COVID-19 Vaccination Did Not Increase the Risk of Potentially Related Serious Adverse Events: 18-Month Cohort Study in an Italian Province
Abstract
:1. Introduction
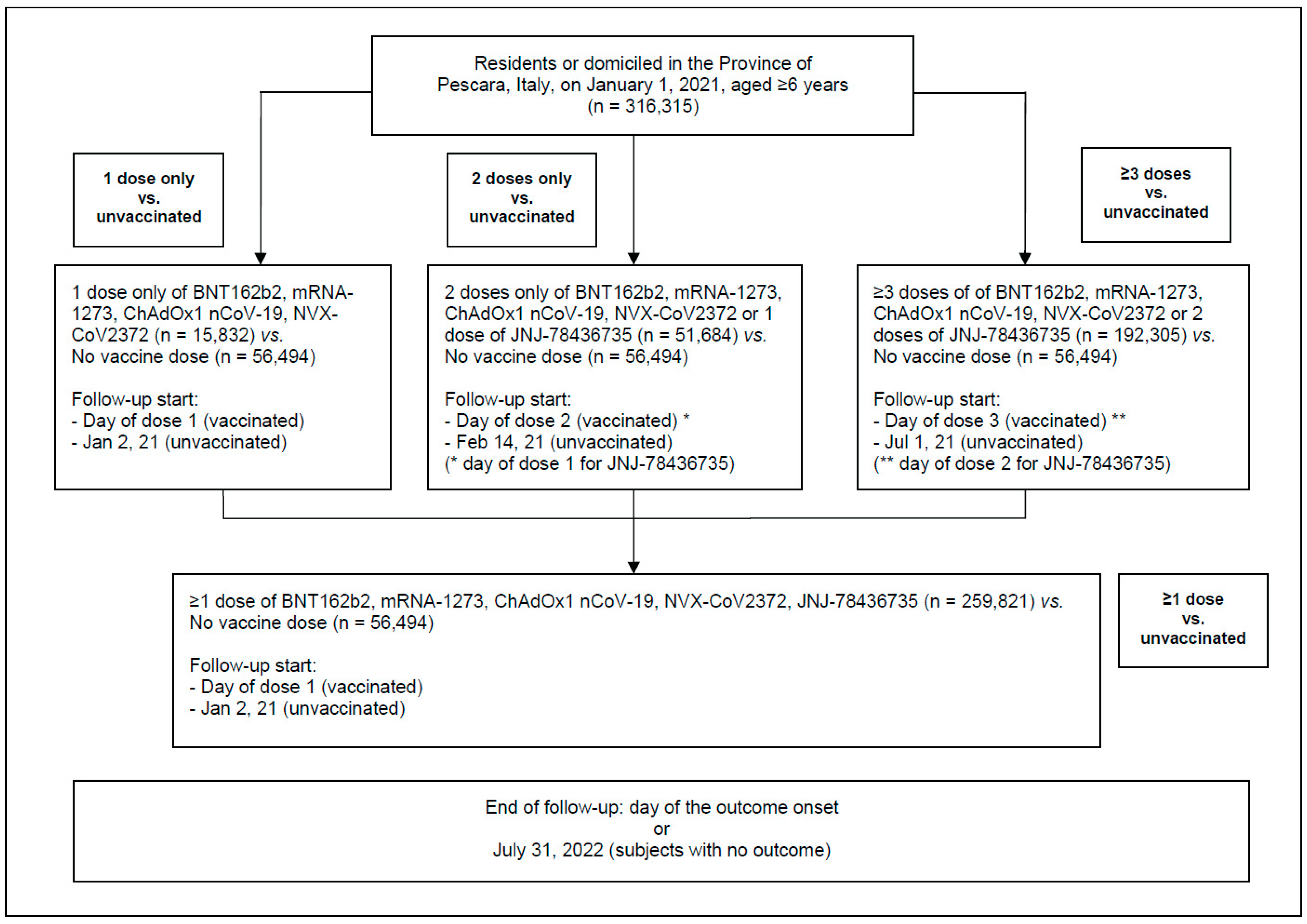
2. Materials and Methods
- individuals who received only one dose of BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, or NVX-CoV2373 were included in the group “1 dose only”;
- individuals who received only one dose of the JNJ-78436735 vaccine or only two doses of BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, or NVX-CoV2373 were included in the group “2 doses only”;
- persons who received three or more doses of BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, or NVX-CoV2373, or two or more vaccine doses, if one of the administered vaccines was JNJ-78436735, were included in the group “≥3 doses”;
- all individuals who received one or more doses of any of the above COVID-19 vaccines, i.e., categories A–C as described above, were included in the group “≥1 dose”.
- When comparing unvaccinated subjects with either those who received one or more doses of vaccine (primary analysis) or those that received one dose only, the follow-up started the day of the first (or single) dose of vaccine for vaccinated subjects and on 2 January 2021 for the unvaccinated.
- When comparing unvaccinated subjects with subjects who received only two doses of vaccine, the follow-up started the day of the second dose of vaccine for vaccinated subjects and on 14 January 2022 (first day of the second dose administration) for the unvaccinated.
- When comparing unvaccinated subjects with those who received three or more doses of vaccine, the follow-up started the day of the third dose of vaccine for vaccinated individuals and on 1 July 2021 (first day of third dose administration) for unvaccinated subjects. The sample varied for the latter two analyses, as subjects who died before the start of the follow-up were excluded.
2.1. Outcomes
2.2. Data Collection
2.3. Statistical Analyses
3. Results
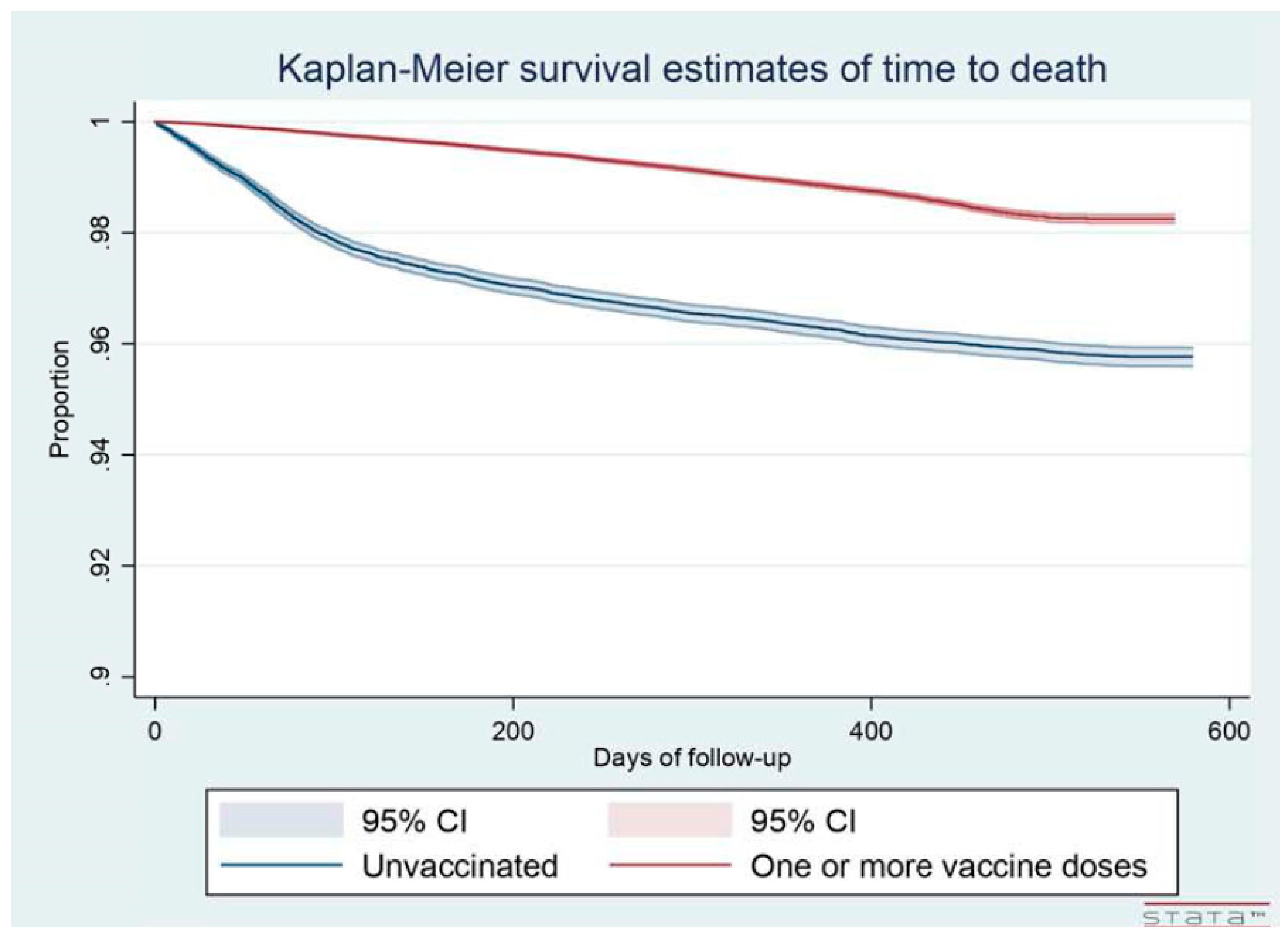
3.1. Risk of Death
3.2. Potentially Vaccine-Related Serious Adverse Events (PVR-SAE)
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acuti Martellucci, C.; Flacco, M.E.; Cappadona, R.; Bravi, F.; Mantovani, L.; Manzoli, L. SARS-CoV-2 pandemic: An overview. Adv. Biol. Regul. 2020, 77, 100736. [Google Scholar] [CrossRef] [PubMed]
- Acuti Martellucci, C.; Flacco, M.E.; Soldato, G.; Di Martino, G.; Carota, R.; Caponetti, A.; Manzoli, L. Effectiveness of COVID-19 Vaccines in the General Population of an Italian Region before and during the Omicron Wave. Vaccines 2022, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Flacco, M.E.; Soldato, G.; Acuti Martellucci, C.; Carota, R.; Di Luzio, R.; Caponetti, A.; Manzoli, L. Interim Estimates of COVID-19 Vaccine Effectiveness in a Mass Vaccination Setting: Data from an Italian Province. Vaccines 2021, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Huang, D.; Jiang, Q.; Guo, Y.; Chen, C. The Vaccine Efficacy Against the SARS-CoV-2 Omicron: A Systemic Review and Meta-Analysis. Front. Public Health 2022, 10, 940956. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Voleti, N.; Groff, D.; Sun, A.; Ba, D.M.; Nunez, J.; Parent, L.J.; Chinchilli, V.M.; Paules, C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 439. [Google Scholar] [CrossRef]
- Zeng, B.; Gao, L.; Zhou, Q.; Yu, K.; Sun, F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: A systematic review and meta-analysis. BMC Med. 2022, 20, 200. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Xu, S.; Huang, R.; Sy, L.S.; Glenn, S.C.; Ryan, D.S.; Morrissette, K.; Shay, D.K.; Vazquez-Benitez, G.; Glanz, J.M.; Klein, N.P.; et al. COVID-19 Vaccination and Non-COVID-19 Mortality Risk—Seven Integrated Health Care Organizations, United States, December 14, 2020-July 31, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1520–1524. [Google Scholar] [CrossRef]
- Karlstad, O.; Hovi, P.; Husby, A.; Harkanen, T.; Selmer, R.M.; Pihlstrom, N.; Hansen, J.V.; Nohynek, H.; Gunnes, N.; Sundstrom, A.; et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022, 7, 600–612. [Google Scholar] [CrossRef]
- Wong, H.L.; Hu, M.; Zhou, C.K.; Lloyd, P.C.; Amend, K.L.; Beachler, D.C.; Secora, A.; McMahill-Walraven, C.N.; Lu, Y.; Wu, Y.; et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: A cohort study in claims databases. Lancet 2022, 399, 2191–2199. [Google Scholar] [CrossRef]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef]
- Husby, A.; Hansen, J.V.; Fosbol, E.; Thiesson, E.M.; Madsen, M.; Thomsen, R.W.; Sorensen, H.T.; Andersen, M.; Wohlfahrt, J.; Gislason, G.; et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: Population based cohort study. BMJ 2021, 375, e068665. [Google Scholar] [CrossRef] [PubMed]
- Italian Government. Decreto Legge: Misure Urgenti per Fronteggiare L’emergenza COVID-19, in Particolare Nei Luoghi Di Lavoro, Nelle Scuole E Negli Istituti Della Formazione Superiore. [Legal Rule: Urgent Measures to Respond to the COVID-19 Emergency, in Particular in Workplaces, Schools, and Higher Education Institutions]. Available online: https://www.gazzettaufficiale.it/eli/id/2022/01/07/22G00002/sg (accessed on 22 December 2022).
- Italian Ministry of Health. Piano Vaccini Anti COVID-19 [Italian National Immunization Plan against COVID-19]. Available online: http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5452&area=nuovoCoronavirus&menu=vuoto (accessed on 22 December 2022).
- Flacco, M.E.; Soldato, G.; Acuti Martellucci, C.; Di Martino, G.; Carota, R.; Caponetti, A.; Manzoli, L. Risk of SARS-CoV-2 Reinfection 18 Months After Primary Infection: Population-Level Observational Study. Front. Public Health 2022, 10, 884121. [Google Scholar] [CrossRef]
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 2022, 40, 5798–5805. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Flacco, M.E.; Carradori, T.; Volta, C.A.; Cosenza, G.; De Togni, A.; Acuti Martellucci, C.; Parruti, G.; Mantovani, L.; Manzoli, L. Predictors of severe or lethal COVID-19, including Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers, in a sample of infected Italian citizens. PLoS ONE 2020, 15, e0235248. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Andrianou, X.; Bella, A.; Del Manso, M.; Urdiales, A.M.; Fabiani, M.; Bellino, S.; Boros, S.; D’Ancona, F.; Rota, M.C.; et al. COVID-19 Integrated Surveillance System. Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza (accessed on 7 January 2022).
- Italian Institute of Health. Characteristics of COVID-19 patients dying in Italy Report based on available data on 10th January, 2022. Report of the Italian Institute of Health, Rome, Italy, 10 January 2022. 2022. Available online: https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_10_january_2022.pdf (accessed on 22 December 2022).
- Italian Government. Raccomandazioni ad interim sui gruppi target della vaccinazione anti SARS-CoV-2/COVID-19. 2021. Guidelines of the Italian Government, Rome, Italy. Gazzetta Ufficiale, Serie Generale, n. 72, 24 March 2021. Available online: https://www.gazzettaufficiale.it/do/gazzetta/serie_generale/3/pdfPaginato?dataPubblicazioneGazzetta=20210324&numeroGazzetta=72&tipoSerie=SG&tipoSupplemento=GU&numeroSupplemento=0&progressivo=0&numPagina=1&edizione=0&rangeAnni= (accessed on 22 December 2022).
- Manzoli, L.; La Vecchia, C.; Flacco, M.E.; Capasso, L.; Simonetti, V.; Boccia, S.; Di Baldassarre, A.; Villari, P.; Mezzetti, A.; Cicolini, G. Multicentric cohort study on the long-term efficacy and safety of electronic cigarettes: Study design and methodology. BMC Public Health 2013, 13, 883. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. (Eds.) Applied Survival Analysis; John Wiley and Sons: New York, NY, USA, 1999. [Google Scholar]
- Sun, C.L.F.; Jaffe, E.; Levi, R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Sci. Rep. 2022, 12, 6978. [Google Scholar] [CrossRef]
- Malhotra, A. Curing the pandemic of misinformation on COVID-19 mRNA vaccines through real evidence-based medicine—Part 1. J. Insul. Resist. 2022, 5, a71. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Bardosh, K.; Krug, A.; Jamrozik, E.; Lemmens, T.; Keshavjee, S.; Prasad, V.; Makary, M.A.; Baral, S.; Hoeg, T.B. COVID-19 Vaccine Boosters for Young Adults: A Risk-Benefit Assessment and Five Ethical Arguments against Mandates at Universities. J. Med. Ethics 2022. [Google Scholar] [CrossRef]
- Dag Berild, J.; Bergstad Larsen, V.; Myrup Thiesson, E.; Lehtonen, T.; Grosland, M.; Helgeland, J.; Wolhlfahrt, J.; Vinslov Hansen, J.; Palmu, A.A.; Hviid, A. Analysis of Thromboembolic and Thrombocytopenic Events After the AZD1222, BNT162b2, and MRNA-1273 COVID-19 Vaccines in 3 Nordic Countries. JAMA Netw. Open 2022, 5, e2217375. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Reconciling estimates of global spread and infection fatality rates of COVID-19: An overview of systematic evaluations. Eur. J. Clin. Investig. 2021, 51, e13554. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef]
- Jabagi, M.J.; Botton, J.; Bertrand, M.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2022, 327, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fu, F.; Ding, L.; Fang, J.; Xiao, J. Booster dose of COVID-19 mRNA vaccine does not increase risks of myocarditis and pericarditis compared with primary vaccination: New insights from the vaccine adverse event reporting system. Front. Immunol. 2022, 13, 938322. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.A.; McBride, S.K.; Leier, H.C.; Guzman, G.; Lyski, Z.L.; Schoen, D.; Winders, B.; Lee, J.Y.; Lee, D.X.; Messer, W.B.; et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci. Immunol. 2022, 7, eabn8014. [Google Scholar] [CrossRef]
- Watson, J.; Whiting, P.F.; Brush, J.E. Interpreting a covid-19 test result. BMJ 2020, 369, m1808. [Google Scholar] [CrossRef]
- Bendavid, E.; Mulaney, B.; Sood, N.; Shah, S.; Bromley-Dulfano, R.; Lai, C.; Weissberg, Z.; Saavedra-Walker, R.; Tedrow, J.; Bogan, A.; et al. COVID-19 antibody seroprevalence in Santa Clara County, California. Int. J. Epidemiol. 2021, 50, 410–419. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Over- and under-estimation of COVID-19 deaths. Eur. J. Epidemiol. 2021, 36, 581–588. [Google Scholar] [CrossRef]
- Axfors, C.; Ioannidis, J.P.A. Infection fatality rate of COVID-19 in community-dwelling elderly populations. Eur. J. Epidemiol. 2022, 37, 235–249. [Google Scholar] [CrossRef]

| Total Sample | Unvaccinated | 1 Dose I | 2 Doses II | ≥3 doses III | ≥1 Dose IV | |
|---|---|---|---|---|---|---|
| (n = 316,315) | (n = 56,494) | (n = 15,832) | (n = 51,684) | (n = 192,305) | (n = 259,821) | |
| Male gender, % | 48.9 | 50.5 | 51.0 | 50.3 | 47.9 | 48.5 |
| Mean age in years (SD) | 48.0 (22.0) | 40.8 (22.8) | 41.8 (20.6) | 38.1 (21.9) | 53.3 (20.2) | 49.6 (21.5) |
| Age category in years, % | ||||||
| 6–29 | 24.0 | 33.6 | 32.7 | 41.1 | 16.0 | 22.0 |
| 30–59 | 44.8 | 46.2 | 47.8 | 42.5 | 44.7 | 44.4 |
| 60 or more | 31.2 | 20.2 | 19.5 | 16.4 | 39.4 | 33.6 |
| Risk factors and comorbiditiesV, % | ||||||
| Hypertension | 13.2 | 7.5 | 8.6 | 7.4 | 16.9 | 14.5 |
| Diabetes | 5.1 | 3.1 | 3.2 | 3.0 | 6.4 | 5.5 |
| CVD | 6.3 | 4.3 | 4.0 | 4.1 | 7.7 | 6.8 |
| COPD | 3.3 | 2.6 | 2.4 | 3.2 | 3.7 | 3.5 |
| Kidney disease | 1.3 | 1.0 | 0.5 | 1.0 | 1.5 | 1.3 |
| Cancer | 4.8 | 3.2 | 3.0 | 2.7 | 6.0 | 5.1 |
| Past potentially vaccine-related serious adverse events (PVR-SAE) VI, % | ||||||
| PVR-SAE, total | 3.8 | 2.6 | 2.9 | 2.4 | 4.5 | 4.0 |
| Myocardial infarction | 1.4 | 0.8 | 1.0 | 0.7 | 1.7 | 1.5 |
| Acute heart failure | 0.06 | 0.03 | 0.03 | 0.04 | 0.07 | 0.06 |
| Cardiac arrest | 0.03 | 0.04 | 0.04 | 0.02 | 0.04 | 0.03 |
| Ischemic stroke | 1.1 | 0.9 | 0.9 | 0.7 | 1.3 | 1.2 |
| Hemorrhagic stroke | 0.4 | 0.3 | 0.3 | 0.3 | 0.5 | 0.4 |
| Coronary artery dissection | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| Aortic aneurysm | 0.5 | 0.3 | 0.3 | 0.3 | 0.7 | 0.6 |
| Peripheral aneurysm | 0.10 | 0.09 | 0.06 | 0.08 | 0.12 | 0.11 |
| Pulmonary embolism | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 |
| Deep vein thrombosis | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 |
| Myocarditis | 0.04 | 0.05 | 0.04 | 0.04 | 0.05 | 0.04 |
| Pericarditis | 0.2 | 0.1 | 0.2 | 0.2 | 0.3 | 0.2 |
| Type of vaccine, % | ||||||
| BNT162b2 | 45.4 | -- | 51.2 | 66.6 | 38.4 | 45.4 |
| mRNA-1273 | 16.0 | -- | 47.4 | 15.5 | 11.9 | 16.0 |
| ChAdOx1 nCoV-19 | 0.9 | -- | 1.2 | 13.0 | 0.0 | 0.9 |
| JNJ-78436735 | 0.2 | -- | -- | 2.1 | 0.0 | 0.2 |
| NVX-CoV2373 | 0.1 | -- | 0.2 | 0.1 | 0.0 | 0.1 |
| Mixed VII | 37.4 | -- | -- | 2.8 | 49.7 | 37.4 |
| Infected with SARS-CoV-2 VIII, % | 34.3 | 40.0 | 74.6 | 51.2 | 24.8 | 33.1 |
| Mean follow-up, days (SD) | 428 (111) | 561 (90) IX | 204 (70) | 279 (91) | 209 (37) | 399 (93) X |
| (A) | Total Sample | Unvaccinated | 1 Dose I | 2 Doses II | ≥3 Doses III | ≥1 Dose IV |
|---|---|---|---|---|---|---|
| (N = 316,315) | (N = 56,494) | (N = 259,821) | ||||
| Death, overall (N) | (15,832) | (51,684) | (192,305) | |||
| % (n) | 1.82 (5743) | 4.23 (2392) | 1.74 (275) | 3.52 (1819) | 0.65 (1257) | 1.29 (3351) |
| Mean monthly rate x1000 | 1.27 | 2.26 | 2.55 | 3.78 | 0.94 | 0.97 |
| Non-COVID-19 Death | ||||||
| % (n) | 1.54 (4873) | 3.21 (1815) | 1.62 (256) | 3.32 (1715) | 0.57 (1087) | 1.18 (3058) |
| Mean monthly rate x1000 | 1.08 | 1.72 | 2.38 | 3.57 | 0.81 | 0.88 |
| Death among the uninfected only (N) | (207,721) | (33,899) | (4015) | (25,243) | (144,564) | (173,822) |
| % (n) | 2.18 (4536) | 4.75 (1611) | 5.70 (229) | 6.65 (1679) | 0.70 (1017) | 1.68 (2925) |
| Mean monthly rate x1000 | 1.51 | 2.55 | 7.34 | 7.95 | 1.01 | 1.23 |
| (B) | Total Sample | Unvaccinated | 1 Dose I | 2 Doses II | ≥3 Doses III | ≥1 Dose IV |
| (N = 316,315) | (N = 56,494) | (N = 259,821) | ||||
| PVR-SAE, total * (N) | (N = 316,315) | (N = 56,494) | (15,913) | (52,067) | (191,841) | (N = 259,821) |
| % (n) | 0.66 (2097) | 0.97 (549) | 0.88 (140) | 1.57 (818) | 0.31 (590) | 0.60 (1548) |
| Mean monthly rate x1000 | 0.46 | 0.52 | 1.29 | 1.69 | 0.44 | 0.45 |
| Myocardial infarction (N) | (15,862) | (51,816) | (192,143) | |||
| % (n) | 0.15 (475) | 0.12 (67) | 0.25 (39) | 0.38 (198) | 0.09 (171) | 0.16 (408) |
| Acute heart failure (N) | (15,832) | (51,688) | (192,301) | |||
| % (n) | 0.01 (38) | 0.01 (4) | 0.01 (1) | 0.03 (18) | 0.01 (15) | 0.01 (34) |
| Cardiac arrest (N) | (15,832) | (51,688) | (192,301) | |||
| % (n) | 0.15 (477) | 0.39 (221) | 0.16 (26) | 0.27 (141) | 0.05 (89) | 0.10 (256) |
| Ischemic stroke (N) | (15,855) | (51,790) | (192,176) | |||
| % (n) | 0.16 (495) | 0.18 (103) | 0.22 (35) | 0.44 (229) | 0.07 (128) | 0.15 (392) |
| Hemorrhagic stroke (N) | (15,840) | (51,733) | (192,248) | |||
| % (n) | 0.10 (306) | 0.13 (75) | 0.11 (18) | 0.23 (120) | 0.05 (93) | 0.09 (231) |
| Coronary dissection (N) | (15,832) | (51,685) | (192,304) | |||
| % (n) | 0.00 (6) | 0.00 (2) | 0.00 (0) | 0.00 (2) | 0.00 (2) | 0.00 (4) |
| Aortic aneurism (N) | (15,846) | (51,741) | (192,234) | |||
| % (n) | 0.06 (192) | 0.04 (21) | 0.11 (18) | 0.16 (81) | 0.04 (72) | 0.07 (171) |
| Peripheral aneurism (N) | (15,835) | (51,697) | (192,289) | |||
| % (n) | 0.01 (47) | 0.01 (8) | 0.02 (3) | 0.04 (20) | 0.01 (16) | 0.02 (39) |
| Pulmonary embolism (N) | (15,835) | (51,711) | (192,275) | |||
| % (n) | 0.05 (162) | 0.10 (56) | 0.06 (9) | 0.12 (63) | 0.02 (34) | 0.04 (106) |
| Deep vein thrombosis (N) | (18,539) | (51,697) | (192,285) | |||
| % (n) | 0.03 (101) | 0.05 (30) | 0.06 (9) | 0.07 (38) | 0.01 (24) | 0.03 (71) |
| Myocarditis (N) | (15,832) | (51,690) | (192,299) | |||
| % (n) | 0.00 (9) | 0.00 (1) | 0.00 (0) | 0.01 (7) | 0.00 (1) | 0.00 (8) |
| Pericarditis (N) | (15,836) | (51,693) | (192,292) | |||
| % (n) | 0.01 (37) | 0.01 (6) | 0.03 (5) | 0.04 (19) | 0.00 (7) | 0.01 (31) |
| 1 Dose III | 2 Doses IV | ≥3 Doses V | ≥1 Dose VI | |
|---|---|---|---|---|
| Outcomes | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Death, total sample | 0.82 (0.72–0.94) | 1.10 (1.06–1.13) | 0.66 (0.64–0.68) | 0.19 (0.18–0.20) |
| Uninfected only VII | 2.08 (1.80–2.39) | 1.29 (1.25–1.34) | 0.66 (0.64–0.69) | 0.22 (0.20–0.23) |
| Infected only VII | 0.19 (0.14–0.25) | 0.55 (0.50–0.60) | 0.66 (0.62–0.70) | 0.11 (0.10–0.13) |
| All PVR-SAEs, total sample | 1.59 (1.30–1.93) | 1.43 (1.35–1.51) | 0.76 (0.72–0.80) | 0.39 (0.36–0.43) |
| Uninfected only VII | 3.69 (3.00–4.55) | 1.74 (1.63–1.85) | 0.82 (0.76–0.87) | 0.52 (0.47–0.59) |
| Infected only VII | 0.23 (0.14–0.39) | 0.54 (0.46–0.65) | 0.58 (0.51–0.66) | 0.12 (0.09–0.15) |
| Myocardial infarction | 4.96 (3.20–7.67) | 2.10 (1.81–2.44) | 0.95 (0.82–1.09) | 0.84 (0.64–1.09) |
| Cardiac arrest | 0.49 (0.32–0.75) | 0.95 (0.85–1.05) | 0.64 (0.57–0.72) | 0.18 (0.15–0.22) |
| Ischemic stroke | 2.60 (1.74–3.88) | 1.71 (1.52–1.92) | 0.76 (0.67–0.86) | 0.50 (0.40–0.63) |
| Hemorrhagic stroke | 1.81 (1.06–3.10) | 1.51 (1.31–1.76) | 0.75 (0.65–0.87) | 0.45 (0.34–0.59) |
| Aortic aneurysm | 7.51 (3.92–14.4) | 2.21 (1.73–2.82) | 0.85 (0.69–1.04) | 0.96 (0.61–1.52) |
| Pulmonary embolism | 1.00 (0.48–2.10) | 1.31 (1.09–1.57) | 0.67 (0.55–0.82) | 0.30 (0.22–0.42) |
| Deep vein thrombosis | 2.78 (1.27–6.09) | 1.45 (1.13–1.85) | 0.71 (0.55–0.91) | 0.37 (0.24–0.57) |
| Myocarditis/Pericarditis | 5.86 (1.73–19.8) | 2.10 (1.37–3.22) | 0.67 (0.42–1.06) | 0.74 (0.32–1.67) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flacco, M.E.; Acuti Martellucci, C.; Soldato, G.; Di Martino, G.; Carota, R.; De Benedictis, M.; Di Marco, G.; Parruti, G.; Di Luzio, R.; Caponetti, A.; et al. COVID-19 Vaccination Did Not Increase the Risk of Potentially Related Serious Adverse Events: 18-Month Cohort Study in an Italian Province. Vaccines 2023, 11, 31. https://doi.org/10.3390/vaccines11010031
Flacco ME, Acuti Martellucci C, Soldato G, Di Martino G, Carota R, De Benedictis M, Di Marco G, Parruti G, Di Luzio R, Caponetti A, et al. COVID-19 Vaccination Did Not Increase the Risk of Potentially Related Serious Adverse Events: 18-Month Cohort Study in an Italian Province. Vaccines. 2023; 11(1):31. https://doi.org/10.3390/vaccines11010031
Chicago/Turabian StyleFlacco, Maria Elena, Cecilia Acuti Martellucci, Graziella Soldato, Giuseppe Di Martino, Roberto Carota, Marco De Benedictis, Graziano Di Marco, Giustino Parruti, Rossano Di Luzio, Antonio Caponetti, and et al. 2023. "COVID-19 Vaccination Did Not Increase the Risk of Potentially Related Serious Adverse Events: 18-Month Cohort Study in an Italian Province" Vaccines 11, no. 1: 31. https://doi.org/10.3390/vaccines11010031
APA StyleFlacco, M. E., Acuti Martellucci, C., Soldato, G., Di Martino, G., Carota, R., De Benedictis, M., Di Marco, G., Parruti, G., Di Luzio, R., Caponetti, A., & Manzoli, L. (2023). COVID-19 Vaccination Did Not Increase the Risk of Potentially Related Serious Adverse Events: 18-Month Cohort Study in an Italian Province. Vaccines, 11(1), 31. https://doi.org/10.3390/vaccines11010031







