Oral Vaccination of Recombinant Saccharomyces cerevisiae Expressing ORF132 Induces Protective Immunity against Cyprinid Herpesvirus-2
Abstract
1. Introduction
2. Material and Methods
2.1. Fish and Virus
2.2. Construction of Recombinant pYD1-ORF132
2.3. Construction of Recombinant EBY100/pYD1-ORF132
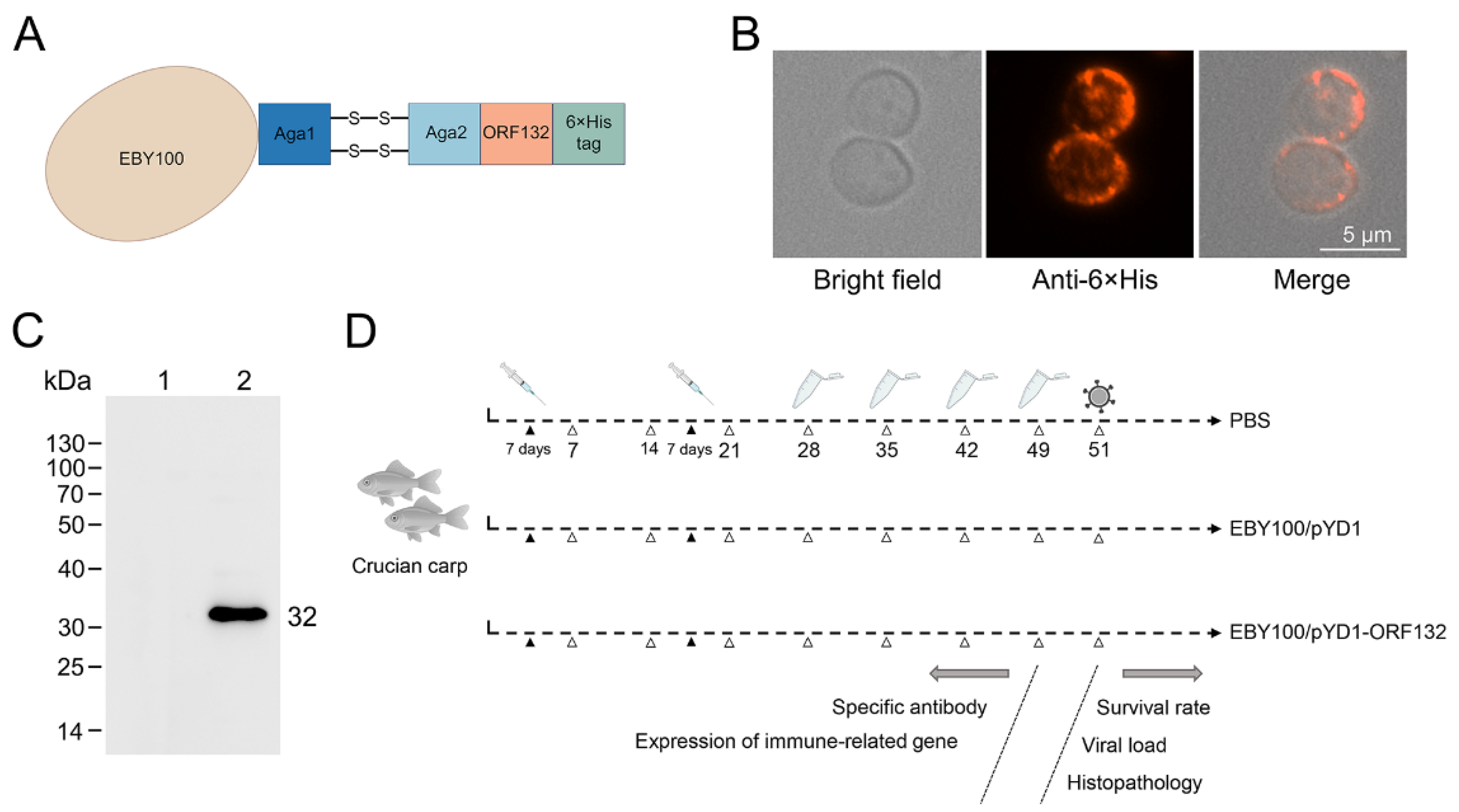
2.4. Immunofluorescence Assay
2.5. Western Blotting
2.6. Oral Immunization and Experimental Design
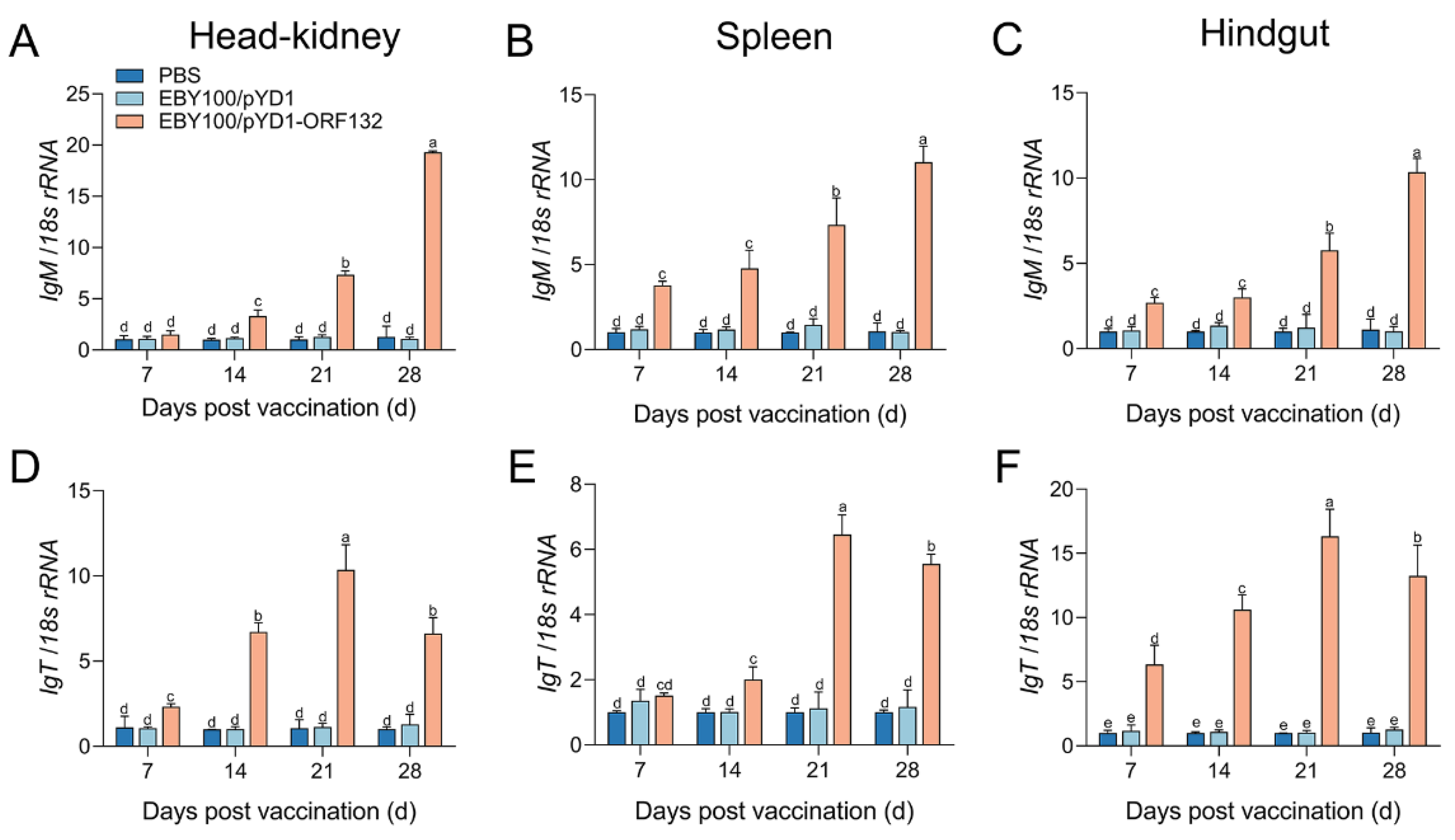
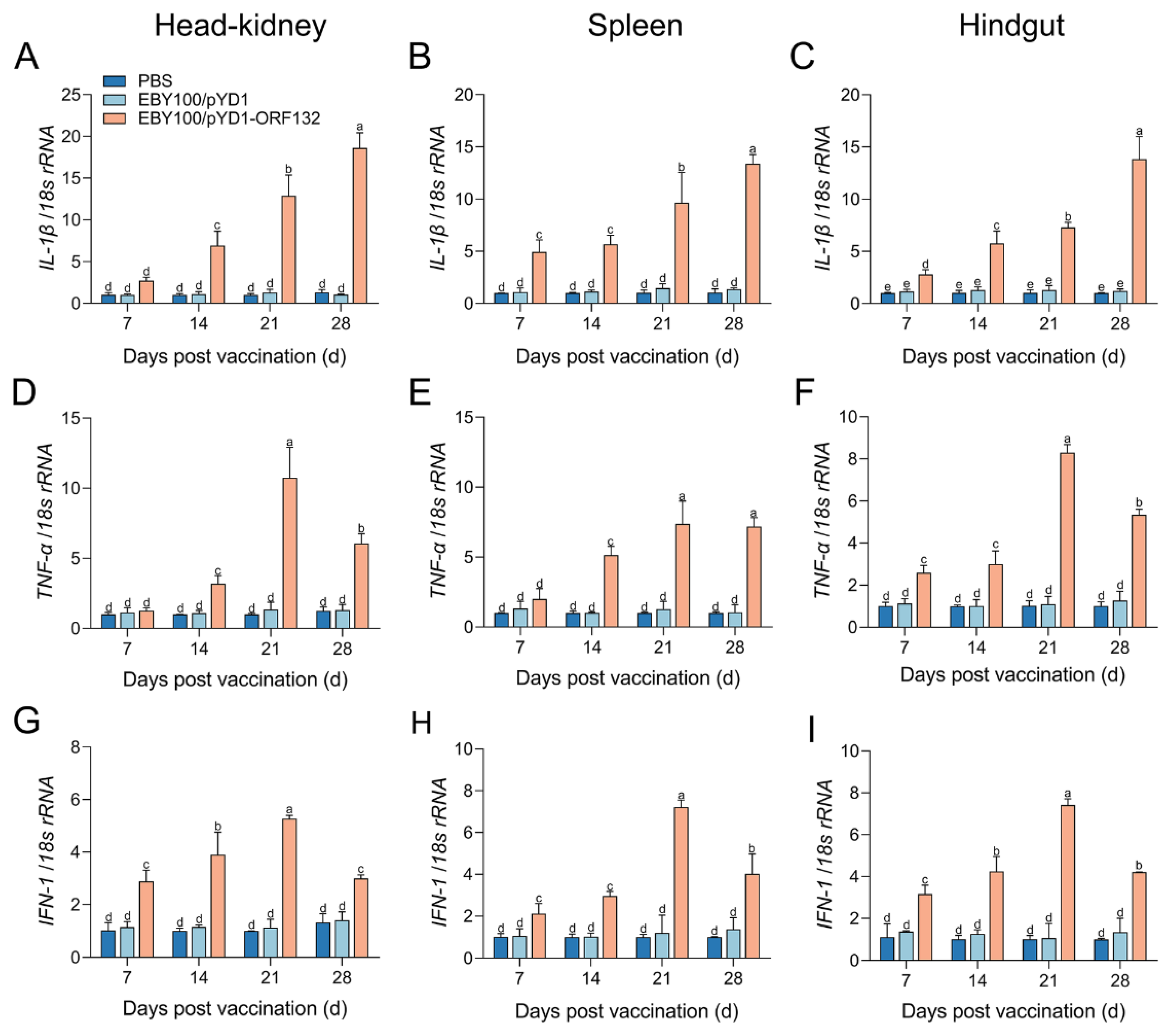
2.7. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (qPCR)
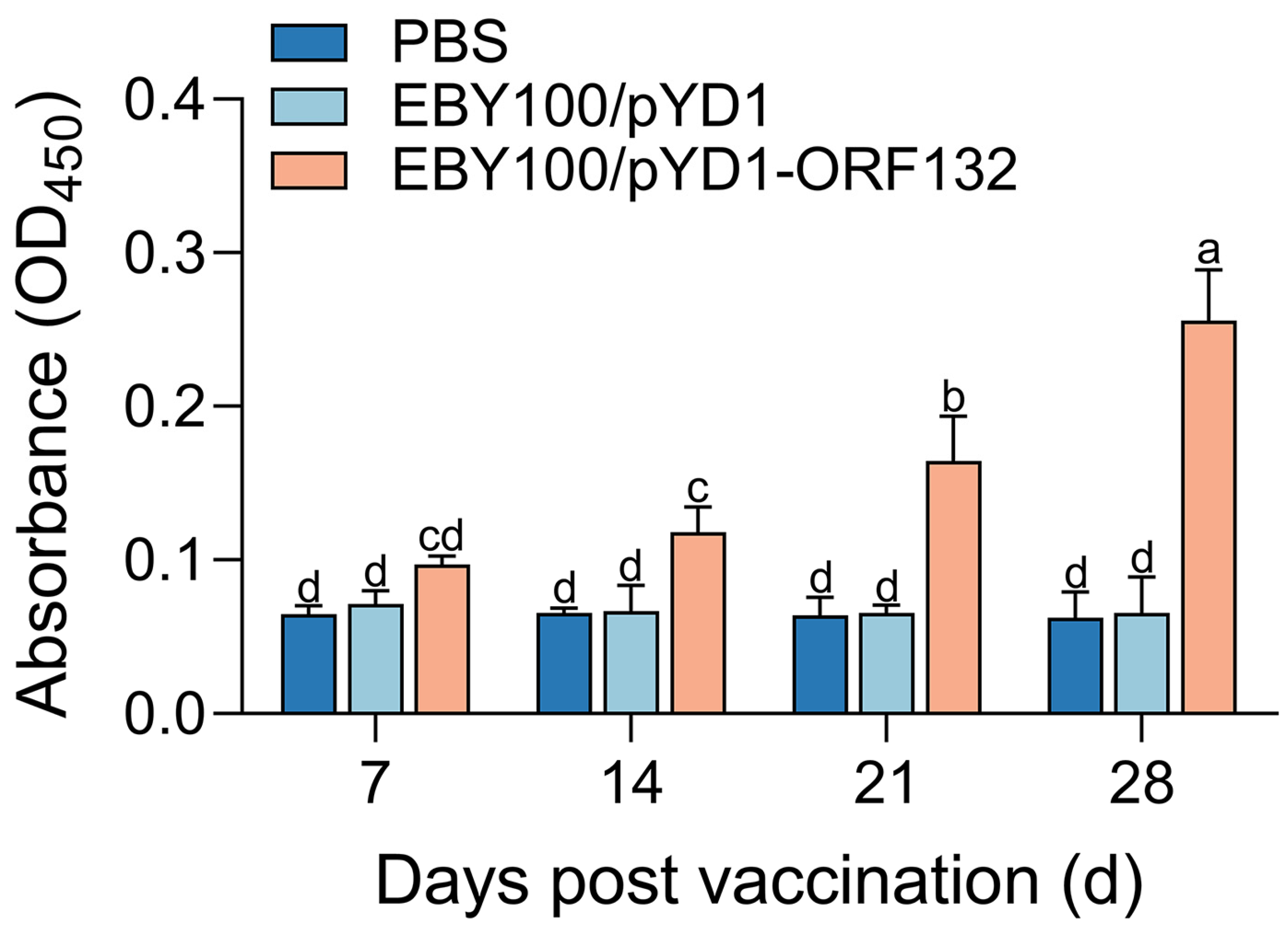
2.8. Detection of ORF132 Specific Antibody
2.9. Histological Examination
2.10. Statistics Analysis
3. Results
3.1. Construction of the Recombinant S. cerevisiae Strain Expressing ORF132
3.2. Specific Antibody Induced by Oral Vaccination
3.3. The Expression of Immune-Related Genes after Vaccination
3.4. Protective Effect of Oral Vaccine on CyHV-2-Infected Crucian Carp
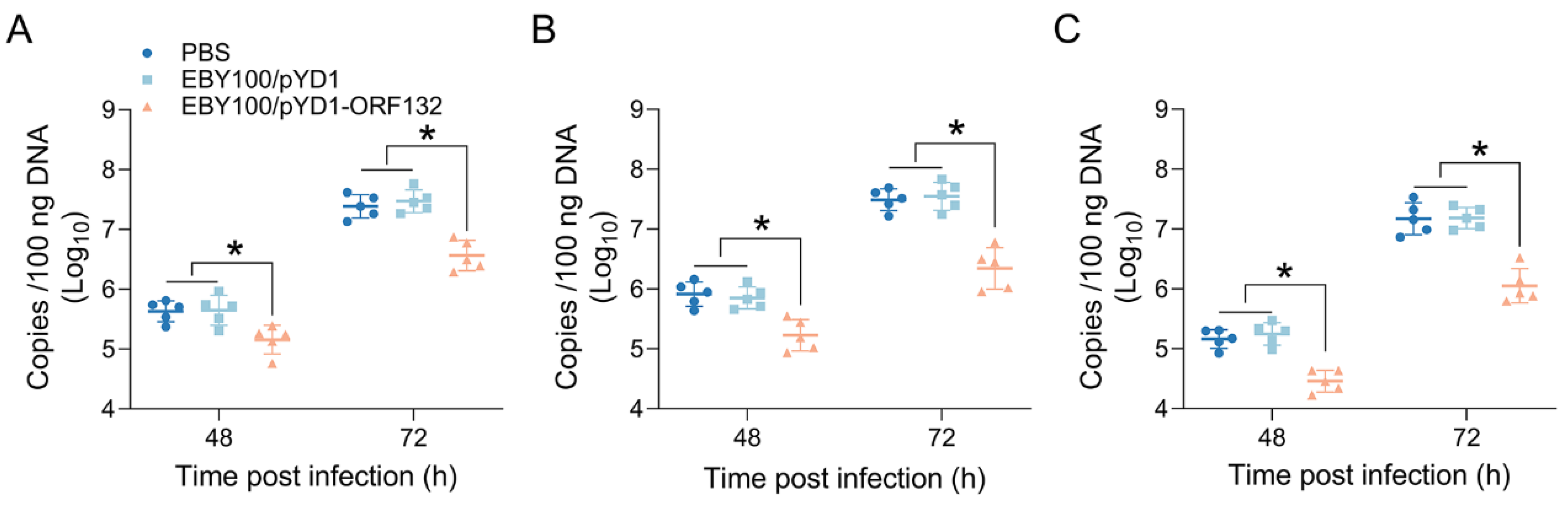
3.5. Oral Vaccine Decreased Virus Load in CyHV-2-Infected Crucian Carp
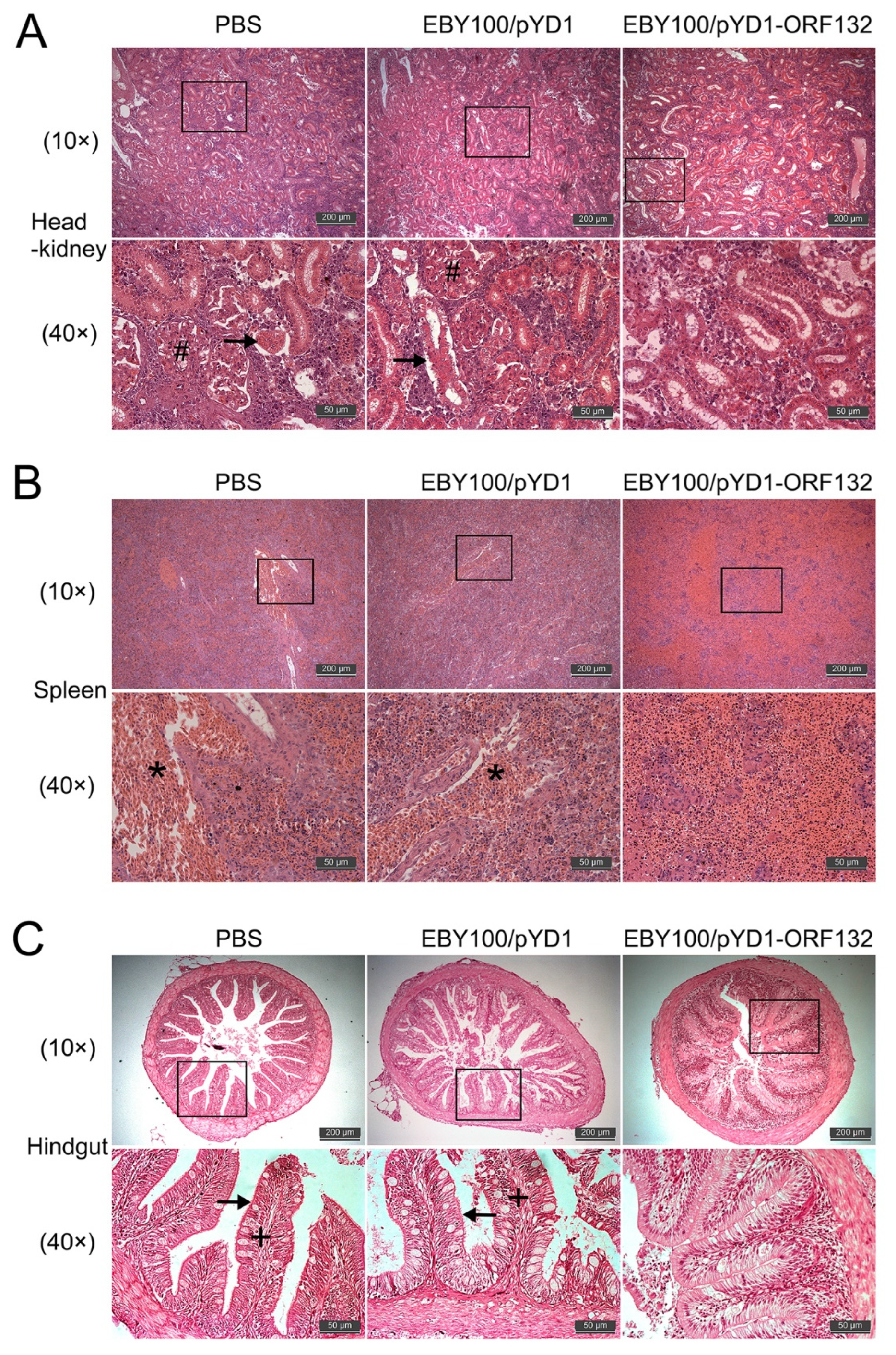
3.6. Effect of Oral Vaccine on the Histopathology of CyHV-2-Infected Crucian Carp
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davison, A.J.; Kurobe, T.; Gatherer, D.; Cunningham, C.; Korf, I.; Fukuda, H.; Hedrick, R.P.; Waltzek, T.B. Comparative genomics of carp herpesviruses. J. Virol. 2013, 87, 2908–2922. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, R.S.; Nithianantham, S.R.; Dharmaratnam, A.; Kumar, R.; Pradhan, P.K.; Thangalazhy Gopakumar, S.; Sood, N. Cyprinid herpesvirus-2 (CyHV-2): A comprehensive review. Rev. Aquac. 2021, 13, 796–821. [Google Scholar] [CrossRef]
- Lu, J.F.; Jin, T.C.; Zhou, T.; Lu, X.J.; Chen, J.P.; Chen, J. Identification and characterization of a tumor necrosis factor receptor like protein encoded by Cyprinid herpesvirus-2. Dev. Comp. Immunol. 2021, 116, 103930. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Shen, Z.Y.; Lu, L.Q.; Xu, D. Cyprinid herpesvirus-2 miR-C12 Attenuates Virus-Mediated Apoptosis and Promotes Virus Propagation by Targeting Caspase 8. Front. Microbiol. 2019, 10, 2923. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, K.R.; Bateman, K.; Bayley, A.; Feist, S.W.; Hulland, J.; Longshaw, C.; Stone, D.; Woolford, G.; Way, K. Isolation of a Cyprinid herpesvirus-2 from goldfish, Carassius auratus (L.), in the UK. J. Fish Dis. 2007, 30, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Miyazaki, T. Herpesviral haematopoietic necrosis of goldfish, Carassius auratus (L.). J. Fish Dis. 1995, 18, 211–220. [Google Scholar] [CrossRef]
- Wen, J.X.; Xu, Y.; Su, M.Z.; Lu, L.Q.; Wang, H. Susceptibility of goldfish to Cyprinid herpesvirus-2 (CyHV-2) SH01 isolated from cultured crucian carp. Viruses 2021, 13, 1761. [Google Scholar] [CrossRef]
- Barnes, A.C.; Silayeva, O.; Landos, M.; Dong, H.T.; Lusiastuti, A.; Phuoc, L.H.; Delamare-Deboutteville, J. Autogenous vaccination in aquaculture: A locally enabled solution towards reduction of the global antimicrobial resistance problem. Rev. Aquac. 2022, 14, 907–918. [Google Scholar] [CrossRef]
- Mondal, H.; Thomas, J. A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Angulo, C.; Tello-Olea, M.; Reyes-Becerril, M.; Monreal-Escalante, E.; Hernandez-Adame, L.; Angulo, M.; Mazon-Suastegui, J.M. Developing oral nanovaccines for fish: A modern trend to fight infectious diseases. Rev. Aquac. 2021, 13, 1172–1192. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, P. Yeast-based vaccines: New perspective in vaccine development and application. FEMS. Yeast. Res. 2019, 19, foz007. [Google Scholar] [CrossRef]
- Gao, T.; Ren, Y.; Li, S.Q.; Lu, X.; Lei, H. Immune response induced by oral administration with a Saccharomyces cerevisiae-based SARS-CoV-2 vaccine in mice. Microb. Cell Fact. 2021, 20, 95. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Xu, L.M.; Liu, M.; Cao, Y.S.; LaPatra, S.E.; Yin, J.S.; Liu, H.B.; Lu, T.Y. Preliminary study of an oral vaccine against infectious hematopoietic necrosis virus using improved yeast surface display technology. Mol. Immunol. 2017, 85, 196–204. [Google Scholar] [CrossRef]
- Ma, Y.P.; Liu, Z.X.; Hao, L.; Wu, J.; Qin, B.T.; Liang, Z.L.; Ma, J.Y.; Ke, H.; Yang, H.W.; Li, Y.G.; et al. Oral vaccination using Artemia coated with recombinant Saccharomyces cerevisiae expressing Cyprinid herpesvirus-3 envelope antigen induces protective immunity in common carp (Cyprinus carpio var. Jian) larvae. Res. Vet. Sci. 2020, 130, 184–192. [Google Scholar] [CrossRef]
- Luo, S.X.; Yan, L.M.; Zhang, X.H.; Yuan, L.; Fang, Q.; Zhang, Y.A.; Dai, H.P. Yeast surface display of capsid protein VP7 of grass carp reovirus: Fundamental investigation for the development of vaccine against hemorrhagic disease. J. Microbiol. Biotechnol. 2015, 25, 2135–2145. [Google Scholar] [CrossRef]
- Gao, W.; Wen, H.; Wang, H.; Lu, J.Q.; Lu, L.Q.; Jiang, Y.S. Identification of structure proteins of Cyprinid herpesvirus-2. Aquaculture 2020, 523, 735184. [Google Scholar] [CrossRef]
- Xu, L.J.; Podok, P.; Xie, J.; Lu, L.Q. Comparative analysis of differential gene expression in kidney tissues of moribund and surviving crucian carp (Carassius auratus gibelio) in response to Cyprinid herpesvirus-2 infection. Arch. Virol. 2014, 159, 1961–1974. [Google Scholar] [CrossRef]
- Lu, J.F.; Xu, D.; Jiang, Y.S.; Kong, S.Y.; Shen, Z.Y.; Xia, S.Y.; Lu, L.Q. Integrated analysis of mRNA and viral miRNAs in the kidney of Carassius auratus gibelio response to Cyprinid herpesvirus-2. Sci. Rep. 2017, 7, 13787. [Google Scholar] [CrossRef]
- Luo, S.; Wang, L.C.; Shuai, Z.H.; Yang, G.J.; Lu, J.F.; Chen, J. A short peptidoglycan recognition protein protects Boleophthalmus pectinirostris against bacterial infection via inhibiting bacterial activity. Fish Shellfish Immunol. 2022, 127, 119–128. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, T.X.; Hu, Y.; Liu, L.; Chen, J. Antiviral effects of natural small molecules on aquatic rhabdovirus by interfering with early viral replication. Zool. Res. 2022, 43, 966–976. [Google Scholar] [CrossRef]
- Wu, X.Y.; Xiong, J.B.; Fei, C.J.; Dai, T.; Zhu, T.F.; Zhao, Z.Y.; Pan, J.; Nie, L.; Chen, J. Prior exposure to ciprofloxacin disrupts intestinal homeostasis and predisposes ayu (Plecoglossus altivelis) to subsequent Pseudomonas plecoglossicida-induced infection. Zool. Res. 2022, 43, 648–665. [Google Scholar] [PubMed]
- Yu, Y.Y.; Huang, Z.Y.; Kong, W.G.; Dong, F.; Zhang, X.T.; Zhai, X.; Cheng, G.F.; Zhan, M.T.; Cao, J.F.; Ding, L.G.; et al. Teleost swim bladder, an ancient air-filled organ that elicits mucosal immune responses. Cell Discov. 2022, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Lei, X.Y.; Zhang, Q.Y. The antiviral defense mechanisms in mandarin fish induced by DNA vaccination against a rhabdovirus. Vet. Microbiol. 2012, 157, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.Y.; Zhang, C.S.; Yuan, X.M.; Huang, L.; Hu, D.Y.; Yu, Z.; Yin, W.L.; Lin, L.Y.; Pan, X.Y.; Yang, G.L.; et al. Oral vaccination with recombinant Pichia pastoris expressing iridovirus major capsid protein elicits protective immunity in Largemouth Bass (Micropterus salmoides). Front. Immunol. 2022, 13, 852300. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.N.; Xu, K.; Li, X.Y.; Liu, Y.W.; Bai, Y.C.; Zhang, X.H.; Han, B.Q.; Chen, Z.L.; Zhang, Z.Y. Recombinant Saccharomyces cerevisiae serves as novel carrier for oral DNA vaccines in Carassius auratus. Fish Shellfish Immunol. 2015, 47, 758–765. [Google Scholar] [CrossRef]
- Dong, Z.R.; Mu, Q.J.; Kong, W.G.; Qin, D.C.; Zhou, Y.; Wang, X.Y.; Cheng, G.F.; Luo, Y.Z.; Ai, T.S.; Xu, Z. Gut mucosal immune responses and protective efficacy of oral yeast Cyprinid herpesvirus-2 (CyHV-2) vaccine in Carassius auratus gibelio. Front. Immunol. 2022, 13, 932722. [Google Scholar] [CrossRef]
- Bøgwald, J.; Dalmo, R.A. Protection of teleost fish against infectious diseases through oral administration of vaccines: Update 2021. Int. J. Mol. Sci. 2021, 22, 10932. [Google Scholar] [CrossRef]
- Liu, Z.X.; Wu, J.; Ma, Y.P.; Hao, L.; Liang, Z.L.; Ma, J.Y.; Ke, H.; Li, Y.G.; Cao, J.M. Protective immunity against CyHV-3 infection via different prime-boost vaccination regimens using CyHV-3 ORF131-based DNA/protein subunit vaccines in carp Cyprinus carpio var. Jian. Fish Shellfish Immunol. 2020, 98, 342–353. [Google Scholar] [CrossRef]
- Jun, J.W.; Kang, J.W.; Giri, S.S.; Kim, S.W.; Kim, S.G.; Kwon, J.; Lee, S.B.; Jung, W.J.; Lee, Y.M.; Jo, S.J.; et al. Preventive effect of starch hydrogel-based oral vaccine against Aeromonas salmonicida infection in rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 555, 738202. [Google Scholar] [CrossRef]
- Salinas, I.; Fernández-Montero, Á.; Ding, Y.; Sunyer, J.O. Mucosal immunoglobulins of teleost fish: A decade of advances. Dev. Comp. Immunol. 2021, 121, 104079. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Langevin, C.; Boudinot, P.; Collet, B. IFN signaling in inflammation and viral infections: New insights from fish models. Viruses 2019, 11, 302. [Google Scholar] [CrossRef]
- Stosik, M.; Tokarz-Deptuła, B.; Deptuła, W. Type I interferons in ray-finned fish (Actinopterygii). Fish Shellfish Immunol. 2021, 110, 35–43. [Google Scholar] [CrossRef]
- Xu, F.; Li, M.Y.; Chen, J. D-dopachrome tautomerase from Japanese sea bass (Lateolabrax japonicus) is a chemokine-like cytokine and functional homolog of macrophage migration inhibitory factor. Zool. Res. 2020, 41, 39–50. [Google Scholar]

| Primer | Gene | Accession Number | Nucleotide Sequence (5′-3′) | Application |
|---|---|---|---|---|
| ORF132-F | ORF132 | KT387800.1 | CGCGGATCCGCTGCCACCCTGGGGACAG a | pYD1 clone |
| ORF132-R | CCGGAATTCGGTATTGTCGTAGCCGC b | |||
| 18s-F | 18s rRNA | XR003291850.1 | GAATGTCTGCCCTATCAACT | RT-qPCR |
| 18s-R | GATGTGGTAGCCGTTTCT | |||
| IgM-F | IgM | GU563726.1 | GTCAATCTTCGGCTTGTCTCA | RT-qPCR |
| IgM-R | GGGTATAATCTCCATCGGGTC | |||
| IgT-F | IgT | GQ201446.1 | ATTCATTGTCAGACCTTCACTCAG | RT-qPCR |
| IgT-R | GCAGTGTCTTCAGTCTTGAGGCT | |||
| IL-1β-F | IL-1β | AB757758.1 | TTTGTGAAGATGCGCTGCTC | RT-qPCR |
| IL-1β-R | CCAATCTCGACCTTCCTGGTG | |||
| TNF-α-F | TNF-α | KF500408.1 | CGCTACTCTGATTCCTATGGC | RT10-qPCR |
| TNF-α-R | GCTTTCGCTGTTGCCTTTCT | |||
| IFN-1-F | IFN-1 | XM026209933.1 | GTGGACATCCAGGGACTGTTAAG | RT-qPCR |
| IFN-1-R | CATTGCTCTGGATTCATGTGT | |||
| CyHV-2-F | ORF71 | KT387800.1 | TTAGCGTCAGGTCCATAG | virus load |
| CyHV-2-R | GGCGTGTAGAAATCAAACT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yang, M.; Luo, S.; Yang, G.; Lu, X.; Lu, J.; Chen, J. Oral Vaccination of Recombinant Saccharomyces cerevisiae Expressing ORF132 Induces Protective Immunity against Cyprinid Herpesvirus-2. Vaccines 2023, 11, 186. https://doi.org/10.3390/vaccines11010186
Wang L, Yang M, Luo S, Yang G, Lu X, Lu J, Chen J. Oral Vaccination of Recombinant Saccharomyces cerevisiae Expressing ORF132 Induces Protective Immunity against Cyprinid Herpesvirus-2. Vaccines. 2023; 11(1):186. https://doi.org/10.3390/vaccines11010186
Chicago/Turabian StyleWang, Licong, Maoxia Yang, Sheng Luo, Guanjun Yang, Xinjiang Lu, Jianfei Lu, and Jiong Chen. 2023. "Oral Vaccination of Recombinant Saccharomyces cerevisiae Expressing ORF132 Induces Protective Immunity against Cyprinid Herpesvirus-2" Vaccines 11, no. 1: 186. https://doi.org/10.3390/vaccines11010186
APA StyleWang, L., Yang, M., Luo, S., Yang, G., Lu, X., Lu, J., & Chen, J. (2023). Oral Vaccination of Recombinant Saccharomyces cerevisiae Expressing ORF132 Induces Protective Immunity against Cyprinid Herpesvirus-2. Vaccines, 11(1), 186. https://doi.org/10.3390/vaccines11010186








