [Google Scholar] [CrossRef]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.-J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef]
- Pfizer. Pfizer and BioNTech Provide Update on Omicron Variant 2021 [Updated Wednesday December 08, 2021]. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-omicron-variant (accessed on 14 September 2022).
- Migueres, M.; Lhomme, S.; Trémeaux, P.; Dimeglio, C.; Ranger, N.; Latour, J.; Dubois, M.; Nicot, F.; Miedouge, M.; Mansuy, J.M.; et al. Evaluation of two RT-PCR screening assays for identifying SARS-CoV-2 variants. J. Clin. Virol. 2021, 143, 104969. [Google Scholar] [CrossRef]
- Migueres, M.; Dimeglio, C.; Mansuy, J.M.; Abravanel, F.; Raymond, S.; Latour, J.; Jeanne, N.; Ranger, N.; Lhomme, S.; Saune, K.; et al. The influence of the nasopharyngeal viral load on the spread of the Omicron BA.2 variant. Clin. Infect. Dis. 2022, ciac563. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.; Czudnochowski, N.; Rosen, L.E.; Zepeda, S.K.; Bowen, J.E.; Walls, A.C.; Hauser, K.; Joshi, A.; Stewart, C.; Dillen, J.R.; et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science 2022, 375, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Stærke, N.B.; Reekie, J.; Nielsen, H.; Benfield, T.; Wiese, L.; Knudsen, L.S.; Iversen, M.B.; Iversen, K.; Fogh, K.; Bodilsen, J.; et al. Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. Nat. Commun. 2022, 13, 4466. [Google Scholar] [CrossRef] [PubMed]
- Dimeglio, C.; Trémeaux, P.; Herin, F.; Da-Silva, I.; Porcheron, M.; Martin-Blondel, G.; Gernigon, C.; Chapuy-Regaud, S.; Villars, H.; Izopet, J. Post-vaccination SARS-CoV-2 antibody kinetics and protection duration against Omicron in elderly population. J. Infect. 2022, S0163-4453(22)00522-9. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Dimeglio, C.; Herin, F.; Da-Silva, I.; Porcheron, M.; Martin-Blondel, G.; Chapuy-Regaud, S.; Izopet, J. Post-vaccination SARS-CoV-2 antibody kinetics and protection duration. Clin. Infect. Dis 2021, ciab984. [Google Scholar] [CrossRef]
- Li, X. Omicron: Call for updated vaccines. J. Med. Virol. 2022, 94, 1261–1263. [Google Scholar] [CrossRef]

| HCWs | Infected/Vaccinated | Vaccinated |
|---|---|---|
| N (%) | 168 (64.9) | 91 (35.1) |
| Age: | ||
| Median (IQR) | 39 (21–58) | 42 (21–61) |
| Female: N (%) | 130 (77.4) | 63 (69.2) |
| Vaccines: n (%) | ||
| BNT162b/BNT162b | 152 (90.5) | 37 (40.7) |
| ChadOx1-S/BNT162b | 16 (9.5) | 46 (50.5) |
| ChadOx1-S/ChadOx1-S | 0 | 8 (8.8) |
| Vaccination status: n (%) | ||
| -Primary vaccinated (2 doses) | 148 (88.1) | 16 (17.6) |
| -Primary vaccinated + booster dose | 20 (11.9) | 75 (82.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimeglio, C.; Migueres, M.; Bouzid, N.; Chapuy-Regaud, S.; Gernigon, C.; Da-Silva, I.; Porcheron, M.; Martin-Blondel, G.; Herin, F.; Izopet, J. Antibody Titers and Protection against Omicron (BA.1 and BA.2) SARS-CoV-2 Infection. Vaccines 2022, 10, 1548. https://doi.org/10.3390/vaccines10091548
Dimeglio C, Migueres M, Bouzid N, Chapuy-Regaud S, Gernigon C, Da-Silva I, Porcheron M, Martin-Blondel G, Herin F, Izopet J. Antibody Titers and Protection against Omicron (BA.1 and BA.2) SARS-CoV-2 Infection. Vaccines. 2022; 10(9):1548. https://doi.org/10.3390/vaccines10091548
Chicago/Turabian StyleDimeglio, Chloé, Marion Migueres, Naémie Bouzid, Sabine Chapuy-Regaud, Caroline Gernigon, Isabelle Da-Silva, Marion Porcheron, Guillaume Martin-Blondel, Fabrice Herin, and Jacques Izopet. 2022. "Antibody Titers and Protection against Omicron (BA.1 and BA.2) SARS-CoV-2 Infection" Vaccines 10, no. 9: 1548. https://doi.org/10.3390/vaccines10091548
APA StyleDimeglio, C., Migueres, M., Bouzid, N., Chapuy-Regaud, S., Gernigon, C., Da-Silva, I., Porcheron, M., Martin-Blondel, G., Herin, F., & Izopet, J. (2022). Antibody Titers and Protection against Omicron (BA.1 and BA.2) SARS-CoV-2 Infection. Vaccines, 10(9), 1548. https://doi.org/10.3390/vaccines10091548






