Pilot Study for Immunogenicity of SARS-CoV-2 Vaccine with Seasonal Influenza and Pertussis Vaccines in Pregnant Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
2.3. Neutralizing Antibody Inhibition Test of SARS-CoV-2 Omicron, Delta, and Wildtype Variants
2.4. Anti-SARS-CoV-2 S Protein Total Antibody
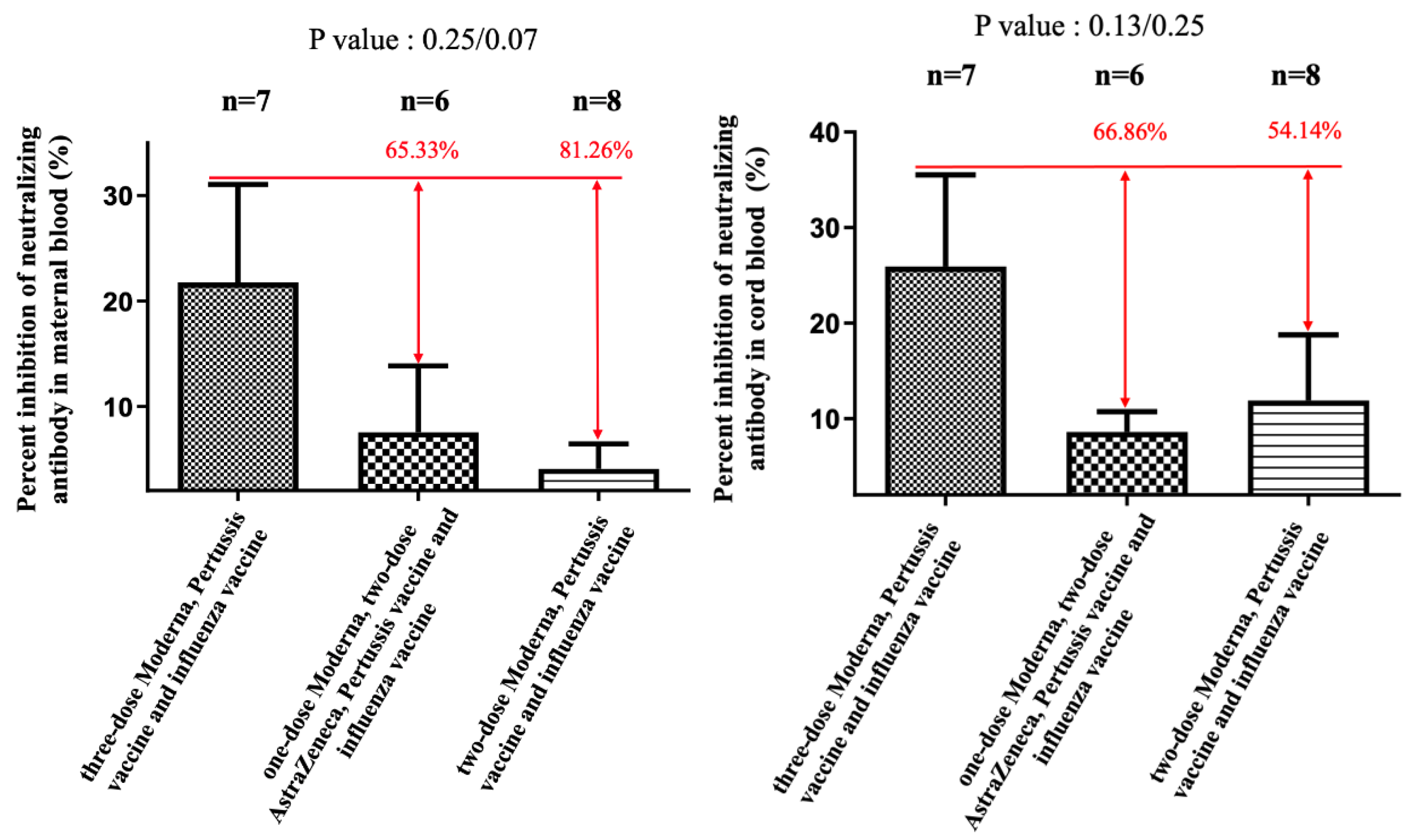
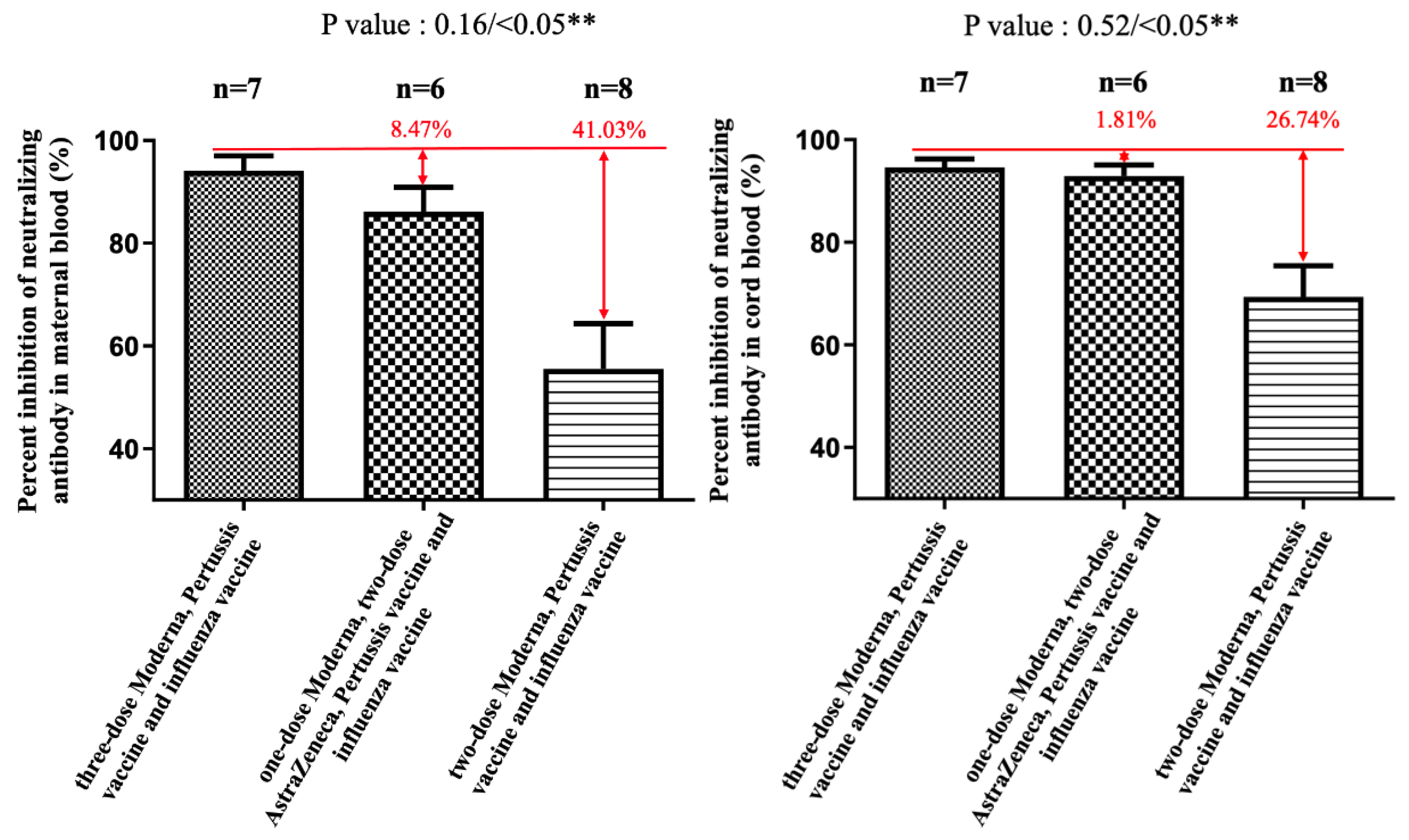
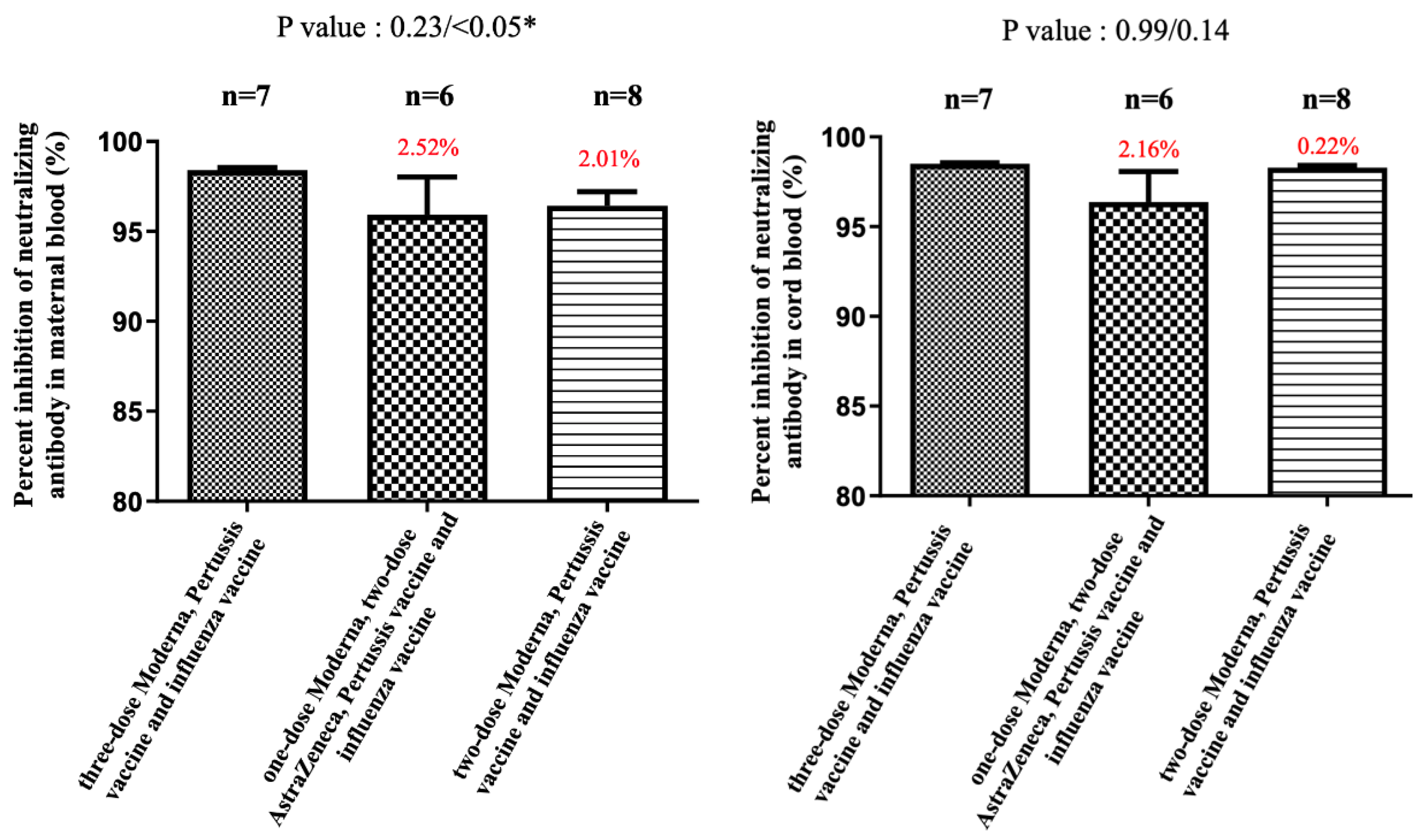
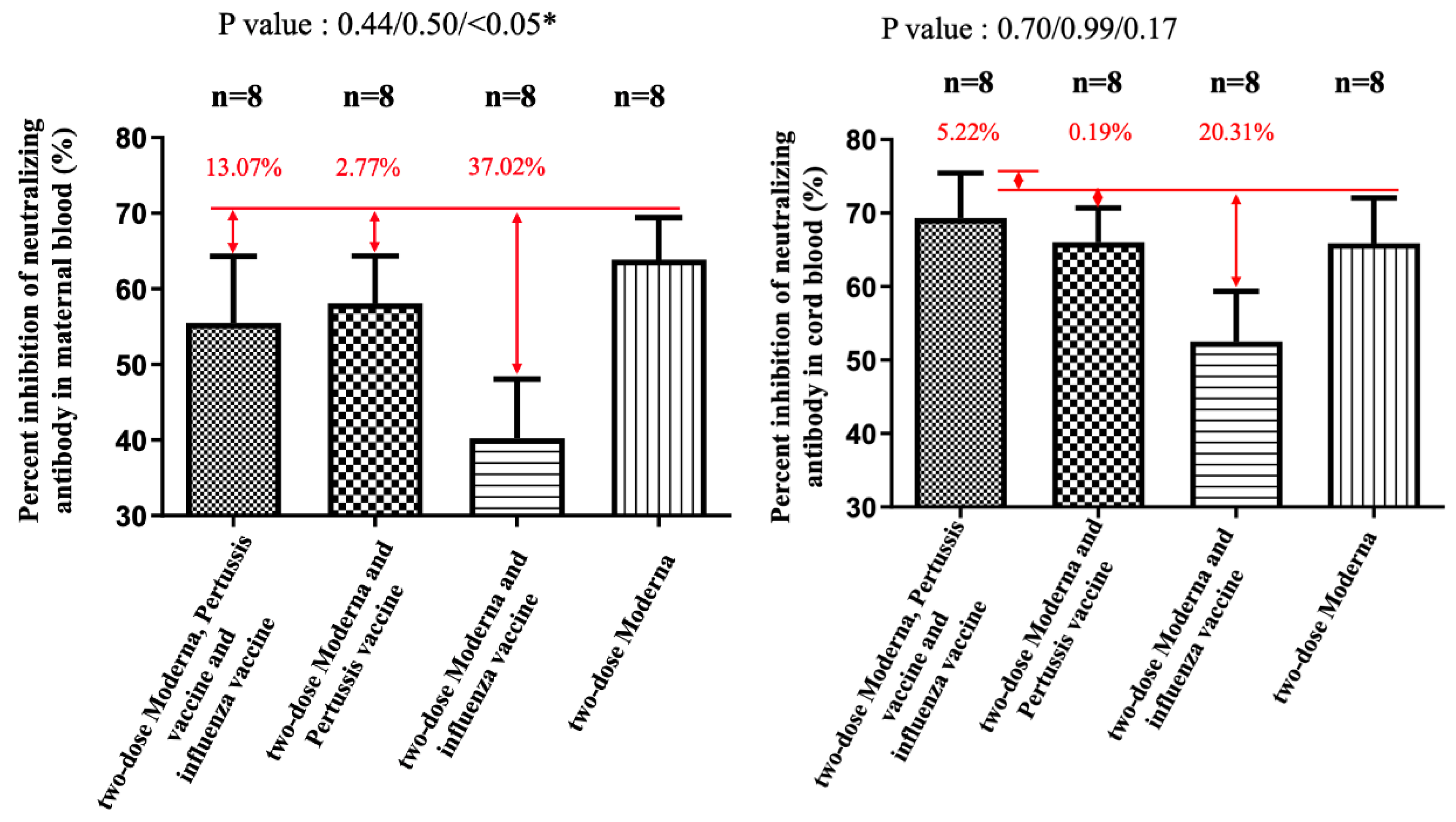
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callaway, E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021, 600, 21. [Google Scholar] [CrossRef] [PubMed]
- Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern; WHO: Geneva, Switzerland, 2021.
- Schmidt, F.; Muecksch, F.; Weisblum, Y.; Da Silva, J.; Bednarski, E.; Cho, A.; Wang, Z.; Gaebler, C.; Caskey, M.; Nussenzweig, M.C.; et al. Plasma Neutralization of the SARS-CoV-2 Omicron Variant. N. Engl. J. Med. 2022, 386, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Chiba, S.; Halfmann, P.; Nagai, H.; et al. Efficacy of Antibodies and Antiviral Drugs against COVID-19 Omicron Variant. N. Engl. J. Med. 2022, 386, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Servellita, V.; Syed, A.M.; Morris, M.K.; Brazer, N.; Saldhi, P.; Garcia-Knight, M.; Sreekumar, B.; Khalid, M.M.; Ciling, A.; Chen, P.Y.; et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. Cell 2022, 185, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). COVID Data Tracker. 2022. Available online: https://covid.cdc.gov/covid-data-tracker/#pregnant-population (accessed on 30 December 2022).
- The American College of Obstetricians and Gynecologists (ACOG). ACOG and SMFM Recommend COVID-19 Vaccination for Pregnant Individuals. 2021. Available online: https://www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals (accessed on 30 December 2022).
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef]
- Flu Vaccine Safety and Pregnancy. 2021. Available online: https://www.cdc.gov/flu/highrisk/qa_vacpregnant.htm#:~:text=Pregnant%20people%20should%20get%20a,by%20up%20to%20one%2Dhalf (accessed on 30 December 2022).
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralizationtest based on antibody-mediated blockage of ACE2-spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Jung, K.; Shin, S.; Nam, M.; Hong, Y.J.; Roh, E.Y.; Park, K.U.; Song, E.Y. Performance Evaluation of Three Automated Quantitative Immunoassays and Their Correlation With a Surrogate Virus Neutralization Test in Coronavirus Disease 19 Patients and Pre-Pandemic Controls. J. Clin. Lab. Anal. 2021, 35, e23921. [Google Scholar] [CrossRef]
- Higgins, V.; Fabros, A.; Kulasingam, V. Quantitative Measurement of Anti-SARS-CoV-2 Antibodies: Analytical and Clinical Evaluation. J. Clin. Microbiol. 2021, 59, e03149-20. [Google Scholar] [CrossRef]
- Munoz, F.M.; Jamieson, D.J. Maternal Immunization. Obstet. Gynecol. 2019, 133, 739–753. [Google Scholar] [CrossRef]
- Marchand, G.; Patil, A.S.; Masoud, A.T.; Ware, K.; King, A.; Ruther, S.; Brazil, G.; Calteux, N.; Ulibarri, H.; Parise, J.; et al. Review and meta-analysis of COVID maternal and neonatal clinical features and pregnancy outcomes to June 3rd 2021. AJOG Glob. Rep. 2021, 3, 100049. [Google Scholar]
- Stock, S.J.; Carruthers, J.; Calvert, C.; Denny, C.; Donaghy, J.; Goulding, A.; Hopcroft, L.E.M.; Hopkins, L.; McLaughlin, T.; Pan, J.; et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med. 2022, 28, 504–512. [Google Scholar] [CrossRef]
- Allen, H.; Turner, C.; Tessier, E. Comparative transmission of SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants and the impact of vaccination: National cohort study, England. medRxiv 2022. [Google Scholar] [CrossRef]
- Seasely, A.R.; Blanchard, C.T.; Arora, N.; Battarbee, A.N.; Casey, B.M.; Dionne-Odom, J.; Leal, S.M.; Moates, D.B.; Sinkey, R.G.; Szychowski, J.M.; et al. Maternal and rerinatal outcomes associated with the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Obstet. Gynecol. 2022, 140, 262–265. [Google Scholar] [CrossRef]
- Birol Ilter, P.; Prasad, S.; Mutlu, M.A.; Tekin, A.B.; O’Brien, P.; von Dadelszen, P.; Magee, L.A.; Tekin, S.; Tug, N.; Kalafat, E.; et al. Maternal and perinatal outcomes of SARS-CoV-2 infection in unvaccinated pregnancies during Delta and Omicron waves. Ultrasound Obs. Gynecol. 2022, 60, 96–102. [Google Scholar] [CrossRef]
- Birol Ilter, P.; Prasad, S.; Berkkan, M.; Mutlu, M.A.; Tekin, A.B.; Celik, E.; Ata, B.; Turgal, M.; Yildiz, S.; Turkgeldi, E.; et al. Clinical severity of SARS-CoV-2 infection among vaccinated and unvaccinated pregnancies during the Omicron wave. Ultrasound Obs. Gynecol. 2022, 59, 560–562. [Google Scholar] [CrossRef]
- Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 2022, 22, 277–282. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Boosters During Pregnancy. Available online: https://www.acog.org/covid-19/covid-19-vaccines-tools-for-your-practice-and-your-patients/boosters-during-pregnancy (accessed on 30 December 2022).
- Beharier, O.; Mayo, R.P.; Raz, T.; Sacks, K.N.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e150319. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Lazarus, R.; Baos, S.; Cappel-Porter, H.; Carson-Stevens, A.; Clout, M.; Culliford, L.; Emmett, S.R.; Garstang, J.; Gbadamoshi, L.; Hallis, B.; et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): A multicentre, randomised, controlled, phase 4 trial. Lancet 2021, 398, 2277–2287. [Google Scholar] [CrossRef]
- Toback, S.; Galiza, E.; Cosgrove, C.; Galloway, J.; Goodman, A.L.; Swift, P.A.; Rajaram, S.; Graves-Jones, A.; Edelman, J.; Burns, F.; et al. Safety, Immunogenicity, and Efficacy of a COVID-19 Vaccine (NVX-CoV2373) Co-administered with Seasonal Influenza Vaccines. medRxiv 2021. [Google Scholar] [CrossRef]
- Shen, C.J.; Fu, Y.C.; Lin, Y.P.; Shen, C.F.; Sun, D.J.; Chen, H.Y.; Cheng, C.M. Evaluation of Transplacental Antibody Transfer in SARS-CoV-2-Immunized Pregnant Women. Vaccines 2022, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Insel, R. Potential Alterations in Immunogenicity by Combining or Simultaneously Administering Vaccine Component. Ann. N.Y. Acad. Sci. 1995, 31, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Eskola, J.; Leclerc, C.; Leroy, O. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect. Immun. 1998, 66, 2093–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Alosaimi, B.; Naeem, A.; Hamed, M.E.; Alkadi, H.S.; Alanazi, T.; Al Rehily, S.S.; Almutairi, A.Z.; Zafar, A. Influenza co-infection associated with severity and mortality in COVID-19 patients. Virol. J. 2021, 18, 127. [Google Scholar] [CrossRef]
- Agarwal, A.; Agarwal, M.; Sharma, A.; Jakhar, R. Impact of influenza A co-infection with COVID-19. Int. J. Tuberc. Lung Dis. 2021, 25, 413–415. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, F.; Wu, K.; Wang, J.; Li, F.; Huang, S.; Lu, J.; Huang, J.; Liu, H.; Zhou, R.; et al. Clinical and virological impact of single and dual infections with influenza A (H1N1) and SARS-CoV-2 in adult inpatients. PLoS Negl. Trop. Dis. 2021, 15, e0009997. [Google Scholar] [CrossRef]
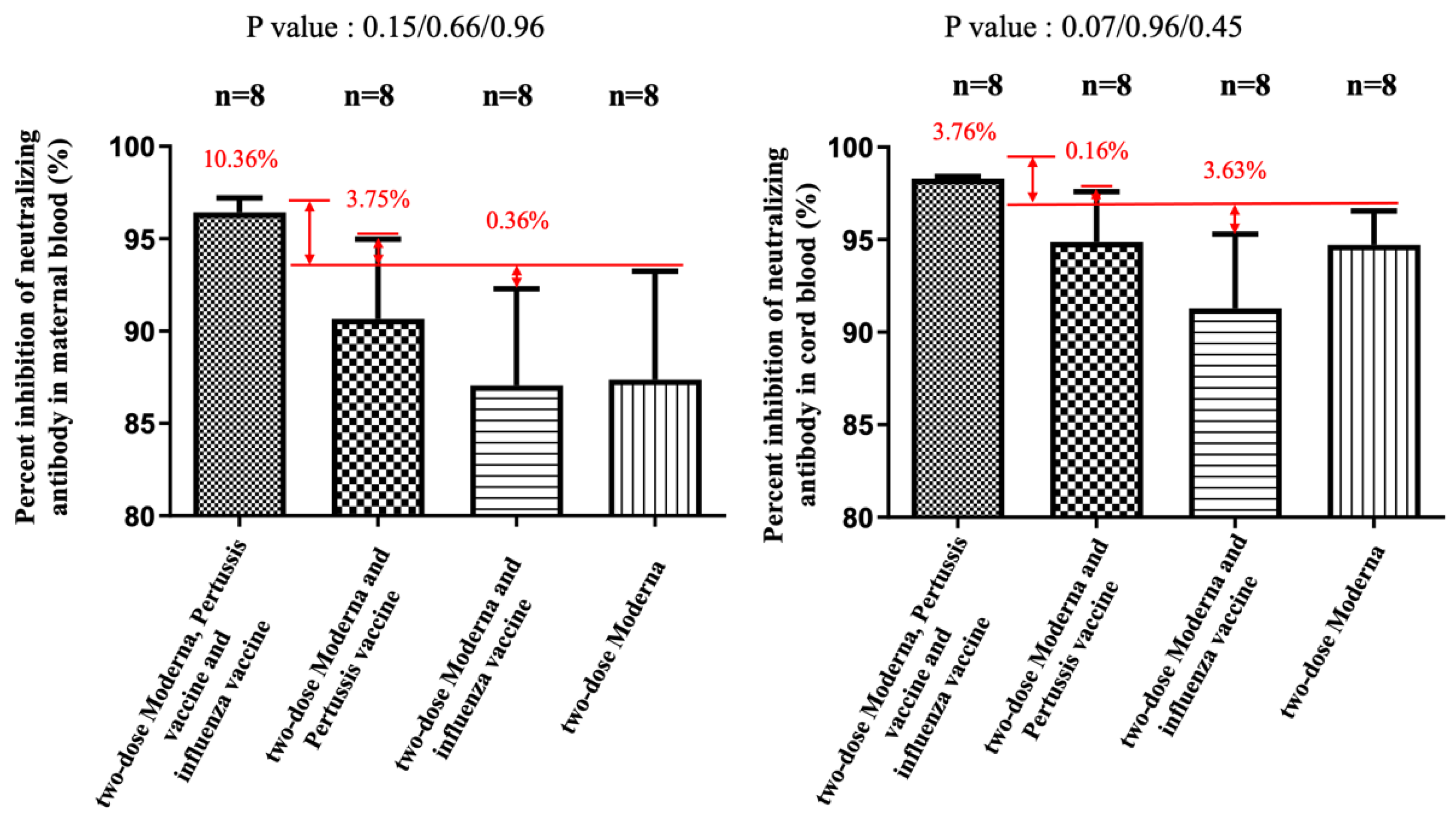
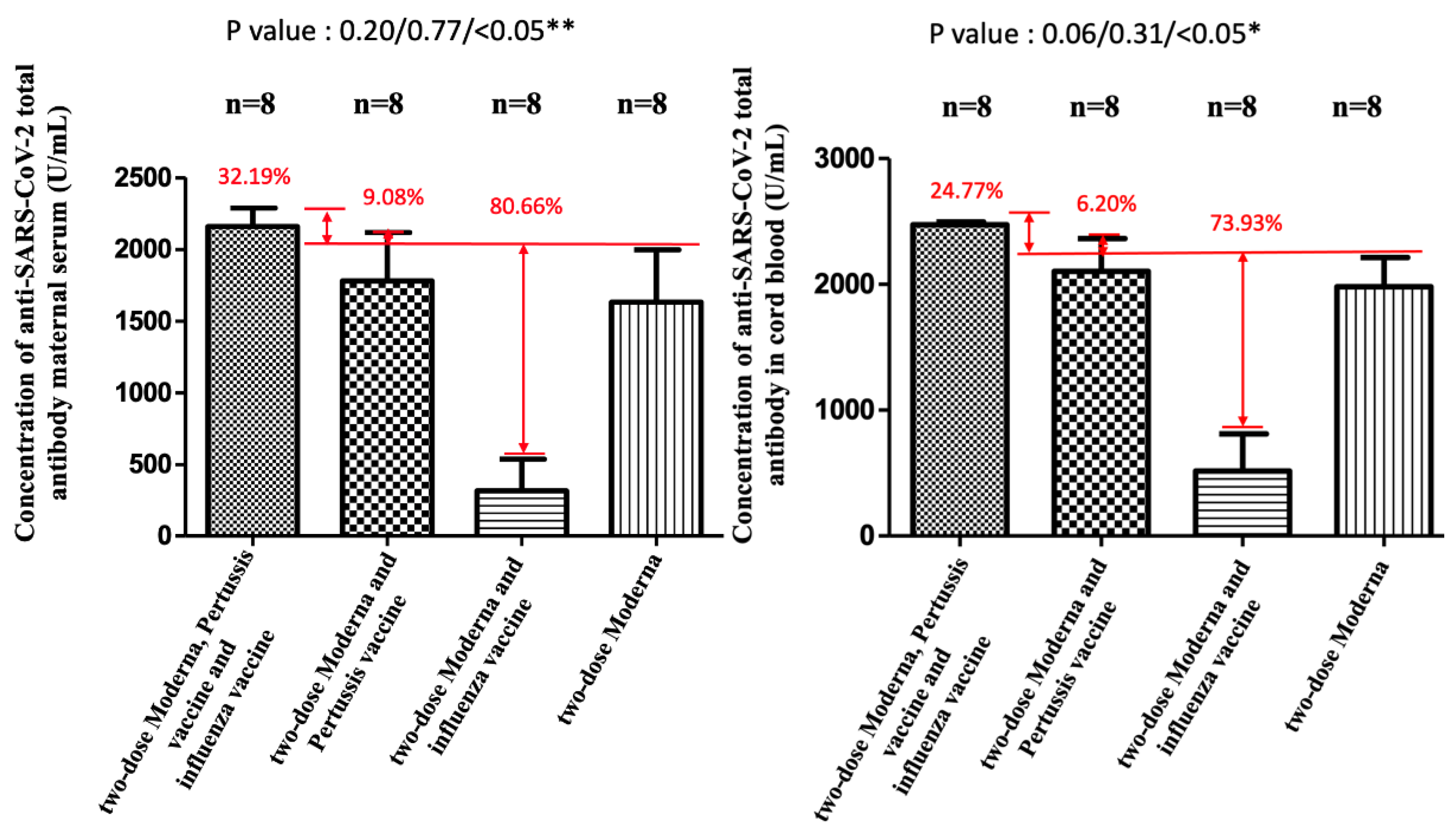

| Variable | Included in the Analysis (Two-Dose) a | Included in the Analysis (Three-Dose) b |
|---|---|---|
| Age of mothers (years) | 31.72 * (±5.27 **) IQR 34–29 | 33.63 * (±4.60 **) IQR 36.5–31 |
| Parity | 0.81 * IQR 2–0 | 1.40 * IQR 2–1 |
| ≥1 | ||
| BMI | 26.76 * (±4.15 **) IQR 29.03–23.38 | 26.89 * (±4.62 **) IQR 30.04–24.53 |
| Weeks of gestation at the first dose of COVID-19 vaccination (weeks) | 23.5 * (±2.79 **) IQR 25.25–21 | 1.28 * (±9.07 **) IQR 7.5–0 |
| Weeks of gestation at the second dose of COVID-19 vaccination (weeks) | 28.75 * (±3.30 **) IQR 31–26 | 13.72 * (±7.38 **) IQR 19.5–7.5 |
| Weeks of gestation at the third dose of COVID-19 vaccination (weeks) | - | 32.54 * (±3.25 **) IQR 35–30.5 |
| Interval between the third dose of COVID-19 vaccination and the collection of blood samples (day of delivery) (weeks) | - | 5.93 * (±2.98 **) IQR 7.5–4 |
| Interval between the second dose of COVID-19 vaccination and the collection of blood samples (day of delivery) (weeks) | 9.84 * (±3.29 **) IQR 12–8 | 24.55 * (±7.37 **) IQR 31–19 |
| Interval between the first dose of COVID-19 vaccination and the collection of blood samples (day of delivery) (weeks) | 15.09 * (±2.97 **) IQR 16.5–13 | 36.96 * (±9.02 **) IQR 42–31 |
| Weeks of gestation at delivery (weeks) | 38.59* (±1.10 **) IQR 39–38 | 38.46 * (±1.17 **) IQR 39–38 |
| Sex of newborn Male Female | 15 (46.9% ***) 17 (53.1% ***) | 33 (50% ***) 33 (50% ***) |
| Weight of newborn (g) | 3039.38 * (±355.78 **) IQR 3170–2823.75 | 3081.42 * (±331.20 **) IQR 3310–2880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-J.; Lin, Y.-P.; Hu, S.-Y.; Shen, C.-F.; Chuang, H.-Y.; Ker, C.-R.; Sun, D.-J.; Yang, Y.-H.; Cheng, C.-M. Pilot Study for Immunogenicity of SARS-CoV-2 Vaccine with Seasonal Influenza and Pertussis Vaccines in Pregnant Women. Vaccines 2023, 11, 119. https://doi.org/10.3390/vaccines11010119
Shen C-J, Lin Y-P, Hu S-Y, Shen C-F, Chuang H-Y, Ker C-R, Sun D-J, Yang Y-H, Cheng C-M. Pilot Study for Immunogenicity of SARS-CoV-2 Vaccine with Seasonal Influenza and Pertussis Vaccines in Pregnant Women. Vaccines. 2023; 11(1):119. https://doi.org/10.3390/vaccines11010119
Chicago/Turabian StyleShen, Ching-Ju, Yen-Pin Lin, Shu-Yu Hu, Ching-Fen Shen, Hui-Yu Chuang, Chin-Ru Ker, Der-Ji Sun, Yu-Hsuan Yang, and Chao-Min Cheng. 2023. "Pilot Study for Immunogenicity of SARS-CoV-2 Vaccine with Seasonal Influenza and Pertussis Vaccines in Pregnant Women" Vaccines 11, no. 1: 119. https://doi.org/10.3390/vaccines11010119








