Immune Response to Vaccination against COVID-19 at Different Second-Dose Intervals and Their Associations with Metabolic Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Blood Sampling
2.3. Antibodies, Biochemical, Anthropometric, and Body Composition Measurements
2.4. Statistics
3. Results
3.1. Clinical Characteristics of Studied Groups
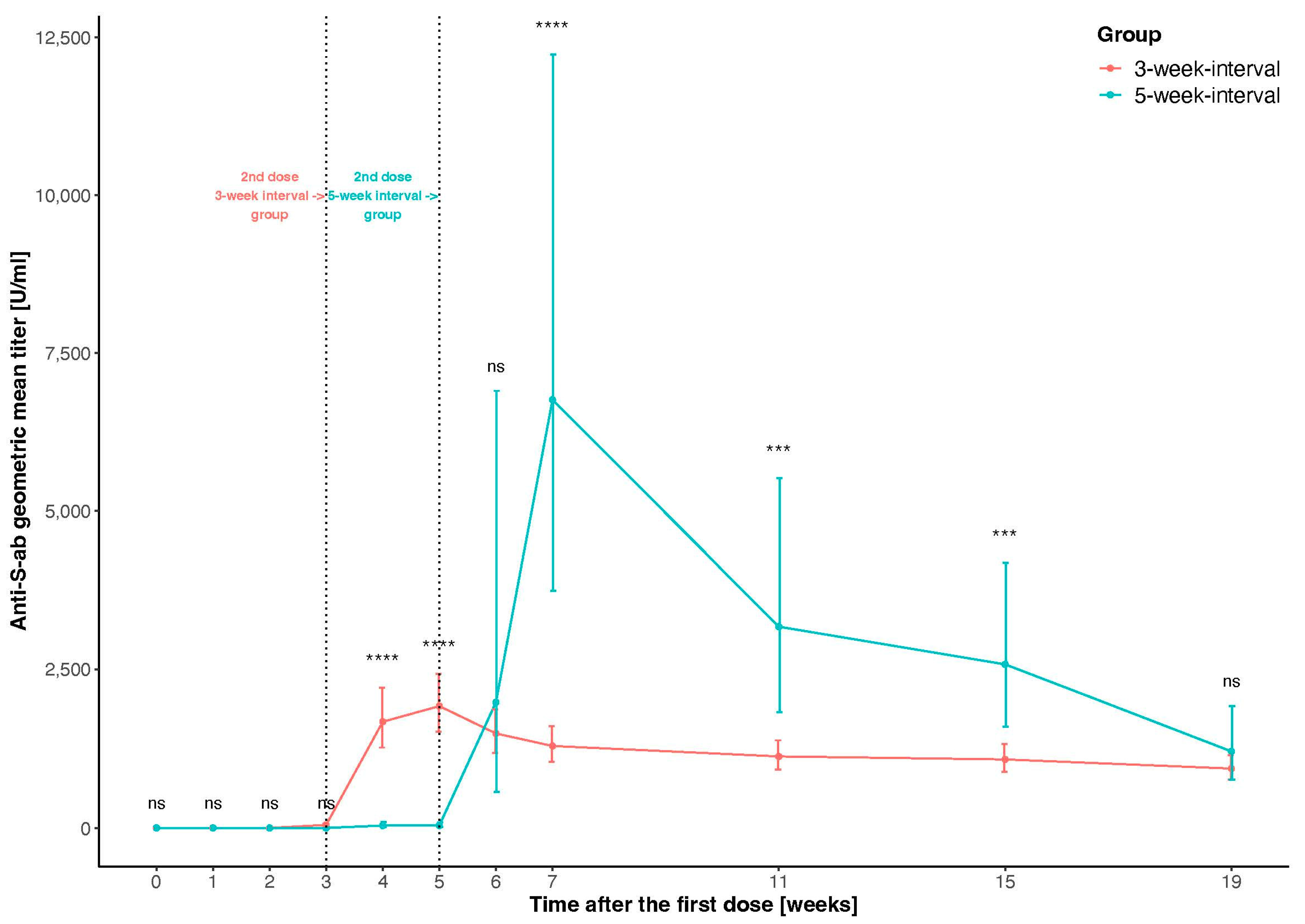
3.2. Comparison of Antibody Response to Vaccination between Groups
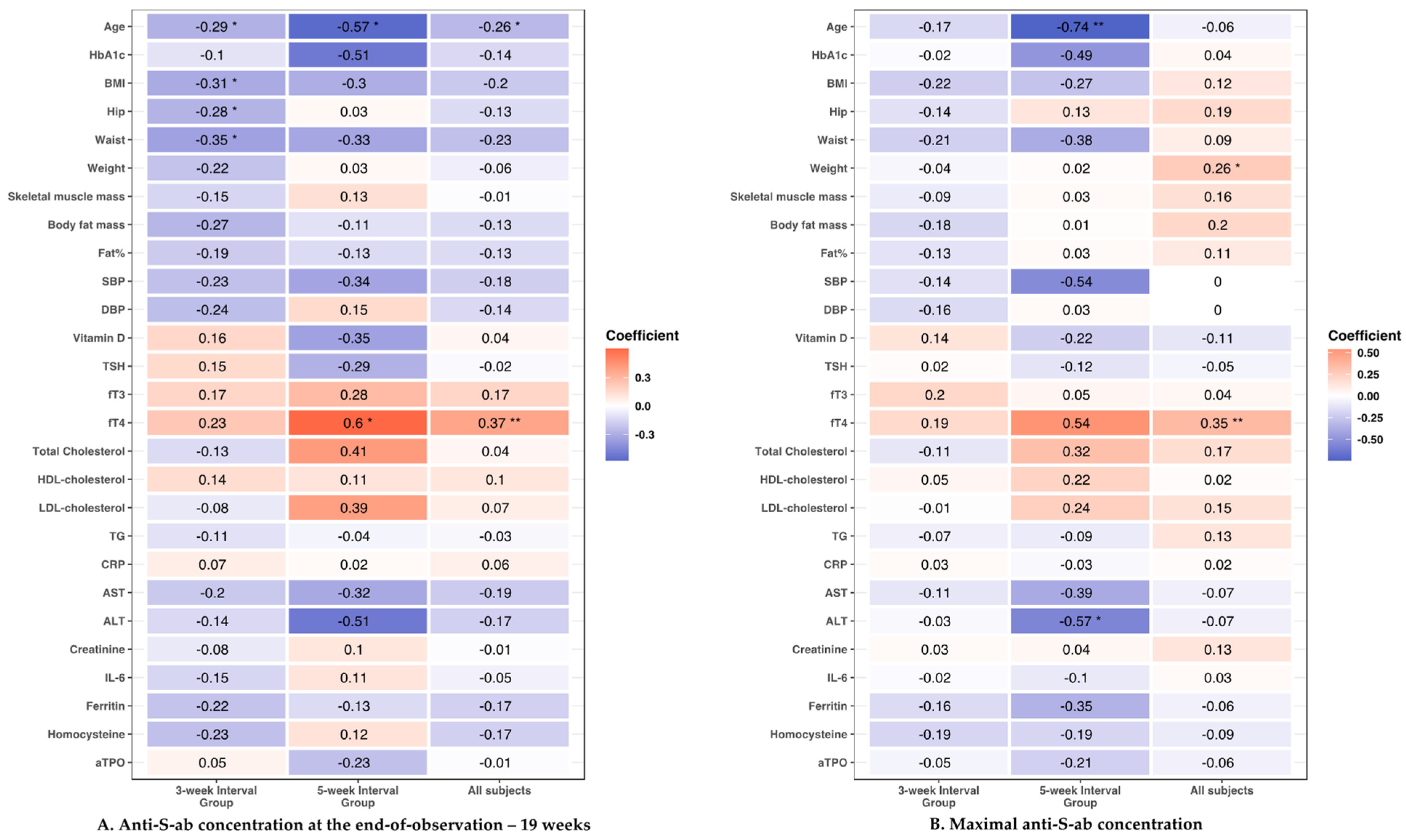
3.3. Clinical Parameters and Antibody Response to the Vaccine
3.3.1. Age
3.3.2. Anthropometric Parameters
3.3.3. Cardiometabolic Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 14.9 Million Excess Deaths Associated with the COVID-19 Pandemic in 2020 and 2021. Available online: https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021 (accessed on 11 June 2022).
- Vokó, Z.; Kiss, Z.; Surján, G.; Surján, O.; Barcza, Z.; Pályi, B.; Formanek-Balku, E.; Molnár, G.A.; Herczeg, R.; Gyenesei, A.; et al. Nationwide Effectiveness of Five SARS-CoV-2 Vaccines in Hungary—The HUN-VE Study. Clin. Microbiol. Infect. 2022, 28, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions—United States, March–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Bajema, K.L.; Dahl, R.M.; Evener, S.L.; Prill, M.M.; Rodriguez-Barradas, M.C.; Marconi, V.C.; Beenhouwer, D.O.; Holodniy, M.; Lucero-Obusan, C.; Brown, S.T.; et al. Comparative Effectiveness and Antibody Responses to Moderna and Pfizer-BioNTech COVID-19 Vaccines among Hospitalized Veterans—Five Veterans Affairs Medical Centers, United States, February 1–September 30, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, M.; Lin, J.; Vaghela, S.; Lingohr-Smith, M.; Nguyen, J.L.; Scassellati Sforzolini, T.; Judy, J.; Cane, A.; Moran, M.M. COVID-19 Vaccine Effectiveness among Immunocompromised Populations: A Targeted Literature Review of Real-World Studies. Expert Rev. Vaccines 2022, 21, 435–451. [Google Scholar] [CrossRef]
- Nanduri, S.; Pilishvili, T.; Derado, G.; Soe, M.M.; Dollard, P.; Wu, H.; Li, Q.; Bagchi, S.; Dubendris, H.; Link-Gelles, R.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant—National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1163–1166. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-World Effectiveness of COVID-19 Vaccines: A Literature Review and Meta-Analysis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca Vaccines on COVID-19 Related Symptoms, Hospital Admissions, and Mortality in Older Adults in England: Test Negative Case-Control Study. BMJ (Clin. Res. Ed.) 2021, 373, n1088. [Google Scholar] [CrossRef]
- Li, D.-D.; Li, Q.-H. SARS-CoV-2: Vaccines in the Pandemic Era. Mil. Med. Res. 2021, 8, 1. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and MRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Bonato, M.; Semenzato, U.; Tinè, M.; Bazzan, E.; Damin, M.; Biondini, D.; Casara, A.; Romagnoli, M.; Turato, G.; Cosio, M.G.; et al. Risk Factors for Development and Severity of COVID-19 in COPD Patients. Front. Med. 2021, 8, 714570. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-Project.Org/ (accessed on 10 August 2022).
- Grunau, B.; Goldfarb, D.M.; Asamoah-Boaheng, M.; Golding, L.; Kirkham, T.L.; Demers, P.A.; Lavoie, P.M. Immunogenicity of Extended MRNA SARS-CoV-2 Vaccine Dosing Intervals. JAMA 2022, 327, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Ikeda, K.; Tanaka, S.; Taniguchi, T.; Igari, H.; Onouchi, Y.; Kaneda, A.; Matsushita, K.; Hanaoka, H.; Nakada, T.-A.; et al. Antibody Responses to BNT162b2 MRNA COVID-19 Vaccine and Their Predictors among Healthcare Workers in a Tertiary Referral Hospital in Japan. Clin. Microbiol. Infect. 2021, 27, 1861.e1–1861.e5. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of Standard and Extended Dosing Intervals of BNT162b2 MRNA Vaccine. Cell 2021, 184, 5699–5714.e11. [Google Scholar] [CrossRef]
- Amirthalingam, G.; Bernal, J.L.; Andrews, N.J.; Whitaker, H.; Gower, C.; Stowe, J.; Tessier, E.; Subbarao, S.; Ireland, G.; Baawuah, F.; et al. Serological Responses and Vaccine Effectiveness for Extended COVID-19 Vaccine Schedules in England. Nat. Commun. 2021, 12, 7217. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Wood, H.; Ierullo, M.; Majchrzak-Kita, B.; Manguiat, K.; Robinson, A.; Kulasingam, V.; Humar, A.; Kumar, D. Delayed-Interval BNT162b2 MRNA COVID-19 Vaccination Enhances Humoral Immunity and Induces Robust T Cell Responses. Nat. Immunol. 2022, 23, 380–385. [Google Scholar] [CrossRef]
- Zhu, F.; Althaus, T.; Tan, C.W.; Costantini, A.; Chia, W.N.; Chau, N.V.V.; Tan, L.V.; Mattiuzzo, G.; Rose, N.J.; Voiglio, E.; et al. WHO International Standard for SARS-CoV-2 Antibodies to Determine Markers of Protection. Lancet Microbe 2022, 3, e81–e82. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial Observations on Age, Gender, BMI and Hypertension in Antibody Responses to SARS-CoV-2 BNT162b2 Vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef]
- Yamamoto, S.; Mizoue, T.; Tanaka, A.; Oshiro, Y.; Inamura, N.; Konishi, M.; Ozeki, M.; Miyo, K.; Sugiura, W.; Sugiyama, H.; et al. Sex-associated Differences between BMI and SARS-CoV-2 Antibody Titers Following the BNT162b2 Vaccine. Obesity 2022, 30, 999–1003. [Google Scholar] [CrossRef]
- Nam, S.Y.; Jeon, S.W.; Lee, H.S.; Lim, H.J.; Lee, D.W.; Yoo, S.S. Demographic and Clinical Factors Associated With Anti–SARS-CoV-2 Antibody Levels After 2 BNT162b2 MRNA Vaccine Doses. JAMA Netw. Open 2022, 5, e2212996. [Google Scholar] [CrossRef]
- Painter, S.D.; Ovsyannikova, I.G.; Poland, G.A. The Weight of Obesity on the Human Immune Response to Vaccination. Vaccine 2015, 33, 4422–4429. [Google Scholar] [CrossRef]
- Neidich, S.D.; Green, W.D.; Rebeles, J.; Karlsson, E.A.; Schultz-Cherry, S.; Noah, T.L.; Chakladar, S.; Hudgens, M.G.; Weir, S.S.; Beck, M.A. Increased Risk of Influenza among Vaccinated Adults Who Are Obese. Int. J. Obes. 2017, 41, 1324–1330. [Google Scholar] [CrossRef] [Green Version]
- Frasca, D.; Blomberg, B.B. Adipose Tissue Inflammation Induces B Cell Inflammation and Decreases B Cell Function in Aging. Front. Immunol. 2017, 8, 1003. [Google Scholar] [CrossRef] [Green Version]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, Inflammation and the Immune System. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central Obesity, Smoking Habit, and Hypertension Are Associated with Lower Antibody Titres in Response to COVID-19 MRNA Vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef]
- Bayart, J.-L.; Morimont, L.; Closset, M.; Wieërs, G.; Roy, T.; Gerin, V.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Ausselet, N.; et al. Confounding Factors Influencing the Kinetics and Magnitude of Serological Response Following Administration of BNT162b2. Microorganisms 2021, 9, 1340. [Google Scholar] [CrossRef]
- Golec, M.; Fronczek, M.; Zembala-John, J.; Chrapiec, M.; Konka, A.; Wystyrk, K.; Botor, H.; Brzoza, Z.; Kasperczyk, S.; Bułdak, R.J. Early and Longitudinal Humoral Response to the SARS-CoV-2 MRNA BNT162b2 Vaccine in Healthcare Workers: Significance of BMI, Adipose Tissue and Muscle Mass on Long-Lasting Post-Vaccinal Immunity. Viruses 2022, 14, 868. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.; Liacos, C.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and Gender-dependent Antibody Responses against SARS-CoV-2 in Health Workers and Octogenarians after Vaccination with the BNT162b2 MRNA Vaccine. Am. J. Hematol. 2021, 96, E257–E259. [Google Scholar] [CrossRef]
- Parthymou, A.; Habeos, E.E.; Habeos, G.I.; Deligakis, A.; Livieratos, E.; Marangos, M.; Chartoumpekis, D.V. Factors Associated with Anti-SARS-CoV-2 Antibody Titres 3 Months Post-Vaccination with the Second Dose of BNT162b2 Vaccine: A Longitudinal Observational Cohort Study in Western Greece. BMJ Open 2022, 12, e057084. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Personalized Vaccinology: A Review. Vaccine 2018, 36, 5350–5357. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Blomberg, B.B. The Generation of Memory B Cells Is Maintained, but the Antibody Response Is Not, in the Elderly after Repeated Influenza Immunizations. Vaccine 2016, 34, 2834–2840. [Google Scholar] [CrossRef]
- Ponti, G.; Ruini, C.; Tomasi, A. Homocysteine as a Potential Predictor of Cardiovascular Risk in Patients with COVID-19. Med. Hypotheses 2020, 143, 109859. [Google Scholar] [CrossRef]
- Ponti, G.; Pastorino, L.; Manfredini, M.; Ozben, T.; Oliva, G.; Kaleci, S.; Iannella, R.; Tomasi, A. COVID-19 Spreading across World Correlates with C677T Allele of the Methylenetetrahydrofolate Reductase (MTHFR) Gene Prevalence. J. Clin. Lab. Anal. 2021, 35, e23798. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, J.; He, Z.; Lü, Y.; Xu, Q.; Ye, C.; Chen, S.; Tang, B.; Yin, K.; Lu, Y.; et al. Predictors for Imaging Progression on Chest CT from Coronavirus Disease 2019 (COVID-19) Patients. Aging 2020, 12, 6037–6048. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, W.; Xu, W. Thyroid Function Analysis in 50 Patients with COVID-19: A Retrospective Study. Thyroid 2021, 31, 8–11. [Google Scholar] [CrossRef]
- Chen, W.; Tian, Y.; Li, Z.; Zhu, J.; Wei, T.; Lei, J. Potential Interaction Between SARS-CoV-2 and Thyroid: A Review. Endocrinology 2021, 162, bqab004. [Google Scholar] [CrossRef]
- İremli, B.G.; Şendur, S.N.; Ünlütürk, U. Three Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccine: Postvaccination ASIA Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 2600–2605. [Google Scholar] [CrossRef]
- Saygılı, E.S.; Karakilic, E. Subacute Thyroiditis after Inactive SARS-CoV-2 Vaccine. BMJ Case Rep. 2021, 14, e244711. [Google Scholar] [CrossRef]
- Bornemann, C.; Woyk, K.; Bouter, C. Case Report: Two Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccination. Front. Med. 2021, 8, 737142. [Google Scholar] [CrossRef]
- Paschou, S.A.; Karalis, V.; Psaltopoulou, T.; Vasileiou, V.; Charitaki, I.; Bagratuni, T.; Ktena, V.; Papandroulaki, F.; Gumeni, S.; Kassi, G.N.; et al. Patients With Autoimmune Thyroiditis Present Similar Immunological Response to COVID-19 BNT162b2 MRNA Vaccine With Healthy Subjects, While Vaccination May Affect Thyroid Function: A Clinical Study. Front. Endocrinol. 2022, 13, 840668. [Google Scholar] [CrossRef]
- Gandon, S.; Lion, S. Targeted Vaccination and the Speed of SARS-CoV-2 Adaptation. Proc. Natl. Acad. Sci. USA 2022, 119, e2110666119. [Google Scholar] [CrossRef]
- Dushoff, J.; Colijn, C.; Earn, D.J.D.; Bolker, B.M. Transmission Dynamics Are Crucial to COVID-19 Vaccination Policy. Proc. Natl. Acad. Sci. USA 2021, 118, e2105878118. [Google Scholar] [CrossRef]
| 3-Week Interval Group | 5-Week Interval Group | p-Value | |||
|---|---|---|---|---|---|
| Parameters: | Mean | sd (+/−) | Mean | sd (+/−) | |
| Age (years) | 35.94 | 13.21 | 50.62 | 16.07 | 0.003 |
| HbA1c (%) | 5.33 | 0.62 | 6.18 | 1.11 | 0.013 |
| BMI (kg/m2) | 25.38 | 4.52 | 30.85 | 5.27 | 0.001 |
| Weight (kg) | 71.82 | 15.32 | 89.82 | 13.52 | 0.001 |
| Skeletal muscle mass (kg) | 27.52 | 6.30 | 32.40 | 7.48 | 0.020 |
| Body fat mass (kg) | 21.90 | 8.86 | 31.79 | 9.96 | 0.005 |
| Fat% | 29.88 | 9.18 | 35.34 | 9.78 | 0.085 |
| SBP (mmHg) | 126.02 | 19.64 | 141.38 | 17.24 | 0.011 |
| DBP (mmHg) | 82.16 | 13.05 | 84.15 | 11.62 | 0.600 |
| Vitamin D (ng/mL) | 28.99 | 12.94 | 24.26 | 9.82 | 0.160 |
| TSH (uIU/mL) | 1.68 | 0.68 | 1.69 | 1.43 | 0.580 |
| fT3 (pg/mL) | 3.51 | 0.48 | 3.44 | 0.38 | 0.760 |
| fT4 (ng/dL) | 1.30 | 0.15 | 1.33 | 0.29 | 0.670 |
| Total cholesterol (mg/dL) | 196.22 | 33.81 | 208.85 | 42.45 | 0.330 |
| HDL-cholesterol (mg/dL) | 60.02 | 13.74 | 55.32 | 18.17 | 0.390 |
| LDL-cholesterol (mg/dL) | 96.99 | 27.39 | 104.41 | 33.53 | 0.470 |
| TG (mg/dL) | 130.94 | 79.38 | 199.69 | 117.98 | 0.036 |
| CRP (mg/L) | 2.12 | 3.21 | 2.38 | 2.54 | 0.550 |
| AST (U/L) | 21.68 | 13.83 | 23.49 | 7.66 | 0.140 |
| ALT (U/L) | 7.53 | 6.66 | 9.95 | 5.50 | 0.025 |
| Creatinine (µmol/L) | 70.88 | 14.89 | 77.73 | 15.36 | 0.100 |
| IL-6 (pg/mL) | 1.23 | 1.59 | 1.78 | 2.25 | 0.450 |
| Ferritin (ng/mL) | 91.05 | 123.83 | 132.52 | 102.04 | 0.033 |
| Homocysteine (µmol/L) | 11.73 | 5.79 | 11.88 | 3.30 | 0.270 |
| aTPO (IU/mL) | 16.75 | 49.76 | 19.54 | 47.41 | 0.510 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczerbiński, Ł.; Okruszko, M.A.; Szabłowski, M.; Warpechowski, J.; Paszko, A.; Citko, A.; Konopka, P.; Bauer, W.; Krętowski, A.J. Immune Response to Vaccination against COVID-19 at Different Second-Dose Intervals and Their Associations with Metabolic Parameters. Vaccines 2023, 11, 149. https://doi.org/10.3390/vaccines11010149
Szczerbiński Ł, Okruszko MA, Szabłowski M, Warpechowski J, Paszko A, Citko A, Konopka P, Bauer W, Krętowski AJ. Immune Response to Vaccination against COVID-19 at Different Second-Dose Intervals and Their Associations with Metabolic Parameters. Vaccines. 2023; 11(1):149. https://doi.org/10.3390/vaccines11010149
Chicago/Turabian StyleSzczerbiński, Łukasz, Michał Andrzej Okruszko, Maciej Szabłowski, Jędrzej Warpechowski, Adam Paszko, Anna Citko, Paulina Konopka, Witold Bauer, and Adam Jacek Krętowski. 2023. "Immune Response to Vaccination against COVID-19 at Different Second-Dose Intervals and Their Associations with Metabolic Parameters" Vaccines 11, no. 1: 149. https://doi.org/10.3390/vaccines11010149





