A Retrospective Study of the Safety and Immunogenicity of MVC-COV1901 Vaccine for People Living with HIV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
3. Results
3.1. Study Population
3.2. Safety
3.3. Immunogenicity
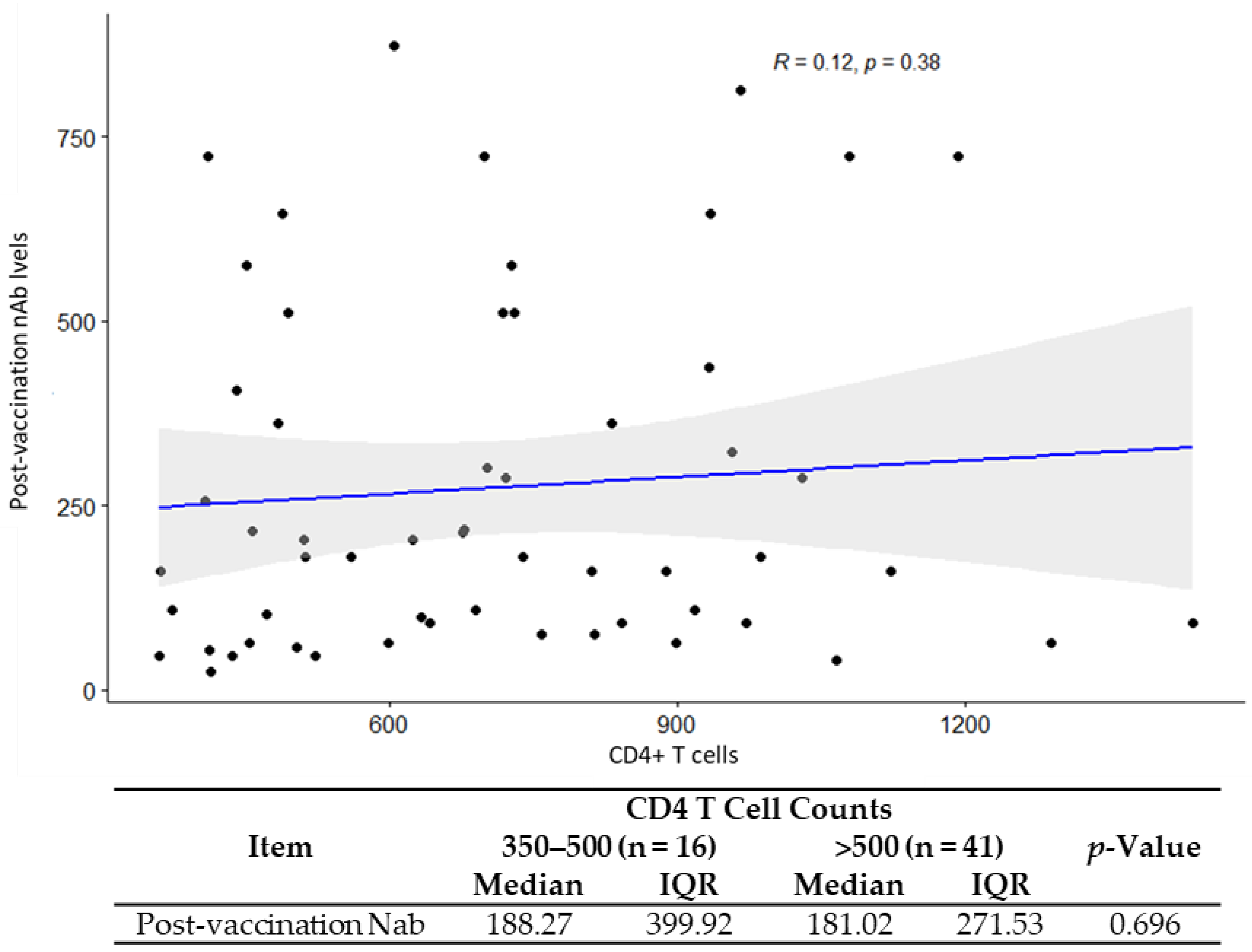
3.4. Correlation of GMT and CD4/CD8 Ratio
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Mission Briefing on COVID-19–11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 5 June 2021).
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Done, E.; Du, I.; Garder, L. COVID-19 Dashboard by the Centrefor Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). John Hopkins University, 2020. Available online: https://coronavirus.jhu.edu/map.html (accessed on 30 November 2022).
- Yancy, C.W. COVID-19 and African Americans. JAMA 2020, 323, 1891–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mdodo, R.; Frazier, E.L.; Dube, S.R.; Mattson, C.L.; Sutton, M.Y.; Brooks, J.T.; Skarbinski, J. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: Cross-sectional surveys. Ann. Intern. Med. 2015, 162, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Shiau, S.; Arpadi, S.M.; Yin, M.T.; Martins, S.S. Patterns of drug use and HIV infection among adults in a nationally representative sample. Addict. Behav. 2017, 68, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasse, B.; Ledergerber, B.; Furrer, H.; Battegay, M.; Hirschel, B.; Cavassini, M.; Bertisch, B.; Bernasconi, E.; Weber, R. Morbidity and aging in HIV-infected persons: The Swiss HIV cohort study. Clin. Infect. Dis. 2011, 53, 1130–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.; Gange, S.J.; Moore, R.D.; Justice, A.C.; Buchacz, K.; Abraham, A.G.; Rebeiro, P.; Koethe, J.R.; Martin, J.N.; Horberg, M.A.; et al. Multimorbidity Among Persons Living with Human Immunodeficiency Virus in the United States. Clin. Infect. Dis. 2017, 66, 1230–1238. [Google Scholar] [CrossRef] [Green Version]
- Eckard, A.R.; McComsey, G.A. Weight gain and integrase inhibitors. Curr. Opin. Infect. Dis. 2020, 33, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; McComsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin. Infect. Dis. 2020, 71, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Spokes, P.; He, W.; Kaldor, J. High risk groups for severe COVID-19 in a whole of population cohort in Australia. BMC Infect. Dis. 2021, 21, 685. [Google Scholar] [CrossRef]
- Kang, I.S.; Kong, K.A. Body mass index and severity/fatality from coronavirus disease 2019: A nationwide epidemiological study in Korea. PLoS ONE 2021, 16, e0253640. [Google Scholar] [CrossRef]
- Dandachi, D.; Geiger, G.; Montgomery, M.W.; Karmen-Tuohy, S.; Golzy, M.; Antar, A.A.; Llibre, J.M.; Camazine, M.; Díaz-De Santiago, A.; Carlucci, P.M.; et al. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients with HIV and Coronavirus Disease-19. Clin. Infect. Dis. 2021, 73, e1964–e1972. [Google Scholar] [CrossRef] [PubMed]
- Geretti, A.M.; Stockdale, A.J.; Kelly, S.H.; Cevik, M.; Collins, S.; Waters, L.; Villa, G.; Docherty, A.; Harrison, E.M.; Turtle, L.; et al. Outcomes of COVID-19 related hospitalization among people with HIV in the ISARIC WHO Clinical Characterization Protocol (UK): A prospective observational study. Clin. Infect. Dis. 2021, 73, e2095–e2106. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, T.Y.; Lin, M.Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.J.; Liu, L.T.; Cheng, J.; Wu, Y.; Wu, C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef]
- Lien, C.E.; Lin, Y.J.; Chen, C.; Lian, W.C.; Kuo, T.Y.; Campbell, J.D.; Traquina, P.; Lin, M.Y.; Liu, L.T.; Chuang, Y.S.; et al. CpG-adjuvanted stable prefusion SARS-CoV-2 spike protein protected hamsters from SARS-CoV-2 challenge. Sci. Rep. 2021, 11, 8761. [Google Scholar] [CrossRef]
- Hsieh, S.-M.; Liu, W.-D.; Huang, Y.-S.; Lin, Y.-J.; Hsieh, E.-F.; Lian, W.-C.; Chen, C.; Janssen, R.; Shih, S.-R.; Huang, C.-G.; et al. Safety and immunogenicity of a Recombinant Stabilized Prefusion SARS-CoV-2 Spike Protein Vaccine (MVC-COV1901) Adjuvanted with CpG 1018 and Aluminum Hydroxide in healthy adults: A Phase 1, dose-escalation study. Eclinicalmedicine 2021, 38, 100989. [Google Scholar] [CrossRef]
- Hsieh, S.-M.; Liu, M.-C.; Chen, Y.-H.; Lee, W.-S.; Hwang, S.-J.; Cheng, S.-H.; Ko, W.-C.; Hwang, K.-P.; Wang, N.-C.; Lee, Y.-L.; et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: Interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir. Med. 2021, 9, 1396–1406. [Google Scholar] [CrossRef]
- Estrada, J.A.; Cheng, C.-Y.; Ku, S.-Y.; Hu, H.-C.; Yeh, H.-W.; Lin, Y.-C.; Chen, C.-P.; Cheng, S.-H.; Janssen, R.; Lin, I.-F. An Immunobridging Study to Evaluate the Neutralizing Antibody Titer in Adults Immunized with Two Doses of Either ChAdOx1-nCov-19 (AstraZeneca) or MVC-COV1901. Vaccines 2022, 10, 655. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Van den Berg, R.; van Hoogstraten, I.; van Agtmael, M. Non-responsiveness to hepatitis B vaccination in HIV seropositive patients; possible causes and solutions. AIDS Rev. 2009, 11, 157–164. [Google Scholar]
- Avelino-Silva, V.I.; Miyaji, K.T.; Hunt, P.W.; Huang, Y.; Simoes, M.; Lima, S.B.; Freire, M.S.; Caiaffa-Filho, H.H.; Hong, M.A.; Costa, D.A.; et al. CD4/CD8 Ratio and KT Ratio Predict Yellow Fever Vaccine Immunogenicity in HIV-Infected Patients. PLoS Negl. Trop. Dis. 2016, 10, e0005219. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, T.; Rini, E.; Okulicz, J.; Messner, O.; Ganesan, A.; Lalani, T.; Bavaro, M.; O’Connell, R.; Agan, B.; Landrum, M. HIV viraemia during hepatitis B vaccination shortens the duration of protective antibody levels. HIV Med. 2015, 16, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroon, F.P.; Van Dissel, J.T.; Labadie, J.; Van Loon, A.M.; Van Furth, R. Antibody response to diphtheria, tetanus, and poliomyelitis vaccines in relation to the number of CD4+ T lymphocytes in adults infected with human immunodeficiency virus. Clin. Infect. Dis. 1995, 21, 1197–1203. [Google Scholar] [CrossRef]
- Kroon, F.P.; van Dissel, J.T.; de Jong, J.C.; van Furth, R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994, 8, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-C.; Chang, S.-Y.; Su, C.-T.; Chen, Y.-Y.; Yang, C.-Y.; Liu, W.-C.; Wu, C.-H. A 5-year longitudinal follow-up study of serological responses to 23-valent pneumococcal polysaccharide vaccination among patients with HIV infection who received highly active antiretroviral therapy. HIV Med. 2009, 11, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Peebody, R. Have COVID-19 Vaccines Been Tested in People with HIV? 2021. Available online: https://www.aidsmap.com/about-hiv/have-covid-19-vaccines-been-tested-people-hiv (accessed on 30 July 2021).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Janssen Biotech, Inc. FDA Briefing Document: Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. 2021. Available online: https://www.fda.gov/media/146217/download (accessed on 27 November 2021).
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Frater, J.; Ewer, K.J.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.K.; Ali, M.; Ansari, M.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: A single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef]
- Madhi, S.A.; Koen, A.L.; Izu, A.; Fairlie, L.; Cutland, C.L.; Baillie, V.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: An interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021, 8, e568–e580. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Karaba, A.H.; Garliss, C.C.; Beck, E.J.; Wang, K.H.; Laeyendecker, O.; Cox, A.L.; Blankson, J.N. The BNT162b2 mRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living with HIV. Clin. Infect. Dis. 2021, 74, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Levy, I.; Finesod, A.W.; Litchevsky, V.; Biber, A.; Indenbaum, V.; Olmer, L.; Huppert, A.; Mor, O.; Goldstein, M.; Levin, E.G.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin. Microbiol. Infect. 2021, 27, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.A.; Peluso, M.J.; Lynch, K.L.; Yun, C.; Glidden, D.V.; Henrich, T.J.; Deeks, S.G.; Gandhi, M. Differences in Post-mRNA Vaccination SARS-CoV-2 IgG Concentrations and Surrogate Virus Neutralization Test Response by HIV Status and Type of Vaccine: A Matched Case-Control Observational Study. Clin. Infect. Dis. 2022, 75, e916–e919. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Huang, X.; Yan, Y.; Su, B.; Xiao, D.; Yu, M.; Jin, X.; Duan, J.; Zhang, X.; Zheng, S.; Fang, Y.; et al. Comparing Immune Responses to Inactivated Vaccines against SARS-CoV-2 between People Living with HIV and HIV-Negative Individuals: A Cross-Sectional Study in China. Viruses 2022, 14, 277. [Google Scholar] [CrossRef] [PubMed]
- Hassold, N.; Brichler, S.; Ouedraogo, E.; Leclerc, D.; Carroue, S.; Gater, Y.; Alloui, C.; Carbonnelle, E.; Bouchaud, O.; Mechai, F.; et al. Impaired antibody response to COVID-19 vaccination in advanced HIV infection. AIDS 2022, 36, F1–F5. [Google Scholar] [CrossRef]
- Díaz, N.A.; de Miguel, R.; Agüero, F.; Sued, O.; Arribas, J.R.; Ambrosioni, J.; Hospital Clinic COVID-19 in HIV Investigators. Prevention and Treatment of SARS-CoV2 Infection in People Living with HIV: The Need for Specific Data. Infect. Dis. Ther. 2021, 28, 1–13. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Peng, X.; Ouyang, J.; Isnard, S.; Lin, J.; Fombuena, B.; Zhu, B.; Routy, J.-P. Sharing CD4+ T Cell Loss: When COVID-19 and HIV Collide on Immune System. Front. Immunol. 2020, 11, 596631. [Google Scholar] [CrossRef]
- Chen, Z.; John, W.E. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020, 20, 529–536. [Google Scholar] [CrossRef]
- Papagno, L.; Spina, C.A.; Marchant, A.; Salio, M.; Rufer, N.; Little, S.; Dong, T.; Chesney, G.; Waters, A.; Easterbrook, P.; et al. Immune Activation and CD8+ T-Cell Differentiation towards Senescence in HIV-1 Infection. PLoS Biol. 2004, 2, E20. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.M. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 2001, 410, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, M.; Van Lunzen, J.; Soghoian, D.Z.; Kuhl, B.D.; Ranasinghe, S.; Kranias, G.; Flanders, M.D.; Cutler, S.; Yudanin, N.; Muller, M.I.; et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Investig. 2012, 122, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdörfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative Expression of Toll-Like Receptor 1–10 mRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyer, R.; McGuire, D.K.; Xing, B.; Jackson, S.; Janssen, R. Safety of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant in adults. Vaccine 2018, 36, 2604–2611. [Google Scholar] [CrossRef]
- Fuster, F.; Vargas, J.I.; Jensen, D.; Sarmiento, V.; Acuna, P.; Peirano, F.; Fuste, F.; Arab, J.P.; Martínez, F. CD4/CD8 ratio as a predictor of the response to HBV vaccination in HIV-positive patients: A prospective cohort study. Vaccine 2016, 34, 1889–1895. [Google Scholar] [CrossRef]
- Hammarlund, E.; Thomas, A.; Amanna, I.J.; Holden, L.A.; Slayden, O.D.; Park, B.; Gao, L.; Slifka, M.K. Plasma cell survival in the absence of B cell memory. Nat. Commun. 2017, 8, 1781. [Google Scholar] [CrossRef] [Green Version]
- World Health Orgnization. WHO Statement on Solidarity Trial Vaccines. Available online: https://www.who.int/news/item/26-10-2021-who-statement-on-solidarity-trial-vaccines (accessed on 26 October 2021).
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.C.; Cravioto, A.; Rees, H.; Higgins, J.P.T.; Boutron, I.; et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Whitley, R.J.; Aletaha, D. SARS-CoV-2 and the rheumatology patient: The last 12 months and a boost in the future. Ann. Rheum. Dis. 2021, 80, 1249–1251. [Google Scholar] [CrossRef]
- Zhang, J.; He, Q.; An, C.; Mao, Q.; Gao, F.; Bian, L.; Wu, X.; Wang, Q.; Liu, P.; Song, L.; et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg. Microbes Infect. 2021, 10, 1598–1608. [Google Scholar] [CrossRef]
- Cruciani, M.; Mengoli, C.; Serpelloni, G.; Lanza, A.; Gomma, M.; Nardi, S.; Rimondo, C.; Bricolo, F.; Consolaro, S.; Trevisan, M.; et al. Serologic response to hepatitis B vaccine with high dose and increasing number of injections in HIV infected adult patients. Vaccine 2009, 27, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Caldera, F.; Mercer, M.; Samson, S.I.; Pitt, J.M.; Hayney, M.S. Influenza vaccination in immunocompromised populations: Strategies to improve immunogenicity. Vaccine 2021, 39, A15–A23. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.; Samji, H.; Cooper, C.L.; Costiniuk, C.T.; Janjua, N.Z.; Kroch, A.E.; Arbess, G.; Benoit, A.C.; Buchan, S.A.; Chung, H.; et al. Coronavirus disease 2019 vaccine effectiveness among a population-based cohort of people living with HIV. AIDS 2022, 36, F17–F26. [Google Scholar] [CrossRef] [PubMed]


| Item | HIV Positive N = 57 | Main Study N = 326 | p-Value |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 38.6 (13.1) | 42.8 (14.9) | 0.058 |
| Median (IQR) | 36 (19.0) | 41 (23.0) | |
| Min~Max | 23.0~72.0 | 23.0~72.0 | |
| Gender | |||
| N (Missing) | 57 (0) | 326 (0) | |
| Male | 54 (94.7%) | 310 (95.1%) | 1 |
| Female | 3 (5.3%) | 16 (4.9%) | |
| BMI (kg/m2) | |||
| Mean (SD) | 25.3 (4.9) | 25.9(4.0) | 0.167 |
| Median (IQR) | 24.5 (6.2) | 25.4 (5.1) | |
| BMI group | |||
| <30 kg/m2 | 46 (80.7%) | 277 (84.9%) | 0.413 |
| ≥30 kg/m2 | 11 (19.3%) | 49 (15.0%) | |
| Comorbidity Category | |||
| Yes | 6 (10.5%) | 59 (18.1%) | 0.16 |
| No | 51 (89.5%) | 267 (81.9%) |
| (a) | ||||||
| All, n (%) | ≥20 to <65 Years, n (%) | ≥65 Years, n (%) | ||||
| MVC-COV1901 (N = 3295) | HIV (N = 58) | MVC-COV1901 (N = 2575) | HIV (N = 54) | MVC-COV1901 (N = 720) | HIV (N = 4) | |
| Any Solicited Local AEs | 2381 (72.3) | 39 (58.6) | 2030 (78.8) | 37 (59.3) | 351 (48.8) | 2 (50.0) |
| Pain/Tenderness | 2346 (71.2) | 39 (67.2) | 2009 (78.0) | 37 (68.5) | 337 (46.8) | 2 (50.0) |
| Grade 1 | 2237 (67.9) | 37 (63.8) | 1907 (74.1) | 35 (64.8) | 330 (45.8) | 2 (50.0) |
| Grade 2 | 97 (2.9) | 2 (34.4) | 91 (3.5) | 2 (3.7) | 6 (0.8) | 0 |
| Grade 3 | 12 (0.4) | 0 | 11 (0.4) | 0 | 1 (0.1) | 0 |
| Induration/Swelling | 347 (10.5) | 1 (1.7) | 286 (11.1) | 1 (1.9) | 61 (8.5) | 0 |
| Grade 1 | 303 (9.2) | 1 (1.7) | 246 (9.6) | 1 (1.9) | 57 (7.9) | 0 |
| Grade 2 | 42 (1.3) | 0 | 38 (1.5) | 0 | 4 (0.6) | 0 |
| Grade 3 | 2 (0.1) | 0 | 2 (0.1) | 0 | 0 | 0 |
| Erythema/Redness | 161 (4.9) | 0 | 138 (5.4) | 0 | 23 (3.2) | 0 |
| Grade 1 | 155 (4.7) | 0 | 132 (5.1) | 0 | 23 (3.2) | 0 |
| Grade 2 | 6 (0.2) | 0 | 6 (0.2) | 0 | 0 | 0 |
| Any Solicited Systemic AEs | 1774 (53.8) | 37 (63.8) | 1484 (57.6) | 35 (65.8) | 290 (40.3) | 2 (50.0) |
| Malaise/Fatigue | 1186 (36.0) | 25 (43.1) | 1036 (40.2) | 24 (44.4) | 150 (20.8) | 1 (25.0) |
| Grade 1 | 961 (29.2) | 22 (43.1) | 831 (32.3) | 24 (44.4) | 130 (18.1) | 1 (25.0) |
| Grade 2 | 203 (6.2) | 3 (5.2) | 185 (7.2) | 2 (3.7) | 18 (2.5) | 1 (25.0) |
| 1Grade 3 | 22 (0.7) | 0 | 20 (0.8) | 0 | 2 (0.3) | 0 |
| Myalgia | 908 (27.6) | 10 (16.7) | 757 (29.4) | 14 (25.9) | 151 (21.0) | 2 (50.0) |
| Grade 1 | 764 (23.2) | 9 (16.7) | 629 (24.4) | 7 (13.0) | 135 (18.8) | 2 (50.0) |
| Grade 2 | 126 (3.8) | 1 (1.7) | 112 (4.3) | 1 (1.9) | 14 (1.9) | 0 |
| Grade 3 | 18 (0.5) | 0 | 16 (0.6) | 0 | 2 (0.3) | 0 |
| Headache | 730 (22.2) | 13 (22.4) | 631 (24.5) | 12 (22.2) | 99 (13.8) | 1 (25.0) |
| Grade 1 | 630 (19.1) | 12 (20.7) | 537 (20.9) | 11 (20.4) | 93 (12.9) | 1 (25.0) |
| Grade 2 | 93 (2.8) | 1 (1.7) | 87 (3.4) | 1 (1.9) | 6 (0.8) | 0 |
| Grade 3 | 7 (0.2) | 0 | 7 (0.3) | 0 | 0 | 0 |
| Diarrhoea | 497 (15.1) | 14 (24.1) | 422 (16.4) | 14 (25.9) | 75 (10.4) | 0 |
| Grade 1 | 411 (12.5) | 12 (20.7) | 350 (13.6) | 12 (22.2) | 61 (8.5) | 0 |
| Grade 2 | 75 (2.3) | 2 (3.4) | 62 (2.4) | 2 (3.7) | 13 (1.8) | 0 |
| Grade 3 | 11 (0.3) | 0 | 10 (0.4) | 0 | 1 (0.1) | 0 |
| Nausea/Vomiting | 254 (7.7) | 6 (10.3) | 219 (8.5) | 6 (11.1) | 35 (4.9) | 0 |
| Grade 1 | 226 (6.9) | 6 (10.3) | 192 (7.5) | 6 (11.1) | 34 (4.7) | 0 |
| Grade 2 | 26 (0.8) | 0 | 25 (1.0) | 0 | 1 (0.1) | 0 |
| Grade 3 | 2 (0.1) | 0 | 2 (0.1) | 0 | 0 | 0 |
| Fever | 23 (0.7) | 0 | 16 (0.6) | 0 | 7 (1.0) | 0 |
| Grade 1 | 14 (0.4) | 0 | 8 (0.3) | 0 | 6 (0.8) | 0 |
| Grade 2 | 6 (0.2) | 0 | 6 (0.2) | 0 | 0 | 0 |
| Grade 3 | 2 (0.1) | 0 | 2 (0.1) | 0 | 0 | 0 |
| Grade 4 | 1 (<0.1) | 0 | 0 | 0 | 1 (<0.1) | 0 |
| (b) | ||||||
| All, n (%) | ≥20 to <65 Years, n (%) | ≥65 Years, n (%) | ||||
| MVC-COV1901 (N = 3295) | HIV in MVC-COV1901 (N = 58) | MVC-COV1901 (N = 2575) | HIV in MVC-COV1901 (N = 54) | MVC-COV1901 (N = 720) | HIV in MVC-COV1901 (N = 4) | |
| Unsolicited AEs | 932 (28.3) | 17 (29.3) | 767 (29.8) | 17 (29.6) | 165 (22.9) | 0 |
| Related unsolicited AEs | 406 (12.3) | 8 (13.8) | 340 (13.2) | 396 (13.2) | 66 (9.2) | 0 |
| Unsolicited AEs ≥ Grade 3 | 93 (2.8) | 3 (5.2) | 86 (3.3) | 96 (3.2) | 7 (1.0) | 0 |
| Related unsolicited AEs ≥ Grade 3 | 21 (0.6) | 0 | 20 (0.8) | 24 (0.8) | 1 (0.1) | 0 |
| SAEs | 18 (0.5) | 1 (1.7) | 16 (0.6) | 17 (0.6) | 2 (0.3) | 0 |
| Related SAEs | 0 | 0 | 0 | 0 | 0 | 0 |
| AESI | 1 (<0.1) | 0 | 0 | 0 | 1 (0.1) | 0 |
| VAED | 0 | 0 | 0 | 0 | 0 | 0 |
| AEs leading to study intervention discontinuation | 2 (0.1) | 0 | 0 | 1 (<0.1) | 2 (0.3) | 0 |
| AEs leading to study withdrawal | 1 (<0.1) | 0 | 0 | 0 | 1 (0.1) | 0 |
| Death | 0 | 0 | 0 | 0 | 0 | 0 |
| Item | Nab Titer (GMT), IU/mL | a Nab GMT Ratio | a,b Adjusted GMT Ratio | |
|---|---|---|---|---|
| HIV-Positive (n = 57) | HIV-Negative (n = 326) | |||
| Estimate | 137.7 | 412.0 | 3.0 | 3.2 |
| 95% CI | 110.7~171.3 | 378.7~448.3 | 2.4~3.8 | 2.5~4.0 |
| HIV Classification Stage | n | Nab GMT (in IU/mL) | 95% CI | * p-Value |
|---|---|---|---|---|
| Stage 1 | 25 | 142.55 | 105.34–192.90 | 0.3486 |
| Stage 2 | 19 | 108.54 | 71.00–165.94 | |
| Stage 3 | 10 | 169.05 | 85.40–334.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.-H.; Lien, C.E.; Hsieh, S.-M.; Cheng, C.-Y.; Liu, W.-D.; Lo, C.-L.; Ko, W.-C.; Chen, Y.-H.; Huang, C.-T.; Chang, H.-T.; et al. A Retrospective Study of the Safety and Immunogenicity of MVC-COV1901 Vaccine for People Living with HIV. Vaccines 2023, 11, 18. https://doi.org/10.3390/vaccines11010018
Cheng S-H, Lien CE, Hsieh S-M, Cheng C-Y, Liu W-D, Lo C-L, Ko W-C, Chen Y-H, Huang C-T, Chang H-T, et al. A Retrospective Study of the Safety and Immunogenicity of MVC-COV1901 Vaccine for People Living with HIV. Vaccines. 2023; 11(1):18. https://doi.org/10.3390/vaccines11010018
Chicago/Turabian StyleCheng, Shu-Hsing, Chia En Lien, Szu-Min Hsieh, Chien-Yu Cheng, Wang-Da Liu, Ching-Lung Lo, Wen-Chien Ko, Yen-Hsu Chen, Ching-Tai Huang, Hsiao-Ting Chang, and et al. 2023. "A Retrospective Study of the Safety and Immunogenicity of MVC-COV1901 Vaccine for People Living with HIV" Vaccines 11, no. 1: 18. https://doi.org/10.3390/vaccines11010018






