Efficacy and Safety of Quadrivalent Conjugate Meningococcal Vaccines: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
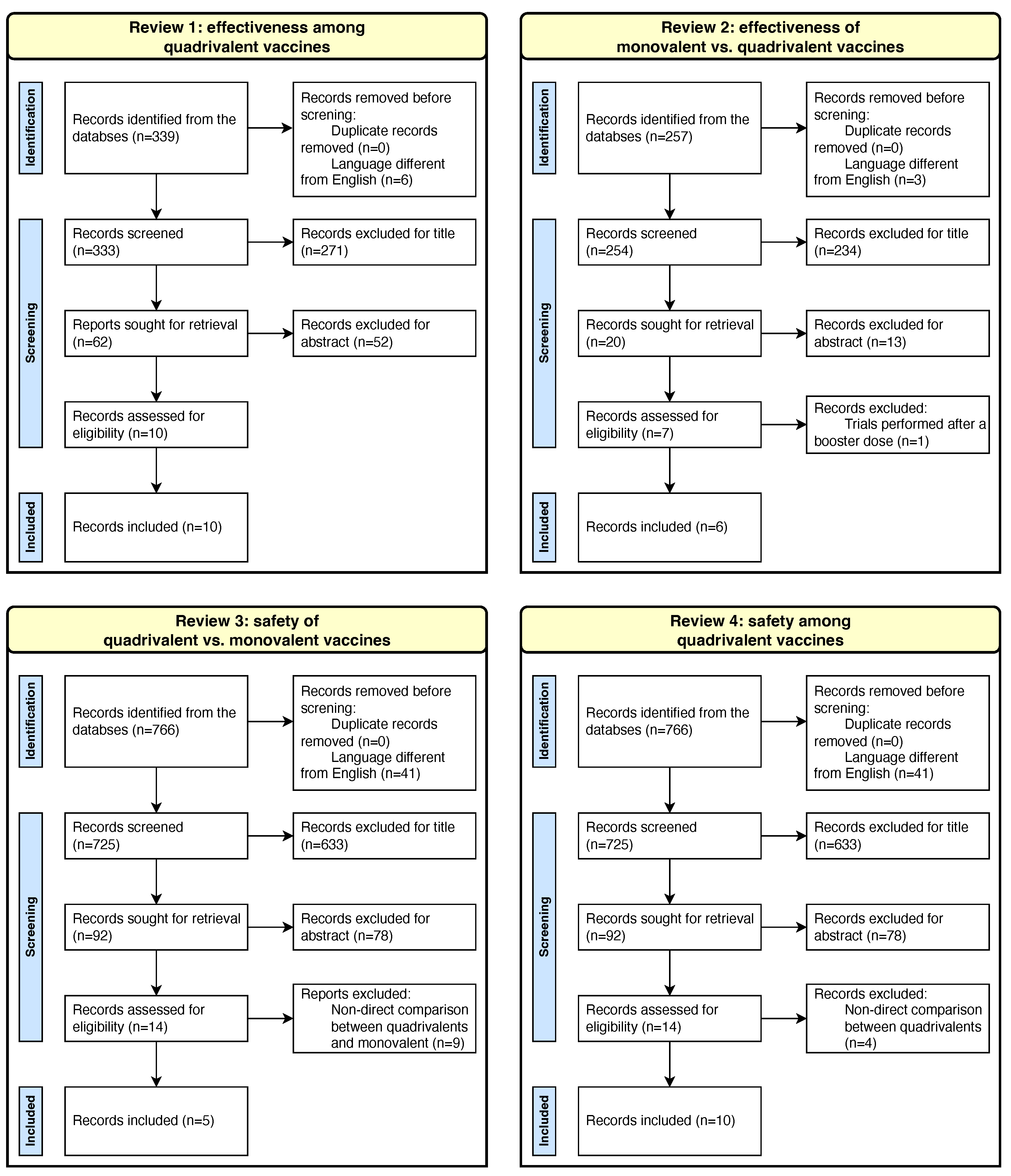
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction, Analysis, and Synthesis
3. Results
3.1. Review 1: Efficacy Comparison among Different MenACWY Vaccines
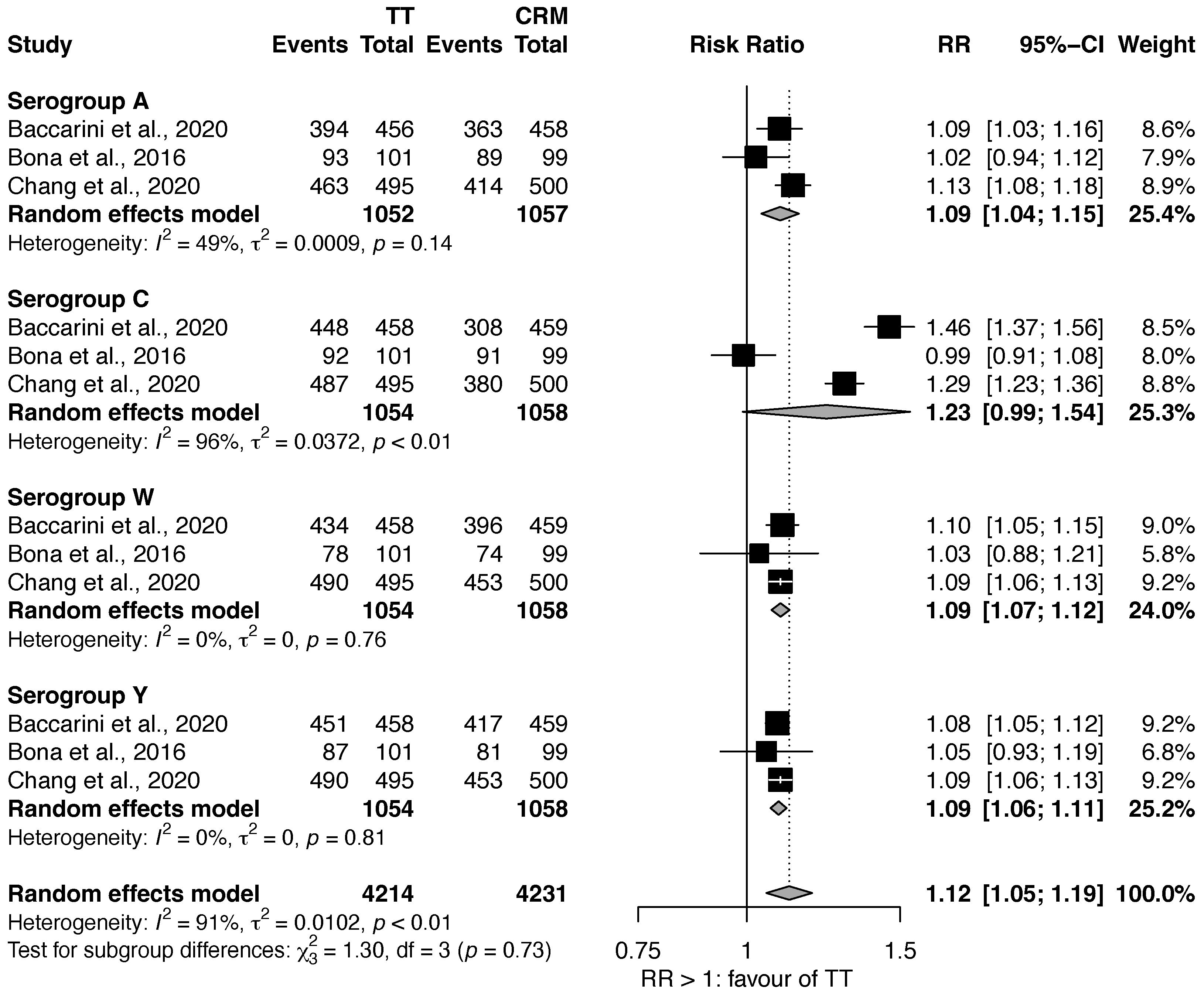
3.1.1. MenACWY-TT vs. MenACWY-CRM
3.1.2. MenACWY-TT vs. MenACWY-D
3.1.3. MenACWY-D vs. MenACWY-CRM
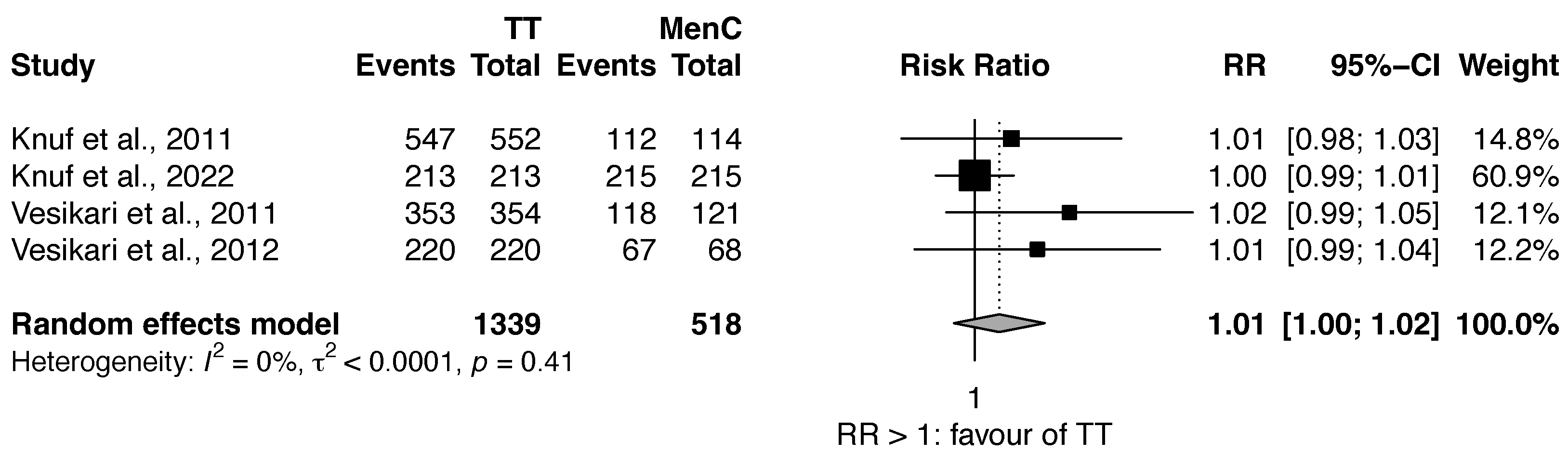
3.2. Review 2: Efficacy Comparison of Quadrivalent MenACWY vs. MenC Vaccines
3.3. Review 3: Safety Comparison of MenACWY vs. MenC
3.4. Review 4: Safety Comparison among Different Quadrivalent Meningococcal Vaccines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IMD | Invasive meningococcal disease |
| MenC | Monovalent serogroup C meningococcal conjugate vaccine |
| MenACWY | Quadrivalent meningococcal vaccine against serogroups A, C, W-135, and Y |
| MenACWY-D | Diphtheria toxoid conjugate vaccine |
| MenACWY-CRM | CRM197 protein conjugate vaccine |
| MenACWY-TT | Tetanus toxoid conjugate vaccine |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SBA | Serum bactericidal activity |
| MenPS | Quadrivalent meningococcal conjugate vaccine |
| AE | Adverse event |
| RR | Risk ratio |
| CI | Confidence interval |
Appendix A

References
- Wang, B.; Santoreneos, R.; Giles, L.; Haji Ali Afzali, H.; Marshall, H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine 2019, 37, 2768–2782. [Google Scholar] [CrossRef]
- Pace, D.; Pollard, A.J. Meningococcal disease: Clinical presentation and sequelae. Vaccine 2012, 30, B3–B9. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Santoreneos, R.; Afzali, H.; Giles, L.; Marshall, H. Costs of Invasive Meningococcal Disease: A Global Systematic Review. PharmacoEconomics 2018, 36, 1201–1222. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, P.; Perrone, V.; Radice, S.; Capuano, A.; Clementi, E. Immunogenicity of meningococcal quadrivalent (serogroup A, C, W135 and Y) tetanus toxoid conjugate vaccine: Systematic review and meta-analysis. Pharmacol. Res. 2015, 92, 31–39. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Meningococcal vaccines: WHO position paper, November 2011. Relev. Epidemiol. Hebd. 2011, 86, 521–539. [Google Scholar]
- Pelton, S.I. The Global Evolution of Meningococcal Epidemiology Following the Introduction of Meningococcal Vaccines. J. Adolesc. Health 2016, 59, S3–S11. [Google Scholar] [CrossRef] [Green Version]
- Pizza, M.; Bekkat-Berkani, R.; Rappuoli, R. Vaccines against Meningococcal Diseases. Microorganisms 2020, 8, 1521. [Google Scholar] [CrossRef]
- Trotter, C.L.; Lingani, C.; Fernandez, K.; Cooper, L.V.; Bita, A.; Tevi-Benissan, C.; Ronveaux, O.; Préziosi, M.P.; Stuart, J.M. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: An analysis of surveillance data. Lancet Infect. Dis. 2017, 17, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Tin Tin Htar, M.; Jackson, S.; Balmer, P.; Serra, L.C.; Vyse, A.; Slack, M.; Riera-Montes, M.; Swerdlow, D.L.; Findlow, J. Systematic literature review of the impact and effectiveness of monovalent meningococcal C conjugated vaccines when used in routine immunization programs. BMC Public Health 2020, 20, 1890. [Google Scholar] [CrossRef]
- Parikh, S.R.; Campbell, H.; Bettinger, J.A.; Harrison, L.H.; Marshall, H.S.; Martinon-Torres, F.; Safadi, M.A.; Shao, Z.; Zhu, B.; von Gottberg, A.; et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J. Infect. 2020, 81, 483–498. [Google Scholar] [CrossRef]
- Campbell, H.; Saliba, V.; Borrow, R.; Ramsay, M.; Ladhani, S.N. Targeted vaccination of teenagers following continued rapid endemic expansion of a single meningococcal group W clone (sequence type 11 clonal complex), United Kingdom 2015. Eurosurveillance 2015, 20, 21188. [Google Scholar] [CrossRef] [PubMed]
- Martinón-Torres, F.; Taha, M.K.; Knuf, M.; Abbing-Karahagopian, V.; Pellegrini, M.; Bekkat-Berkani, R.; Abitbol, V. Evolving strategies for meningococcal vaccination in Europe: Overview and key determinants for current and future considerations. Pathog. Glob. Health 2022, 116, 85–98. [Google Scholar] [CrossRef]
- Zahlanie, Y.C.; Hammadi, M.M.; Ghanem, S.T.; Dbaibo, G.S. Review of meningococcal vaccines with updates on immunization in adults. Hum. Vaccines Immunother. 2014, 10, 995–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 10, 89. [Google Scholar] [CrossRef]
- Granoff, D.M. Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease. Vaccine 2009, 27, B117–B125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrow, R.; Balmer, P.; Miller, E. Meningococcal surrogates of protection?serum bactericidal antibody activity. Vaccine 2005, 23, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccarini, C.I.; Simon, M.W.; Brandon, D.; Christensen, S.; Jordanov, E.; Dhingra, M.S. Safety and Immunogenicity of a Quadrivalent Meningococcal Conjugate Vaccine in Healthy Meningococcal-Naïve Children 2–9 Years of Age: A Phase III, Randomized Study. Pediatr. Infect. Dis. J. 2020, 39, 955–960. [Google Scholar] [CrossRef]
- Baxter, R.; Baine, Y.; Ensor, K.; Bianco, V.; Friedland, L.R.; Miller, J.M. Immunogenicity and Safety of an Investigational Quadrivalent Meningococcal ACWY Tetanus Toxoid Conjugate Vaccine in Healthy Adolescents and Young Adults 10 to 25 Years of Age. Pediatr. Infect. Dis. J. 2011, 30, e41–e48. [Google Scholar] [CrossRef]
- Bona, G.; Castiglia, P.; Zoppi, G.; de Martino, M.; Tasciotti, A.; D’Agostino, D.; Han, L.; Smolenov, I. Safety and immunogenicity of a CRM or TT conjugated meningococcal vaccine in healthy toddlers. Vaccine 2016, 34, 3363–3370. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.J.; Hedrick, J.; Christensen, S.; Pan, J.; Jordanov, E.; Dhingra, M.S. A Phase II, randomized, immunogenicity and safety study of a quadrivalent meningococcal conjugate vaccine, MenACYW-TT, in healthy adolescents in the United States. Vaccine 2020, 38, 3560–3569. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, M.S.; Peterson, J.; Hedrick, J.; Pan, J.; Neveu, D.; Jordanov, E. Immunogenicity, safety and inter-lot consistency of a meningococcal conjugate vaccine (MenACYW-TT) in adolescents and adults: A Phase III randomized study. Vaccine 2020, 38, 5194–5201. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Gupta, A.; Jeanfreau, R.; Klein, N.P.; Reisinger, K.; Walter, E.; Bedell, L.; Gill, C.; Dull, P.M. Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2–10 years of age. Vaccine 2010, 28, 7865–7872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halperin, S.A.; Baine, Y.; Domachowske, J.B.; Aggarwal, N.; Simon, M.; Langley, J.M.; McNeil, S.A.; Friedland, L.R.; Bianco, V.; Baccarini, C.I.; et al. Comparison of the Safety and Immunogenicity of a Novel Quadrivalent Meningococcal ACWY-Tetanus Toxoid Conjugate Vaccine and a Marketed Quadrivalent Meningococcal ACWY-Diphtheria Toxoid Conjugate Vaccine in Healthy Individuals 10–25 Years of Age. J. Pediatr. Infect. Dis. Soc. 2014, 3, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Jackson, L.; Baxter, R.; Reisinger, K.; Karsten, A.; Shah, J.; Bedell, L.; Dull, P.; the V59P13 Study Group. Phase III Comparison of an Investigational Quadrivalent Meningococcal Conjugate Vaccine with the Licensed Meningococcal ACWY Conjugate Vaccine in Adolescents. Clin. Infect. Dis. 2009, 49, e1–e10. [Google Scholar] [CrossRef] [Green Version]
- Knuf, M.; Kieninger-Baum, D.; Habermehl, P.; Muttonen, P.; Maurer, H.; Vink, P.; Poolman, J.; Boutriau, D. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine 2010, 28, 744–753. [Google Scholar] [CrossRef]
- Knuf, M.; Pantazi-Chatzikonstantinou, A.; Pfletschinger, U.; Tichmann-Schumann, I.; Maurer, H.; Maurer, L.; Fischbach, T.; Zinke, H.; Pankow-Culot, H.; Papaevangelou, V.; et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix™ hexa is immunogenic, with an acceptable safety profile in 12–23-month-old children. Vaccine 2011, 29, 4264–4273. [Google Scholar] [CrossRef]
- Knuf, M.; Romain, O.; Kindler, K.; Walther, U.; Tran, P.M.; Pankow-Culot, H.; Fischbach, T.; Kieninger-Baum, D.; Bianco, V.; Baine, Y.; et al. Immunogenicity and safety of the quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) in 2–10-year-old children: Results of an open, randomised, controlled study. Eur. J. Pediatr. 2013, 172, 601–612. [Google Scholar] [CrossRef] [Green Version]
- Knuf, M.; Rämet, M.; Breinholt Stærke, N.; Bertrand-Gerentes, I.; Thollot, Y.; B’Chir, S.; Arroum, H.; Oster, P. Comparing the meningococcal serogroup C immune response elicited by a tetanus toxoid conjugate quadrivalent meningococcal vaccine (MenACYW-TT) versus a quadrivalent or monovalent C tetanus toxoid conjugate meningococcal vaccine in healthy meningococcal vaccine-naïve toddlers: A randomised, controlled trial. Hum. Vaccines Immunother. 2022, 18, 2052657. [Google Scholar] [CrossRef]
- Reisinger, K.S.; Baxter, R.; Block, S.L.; Shah, J.; Bedell, L.; Dull, P.M. Quadrivalent Meningococcal Vaccination of Adults: Phase III Comparison of an Investigational Conjugate Vaccine, MenACWY-CRM, with the Licensed Vaccine, Menactra. Clin. Vaccine Immunol. 2009, 16, 1810–1815. [Google Scholar] [CrossRef] [Green Version]
- Stamboulian, D.; Lopardo, G.; Lopez, P.; Cortes-Barbosa, C.; Valencia, A.; Bedell, L.; Karsten, A.; Dull, P. Safety and immunogenicity of an investigational quadrivalent meningococcal CRM197 conjugate vaccine, MenACWY-CRM, compared with licensed vaccines in adults in Latin America. Int. J. Infect. Dis. 2010, 14, e868–e875. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Karvonen, A.; Bianco, V.; Van der Wielen, M.; Miller, J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles–mumps–rubella–varicella vaccine during the second year of life: An open, randomized controlled trial. Vaccine 2011, 29, 4274–4284. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Forstén, A.; Boutriau, D.; Bianco, V.; Van der Wielen, M.; Miller, J.M. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum. Vaccines Immunother. 2012, 8, 1892–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbaeyi, S.A.; Bozio, C.H.; Duffy, J.; Rubin, L.G.; Hariri, S.; Stephens, D.S.; MacNeil, J.R. Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm. Rep. 2020, 69, 1–41. [Google Scholar] [CrossRef]
- World Health Organization. Global Manual on Surveillance of Adverse Events Following Immunization; WHO Document Production Services: Geneva, Switzerland, 2016; ISBN 978-92-4150776-9. [Google Scholar]
- Kim, J.J. The Role of Cost-Effectiveness in U.S. Vaccination Policy. N. Engl. J. Med. 2011, 365, 1760–1761. [Google Scholar] [CrossRef] [PubMed]
- Watle, S.V.; Næss, L.M.; Tunheim, G.; Caugant, D.A.; Wisløff, T. Cost-effectiveness of meningococcal vaccination of Norwegian teenagers with a quadrivalent ACWY conjugate vaccine. Hum. Vaccines Immunother. 2021, 17, 2777–2787. [Google Scholar] [CrossRef] [PubMed]
- Hepkema, H.; Pouwels, K.B.; van der Ende, A.; Westra, T.A.; Postma, M.J. Meningococcal Serogroup A, C, W135 and Y Conjugated Vaccine: A Cost-Effectiveness Analysis in the Netherlands. PLoS ONE 2013, 8, e65036. [Google Scholar] [CrossRef] [Green Version]
- Si, S.; Zomer, E.; Fletcher, S.; Lee, J.; Liew, D. Cost-effectiveness of meningococcal polysaccharide serogroups A, C, W-135 and Y conjugate vaccine in Australian adolescents. Vaccine 2019, 37, 5009–5015. [Google Scholar] [CrossRef]
- Delea, T.E.; Weycker, D.; Atwood, M.; Neame, D.; Alvarez, F.P.; Forget, E.; Langley, J.M.; Chit, A. Cost-effectiveness of alternate strategies for childhood immunization against meningococcal disease with monovalent and quadrivalent conjugate vaccines in Canada. PLoS ONE 2017, 12, e0175721. [Google Scholar] [CrossRef] [Green Version]
- CDC Vaccine Price List. Available online: https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html (accessed on 15 December 2022).
- Findlow, J.; Balmer, P.; Borrow, R. A review of complement sources used in serum bactericidal assays for evaluating immune responses to meningococcal ACWY conjugate vaccines. Hum. Vaccines Immunother. 2019, 15, 2491–2500. [Google Scholar] [CrossRef] [Green Version]
- Bekkat-Berkani, R.; Fragapane, E.; Preiss, S.; Rappuoli, R.; Sohn, W.Y.; Soumahoro, L.; Vadivelu, K. Public health perspective of a pentavalent meningococcal vaccine combining antigens of MenACWY-CRM and 4CMenB. J. Infect. 2022, 85, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Deal, A.; Halliday, R.; Crawshaw, A.F.; Hayward, S.E.; Burnard, A.; Rustage, K.; Carter, J.; Mehrotra, A.; Knights, F.; Campos-Matos, I.; et al. Migration and outbreaks of vaccine-preventable disease in Europe: A systematic review. Lancet Infect. Dis. 2021, 21, e387–e398. [Google Scholar] [CrossRef] [PubMed]
| Review | String | Objective |
|---|---|---|
| 1 | “Meningococcal Vaccines”[Mesh] AND quadrivalent | To compare the efficacy of different quadrivalent vaccines. |
| 2 | “Vaccines”[Majr] AND menc | To compare the efficacy of quadrivalent and monovalent vaccines. |
| 3 | “Meningococcal Vaccines/adverse effects”[Mesh] OR (“Meningococcal Vaccines”[Mesh] AND safety) | To compare safety of monovalent and quadrivalent vaccines. |
| 4 | “Meningococcal Vaccines/adverse effects”[Mesh] OR (“Meningococcal Vaccines”[Mesh] AND safety) | To compare safety among different quadrivalent vaccines. |
| Study | Countries | N | Subject Age | Aim | Review | Vaccines | Study Design |
|---|---|---|---|---|---|---|---|
| Baccarini et al. [18] | United States, Puerto Rico | 1000 | 2–9 years | E + S | 1, 4 | MenACWY-TT, MenACWY-CRM | Ph3, double-blind |
| Baxter et al. [19] | United States | 784 | 10–25 years | E + S | 1, 4 | MenACWY-TT MenACWY-D, | Ph2 single-blind |
| Bona et al. [20] | Italy | 202 | 12–15 months | E + S | 1, 4 | MenACWY-TT, MenACWY-CRM | Ph2 single-blind |
| Chang et al. [21] | United States | 1715 | 10–17 years | E + S | 1, 4 | MenACWY-TT, MenACWY-CRM | Ph2 open-label |
| Dhingra et al. [22] | United States | 3344 | 10–55 years | E + S | 1, 4 | MenACWY-TT MenACWY-D, | Ph3 modified double-blind |
| Halperin et al. [23] | United States, Canada | 2907 | 2–5 years | E + S | 1, 4 | MenACWY-D, MenACWY-CRM | Ph3 single-blind |
| Halperin et al. [24] | United States, Canada | 1016 | 10–25 years | E + S | 1, 4 | MenACWY-TT, MenACWY-D | Ph2 observer-blind |
| Jackson et al. [25] | United States | 2180 | 11–18 years | E + S | 1, 4 | MenACWY-D MenACWY-CRM, | Ph3 observer-blind |
| Knuf et al. [26] | Germany, Austria | 508 | 1–5 years | E + S | 2, 3 | MenACWY-TT, MenC | Ph2 double-blind |
| Knuf et al. [27] | Austria, Germany, Greece | 793 | 12–23 months | E + S | 2 | MenACWY-TT, MenC | Ph3 open |
| Knuf et al. [28] | Germany, France | 413 | 2–10 years | E | 2, 3 | MenACWY-TT, MenC | Ph3 open |
| Knuf et al. [29] | Denmark, Germany, Finland | 707 | 12–23 months | E + S | 2, 3 | MenACWY-TT, MenC | Ph3 double-blind |
| Reisinger et al. [30] | United States | 1359 | 19–55 years | E + S | 1, 4 | MenACWY-D, MenACWY-CRM | Ph3 open |
| Stamboulian et al. [31] | Latin America | 2505 | 19–65 years | E + S | 1, 4 | MenACWY-D, MenACWY-CRM | Ph3 observer-blind |
| Vesikari et al. [32] | Finland | 1000 | 12–23 months | E + S | 2, 3 | MenACWY-TT, MenC | Ph3 single-blind |
| Vesikari et al. [33] | Finland | 304 | 12–23 months | E + S | 2, 3 | MenACWY-TT, MenC | Ph2 open |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, A.; Broglia, G.; Sacchi, C.; Risi, F.; Barone-Adesi, F.; Panella, M. Efficacy and Safety of Quadrivalent Conjugate Meningococcal Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 178. https://doi.org/10.3390/vaccines11010178
Conti A, Broglia G, Sacchi C, Risi F, Barone-Adesi F, Panella M. Efficacy and Safety of Quadrivalent Conjugate Meningococcal Vaccines: A Systematic Review and Meta-Analysis. Vaccines. 2023; 11(1):178. https://doi.org/10.3390/vaccines11010178
Chicago/Turabian StyleConti, Andrea, Gaia Broglia, Chiara Sacchi, Fabrizia Risi, Francesco Barone-Adesi, and Massimiliano Panella. 2023. "Efficacy and Safety of Quadrivalent Conjugate Meningococcal Vaccines: A Systematic Review and Meta-Analysis" Vaccines 11, no. 1: 178. https://doi.org/10.3390/vaccines11010178
APA StyleConti, A., Broglia, G., Sacchi, C., Risi, F., Barone-Adesi, F., & Panella, M. (2023). Efficacy and Safety of Quadrivalent Conjugate Meningococcal Vaccines: A Systematic Review and Meta-Analysis. Vaccines, 11(1), 178. https://doi.org/10.3390/vaccines11010178