Abstract
The effects of cytosine phosphoguanine oligodeoxynucleotides (CPG ODNs) on immune response have been demonstrated for different vaccines; however, such information is limited for the vector-based Coronavirus disease 2019 (COVID-19). This paper aims to demonstrate the potential effect of CPG ODNs on immunological response against the vector-based COVID-19 vaccine on Balb/c mice using a JNJ-78436735 Ad26.COV2-S recombinant as a model vaccine. A total of 18 BALB/c mice clustered into six groups were used. All groups were observed for 14- and 28-days post immunization. Qualitative determination of IgG was performed using indirect Enzyme-Linked Immunosorbent Assay (ELISA) and qPCR for cytokine profiling. A significant (p ≤ 0.001) rise in antibody response was observed for groups 3 and 4, who also showed increased expression levels of Tumor Necrosis Factor (TNF) and Interferon Gamma (IFN-γ). Immunological parameters for toxicity were normal in all treatment groups. We conclude that supplementing vector-based COVID-19 vaccines with CpG ODNs has the potential to boost the body’s immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
1. Introduction
The 2019 coronavirus disease (COVID-19) pandemic is a public health concern that has not only led to high morbidity and mortality in the populations, but has negatively affected the global economy [1,2]. The health and economic burden caused by this pandemic therefore calls for an urgent launch of effective measures against SARS- CoV-2 [2], with the aim of ending the pandemic, or reducing its intensity on the economy and disease severity in the populations [3,4]. The spike protein has been demonstrated to be the most effective SARS-CoV-2 antigen and is thought to be the crucial target for SARS-CoV-2 vaccines [5,6]. The development of vaccines against SARS-CoV-2 is a strategy for preventing and ending the pandemic where different vaccine technologies have been developed such as inactivated vaccines, recombinant protein vaccines, live-attenuated vaccines, viral vectors (adenovirus) vaccines, DNA vaccines, and mRNA vaccines [5]. Adenovirus-vector-based vaccines elicit powerful immunological responses due to the presence of viral proteins and the stimulation of an innate immune response [6].
Angiotensin-Converting Enzyme 2 (ACE2) and the Receptor-Binding Domain (RBD) of the spike glycoprotein are both present on the surface of human host cells, and their interactions with the spike receptor glycoproteins of SARS-CoV-2 allow the virus to infect and enter the cells causing infection [7]. The spike glycoprotein of SARS-CoV-2 is therefore an appropriate target for a vaccine owing to the viral mechanism of cell invasion [8]. Clinical trials have shown that recipients of a single dose of the COVID-19 Janssen Vaccine were 67% protected against symptoms of SARS-CoV-2 infection, 77% from severe/critical COVID-19 at 14 days, 85% protected after 28 days, and 93% were protected against hospitalizations [9]. Lower vaccine efficacy has been noted however with the development of new variants [10]. To boost the development of protective and therapeutic vaccination responses, it is desirable to find adjuvants that could help antigen-presenting cells (APC) to stimulate CD8+ T-cell responses in the absence of T-cell help [11].
The cytosine phosphoguanine oligodeoxynucleotides (CpG ODN) have been shown to maximize immune response [12]. They are single-stranded synthetic DNA molecules with unmethylated CpG dinucleotides in specific sequence contexts (CpG motifs) [13]. They can cause Toll-like Receptor-9 (TLR-9)-expressing cells, such as human plasmacytoid dendritic cells and B cells, to produce an innate immune response that includes the production of T helper-1 and proinflammatory cytokines [14]. Their potential as a vaccine adjuvant has been proven in trials using ovalbumin, heterologous gammaglobulin, and hen egg lysozyme as model antigens [15]. In all the latter studies it has been demonstrated that CpG ODN is a greater Th1-like adjuvant compared to the “gold standard” (complete Freund’s adjuvant (CFA)) based on its capacity to promote the development of Interferon-secreting T cells and cytotoxic T cells. Furthermore, other studies have demonstrated that CpG ODN achieved an increased antigen-specific activation level without triggering any of the severe local inflammatory effects seen with CFA [16]. Co-administering CpG ODNs with vaccines boosts the formation of humoral and cellular vaccine-specific immune responses and improves the activity of professional antigen-presenting cells [17].
In this study, an increased effect of the CpG oligodeoxynucleotides (ODNs) adjuvant on immunological response against viral vector-based SARS-CoV-2 vaccine was demonstrated in BALB/c mice.
2. Materials and Methods
2.1. Study Approval
Approval for animal use was obtained from the Mount Kenya University scientific Review Committee. (Approval number 1386).
2.2. Animal Model, Sample Stratification, and Immunization
A total of 18 female BALB/c mice (20 ± 2 g) purchased from the Institute of Primate Research (IPR) in Kenya were used in this study. The mice were acclimated for seven (7) days in a standard facility at the Kenya Medical Research Institute (KEMRI). During this time, mice were fed on commercial pellets and had access to water. They were clustered into 6 groups. Mice in group 1 and group 2 received a single subcutaneous injection of 4 × 109 VP and 8 × 109 VP Janssen vaccine (JNJ-78436735 Ad26.COV2-S, recombinant; Janssen Pharmaceutical Companies, USA), respectively. Mice in group 3 and group 4 first received a single subcutaneous injection of 4 × 109 VP and 8 × 109 VP Janssen vaccine, respectively, followed by a single dose of 0.1 nM/µL of CpG ODNs (Table 1). Group 5 mice received a single dose of 0.1 nM/µL of CpG ODNs while group 6 mice received 1X PBS.

Table 1.
Types of cytosine phosphoguanine oligodeoxynucleotides (CpG ODNs) used in the study.
2.3. Sample Collection
Mice were bled on day 14 and day 28 after vaccination, and the blood was separated to obtain plasma or retained as whole blood. Blood for plasma was collected by bleeding from the tail whereas whole blood was collected via cardiac puncture. The tail blood was mixed with the heparin injection in a 1.25 mL cryovial tube, centrifuged at 1400 rpm for 10 min, and the supernatant was collected. Before collection of the whole blood, mice were euthanized using CO2 and 700 μL collected in 1.5 mL EDTA-containing collection tubes. Plasma and whole blood collected were stored at −20 °C and −80 °C, respectively. Moreover, spleen tissues were obtained by dissecting the mice and placing the spleen in sterile 1.5 mL Eppendorf tubes while still on ice. The spleen samples were stored at −80 °C before they were used for total RNA extraction and other downstream experiments.
2.4. Evaluation of Humoral Immune Responses to the Vaccine
Enzyme Linked Immunosorbent Assay (ELISA) was used to determine SARS-CoV-2 anti-spike IgG levels using the Mouse Anti-SARS-CoV-2 Spike Protein Antibody IgG Titer Serologic ELISA Kit (Solarbio Science & Technology Co., Ltd., Beijing, China). The ELISA plates were read at 450 nm using the VersaMax™ ELISA Microplate Reader (Sunnyvale, CA, USA). Samples from each mouse were assayed in duplicate and the mean OD (Optical Density) value was used to represent each experimental unit.
2.5. mRNA Expression of TNF and INF-γ on Immunized Mice
Total RNA was extracted using the Total RNA Extraction Kit (Solarbio Science & Technology Co., Ltd., Beijing, China) as per the manufacturer’s instructions. The RNA concentration and purity were assessed using the NanoDrop™ 2000/2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at absorbance 260/280 and samples stored at −80 °C for further experiments. Synthesis of cDNA from extracted total RNA samples was completed using the Universal RT-PCR Kit and protocol (Solarbio Science & Technology (Beijing, China).
2.6. Quantitative Polymerase Chain Reaction (qPCR)
A 2X SYBR Green quantitative PCR Mastermix (Solarbio Science & Technology Co., Ltd., Beijing, China) was used in a 25μL total reaction volume (Table 2). Amplification was completed on the Applied Biosystems Quant Studio 5 (PE Applied Biosystems, Waltham, MA, USA) qPCR platform (Table 2). The primers used to amplify TNF, INF-γ, and the housekeeping genes were as shown in Table 3. Relative quantification of gene expression was calculated using the delta–delta threshold cycle (∆∆Ct) formula ∆Ct = Ct (gene of interest)—Ct (housekeeping gene) according to [17].

Table 2.
Quantitative Real-Time PCR Thermal Profile.

Table 3.
Primers and expected Amplicon sizes for TNF and INF-γ toxicity analysis.
2.7. Assessment of Hematological Profiles and Biochemical Tests
Whole blood count analysis was performed using the HumaCount 30TS (Human Diagnostics Worldwide, Wiesbaden, Germany) hematology analyzer machine following the laboratory protocol [19]. The serum levels of Aspartate Transaminase (AST), Alanine Transferase (ALT), Gamma-Glutamyltransfearse (GGT), Creatinine, and Urea were determined using the Reflotron colorimetric test strips (Woodley Equipment Company, Lancashire, England) and protocols.
2.8. Data Analysis
Antibody levels of the vaccinated groups and the control groups were compared using a one-way analysis of variance (ANOVA) 1-factor test for three or more groups and t-tests for two (2) groups were used to determine the significant differences by using GraphPad prism software. Values were considered significant at 95% confidence intervals (p ≤ 0.05).
3. Results
3.1. Determination of IgG Antibodies
3.1.1. Reliability Testing for the Results Obtained
For each group, a minimum of three mice were inoculated with similar concentrations and a coefficient of variation among the different doses was recorded (Table 4 and Table 5). It was noted that the use of Vac 40/CPG and Vac 80/CPG was reliable, whether on day 14 (CV = 0.6% and CV = 1.6%) or on day 28 (CV = 3.8% and CV = 2.0%), respectively (Table 4 and Table 5).

Table 4.
Reliability of the vaccine concentrations among BALB/c mice at day 14.

Table 5.
Reliability of the vaccine concentrations among BALB/c mice at day 28.
3.1.2. Levels of IgG Generated within Different Experimental Groups
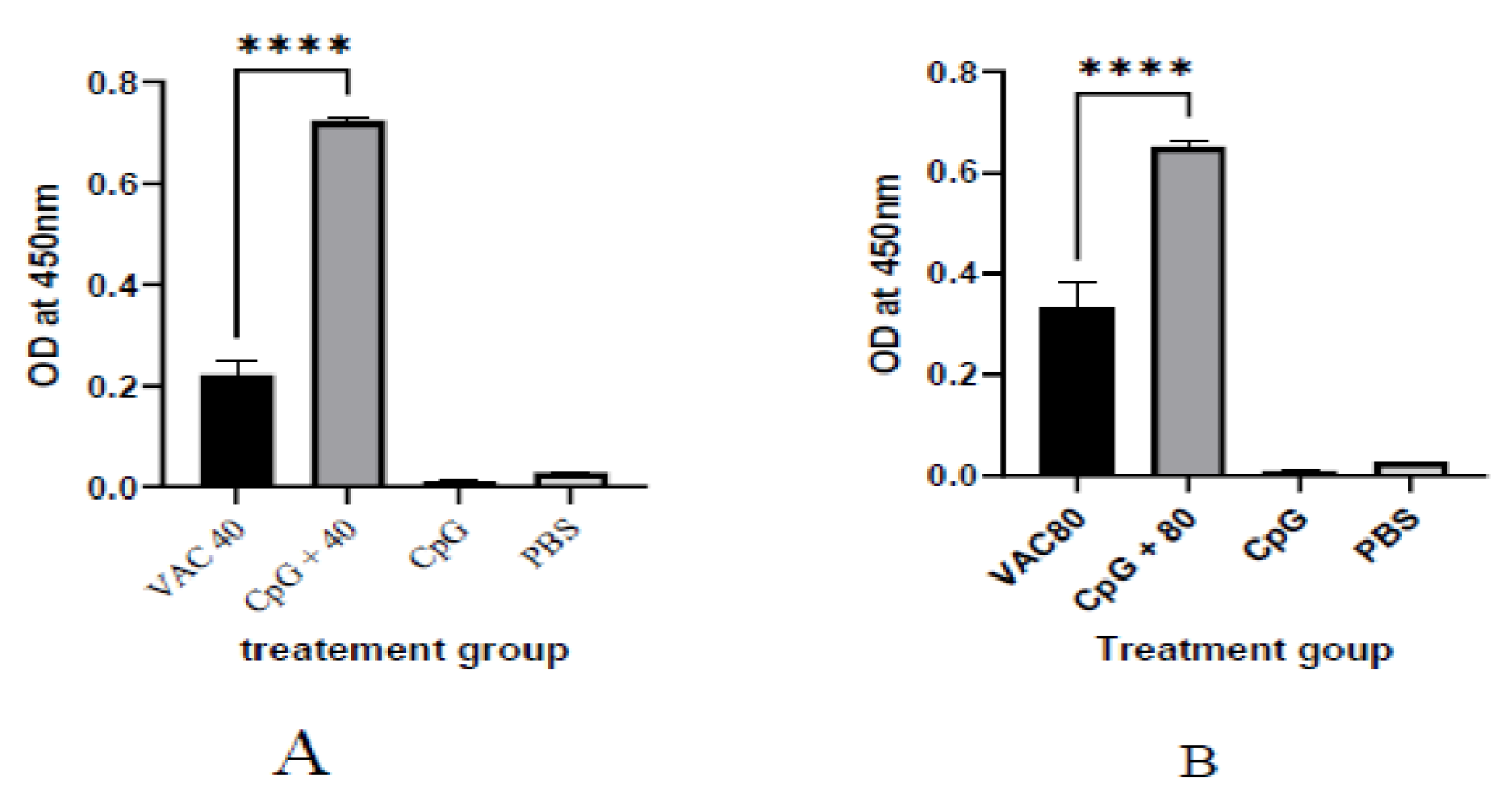
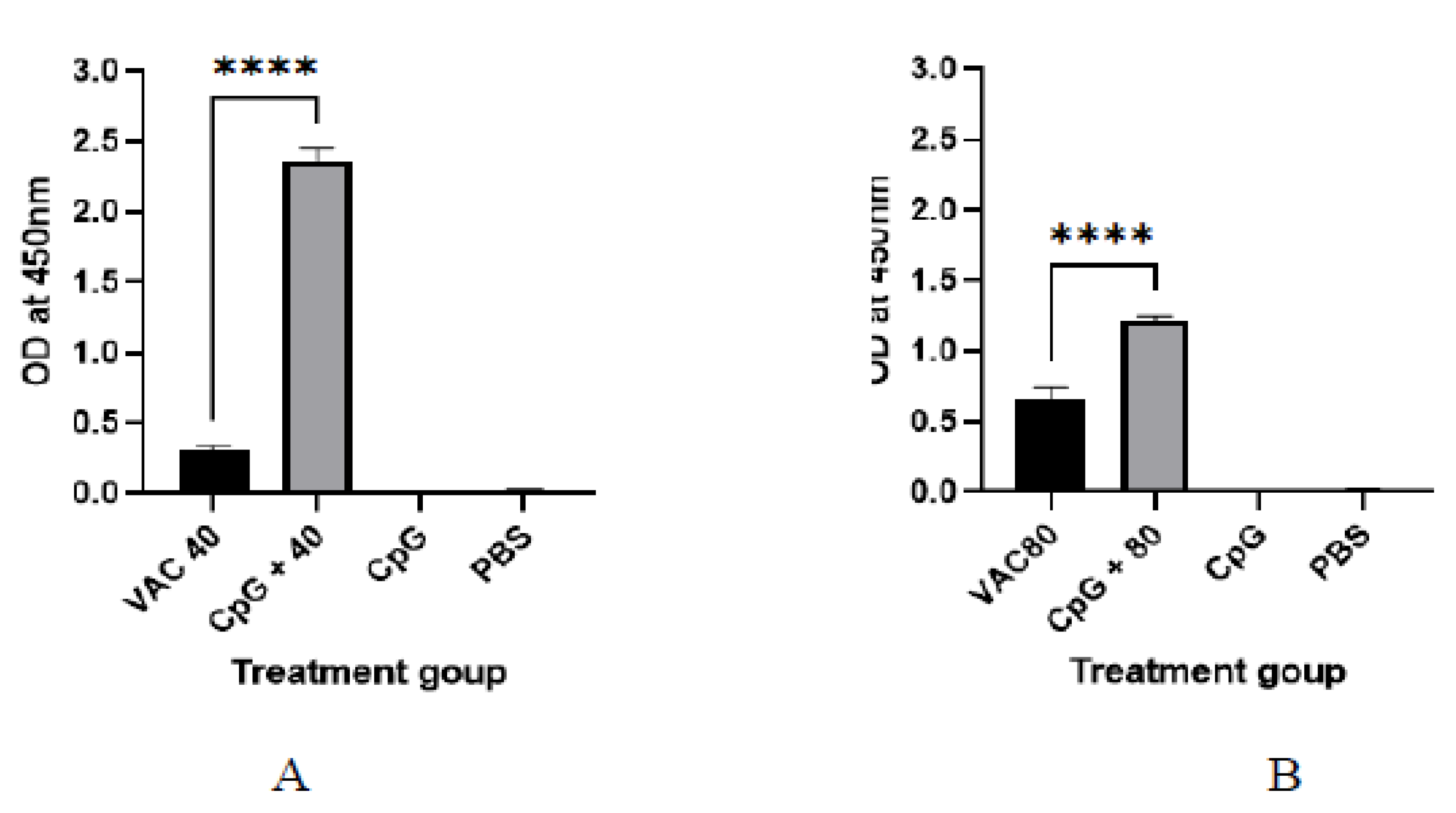
It was shown that among the 4 × 109 VP group at day 14, a statistically significant (p ≤ 0.001), 2-fold increase in IgG was shown for the CPG-supplemented group (OD = 0.7) against the vaccine-only group (OD = 0.22). On the other hand, only about a 1.5-fold increase for the CPG-supplemented against vaccine-only group (OD = 0.33 versus OD = 0.55) was shown among the 4 × 109 VP group (Figure 1). At day 28, the group of 4 × 109 VP was statistically significant (p ≤ 0.001) where a 5.6-fold increase in IgG was shown for the supplemented group (OD = 1.7) against the vaccine-only group (OD = 0.3). On the other hand, there was only a 1.2-fold increase for the supplemented group (OD = 0.7) against the vaccine-only group (0.6) observed among the 8 × 109 VP group (Figure 2).
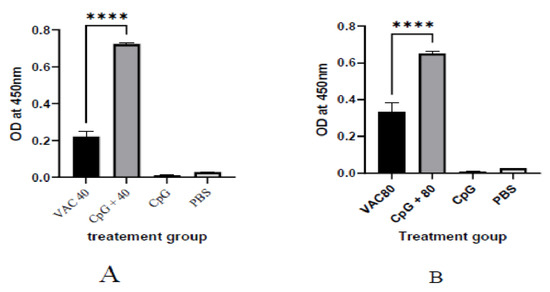
Figure 1.
The IgG antibody immune response to SARS-CoV-2 at 14 days after immunization. (A) The results of SARS-CoV-2 spike protein-specific IgG antibodies in the plasma of 4 × 109 VP of vaccine plus the CpG ODNS group showed statistical significance (p ≤ 0.001), 2-fold increase in IgG against 4 × 109 VP of vaccine. (B) The results of SARS-CoV-2 spike protein-specific IgG antibodies in the plasma of 8 × 109 VP of vaccine plus the CpG ODNs group. There was a 1.5-fold increase for the CPG-supplemented against 8 × 109 VP of the vaccine-only group. A 95% CI was considered for all tests. (**** p < 0.0001).
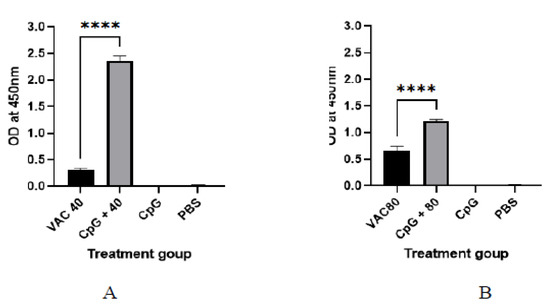
Figure 2.
The IgG antibody immune response to SARS-CoV-2 at 28 days after immunization. (A) The results of SARS-CoV-2 spike protein-specific IgG antibodies in the plasma of 4 × 109 VP of vaccine plus the CpG ODNS group showed statistical significance (p ≤ 0.001) with a 5.6-fold increase in IgG against 4 × 109 VP of vaccine. (B) The results of SARS-CoV-2 spike protein-specific IgG antibodies in the plasma of 8 × 109 VP of vaccine plus the CpG ODNs group. There was a 1.2-fold increase against 8 × 109 VP of the vaccine group. A 95% CI was considered for all tests. A 95% CI was considered for all tests. (**** p < 0.0001).
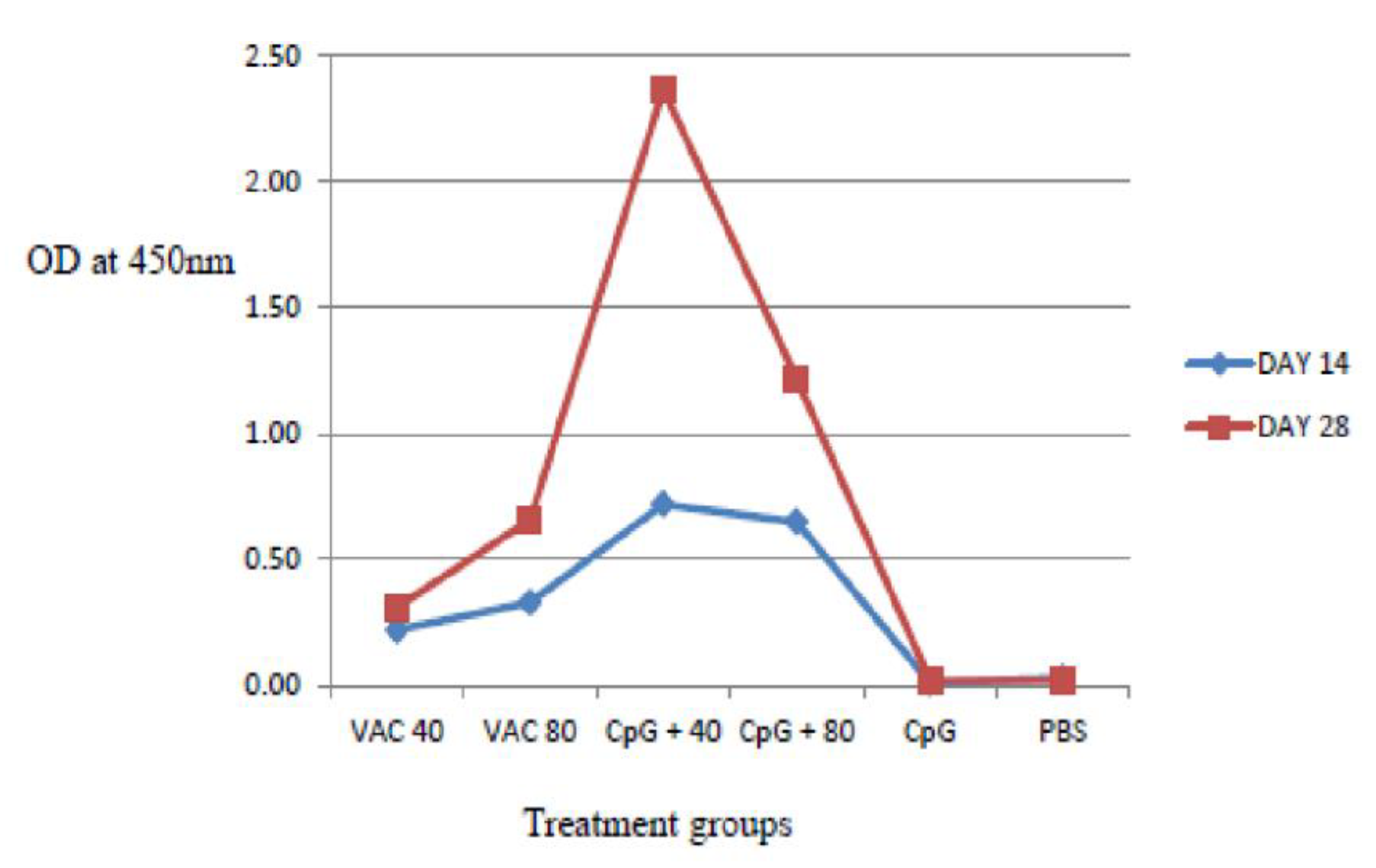
IgG antibody titers on day 28 were found to be higher compared to day 14 both in the vaccine-only and the vaccine plus CpG ODNs groups. The IgG response was significantly stronger in the 4 × 109 VP of vaccine supplemented by CpG ODNs mice, compared to the CpG ODNs and 8 × 109 VP of vaccinated mice (p < 0.06) (Figure 3). For the 8 × 109 VP vaccine concentration, high IgG antibody titers were shown compared to 4 × 109 VP.
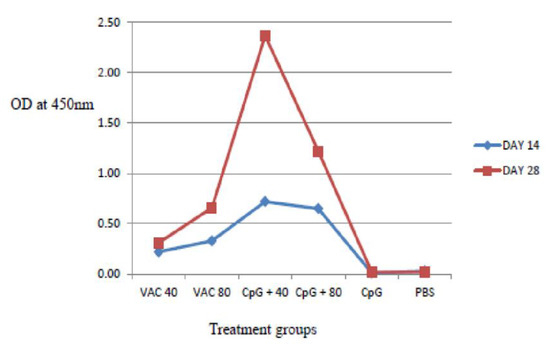
Figure 3.
IgG antibody response to SARS-CoV-2 using ELISA assays on days 14 and 28. The IgG response was significantly stronger in the 4 × 109 VP of vaccine supplemented by CpG ODNs mice, compared to the CpG ODNs and 8 × 109 VP of vaccinated mice (p < 0.06).
3.2. Evaluation of mRNA Expression on Immunized Mice
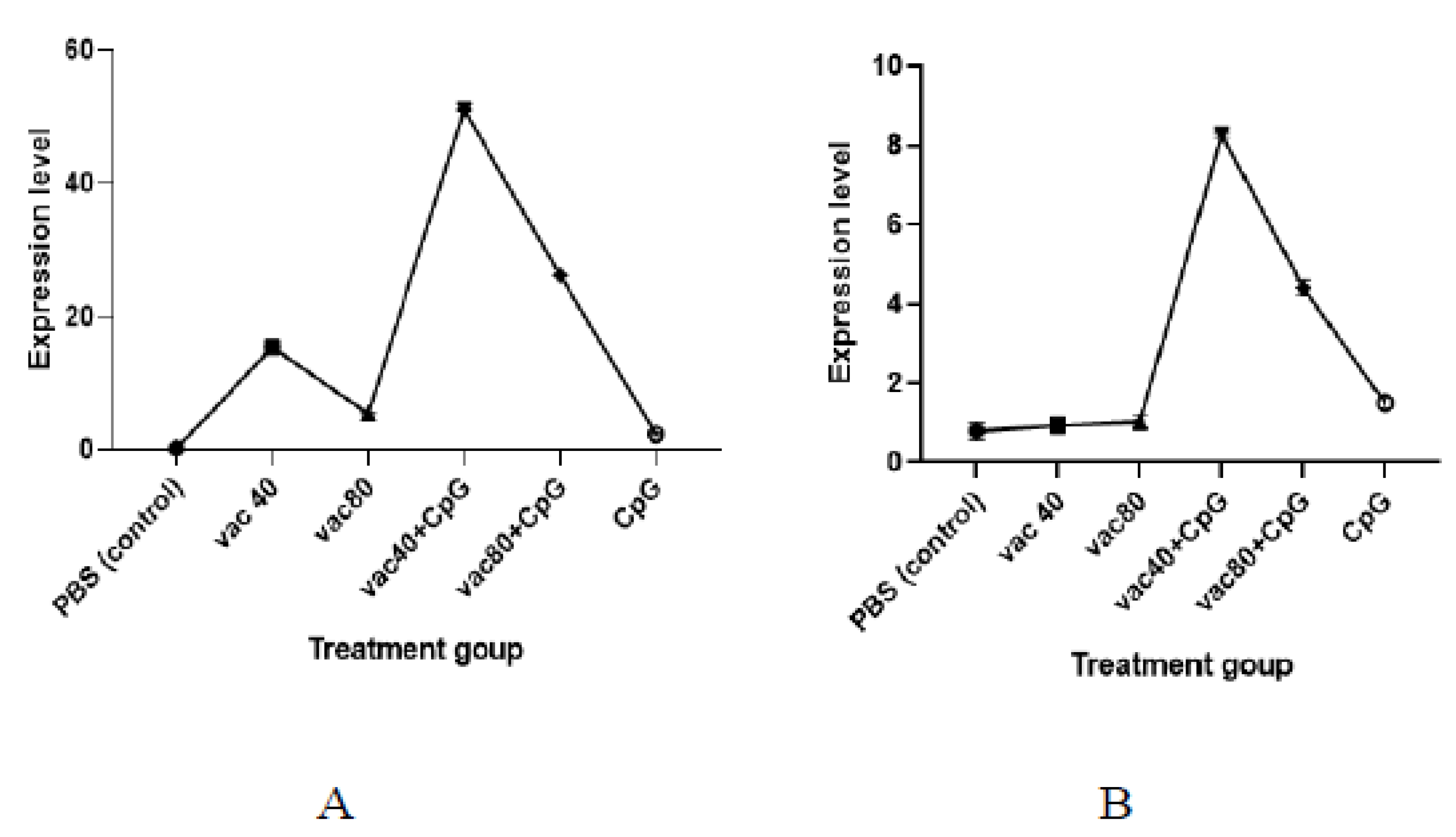
A lower expression level of TNF was observed among the CpG-only treated group compared to the expression level observed for the group treated with 4 × 109 VP and 8 × 109 VP of vaccines (2.3 against 15.4 and 5.5, respectively) (Figure 4). Similarly, the numbers of the expression level of TNF generated in mice immunized with both groups of vaccine (4 × 109 VP and 8 × 109 VP) plus CpG ODNs were higher (51.0 and 26.2, respectively) compared to the vaccine-only treated group. Further, the expression level of TNF for the 4 × 109 VP of vaccine concentration was significantly higher compared to the 8 × 109 VP of vaccine concentration (p< 0.0001). For INF-γ, the group of mice treated with CpG ODNs only showed a higher expression of INF-γ compared to the mice treated with 4 × 109 VP and 8 × 109 VP of vaccine concentrations (1.5 against 0.9 and 1.0, respectively)). Mice treated with 4 × 109 VP of vaccine plus CpG showed a higher expression level compared to the mice treated with 8 × 109 VP of vaccine and CpG (8.3 against 4.4) (p = 0.000997). The findings demonstrate the ability of CpG ODNs to enhance the secretion of TNF and INF-γ.
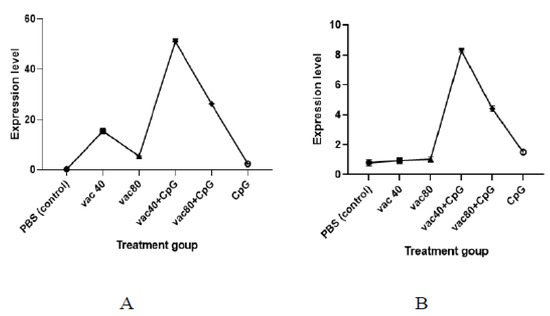
Figure 4.
mRNA expression of TNF (A) and INF-γ (B). VACC 40 and VACC 80: groups treated with 4 × 109 VP and 8 × 109 VP of vaccine concentration, respectively, VACC 40 +CpG and VACC 80 + CpG: groups treated with 4 × 109 VP and 8 × 109 VP of vaccine plus CpG ODNs, CpG: group treated with CpG ODNs, and PBS: group treated with PBS.
3.3. Evaluation of Hematology and Biochemical Parameters
Hematological parameters of the female BALB/c mice immunized with 4 × 109 VP and 8 × 109 VP of the vaccine only (group 1 and group 2), 4 × 109 VP and 8 × 109 VP of vaccine with CpG ODNs (group 3 and group 4), CpG ODNs only (group 5) and PBS (group 6) are shown in Table 6. Analysis of RBC revealed that group 2 was 1.1 times higher than all other groups; all the parameters for erythrocyte were within the range of normal untreated female BALB/c mice (Table 6). Higher platelet counts were observed in vaccine of 4 × 109 VP supplemented with CpG ODN (group 4) with lower counts observed in the vaccine-only group of 4 × 109 VP. Leukocyte count showed that the vaccine group of 4 × 109 VP supplemented with CpG ODN had a higher number of immune cells (WBC) and in all groups, no basophils were found. Overall analysis showed no statistical difference (p ≥ 0.99) between treated mice and untreated mice.

Table 6.
Results from Hematological Profiling.
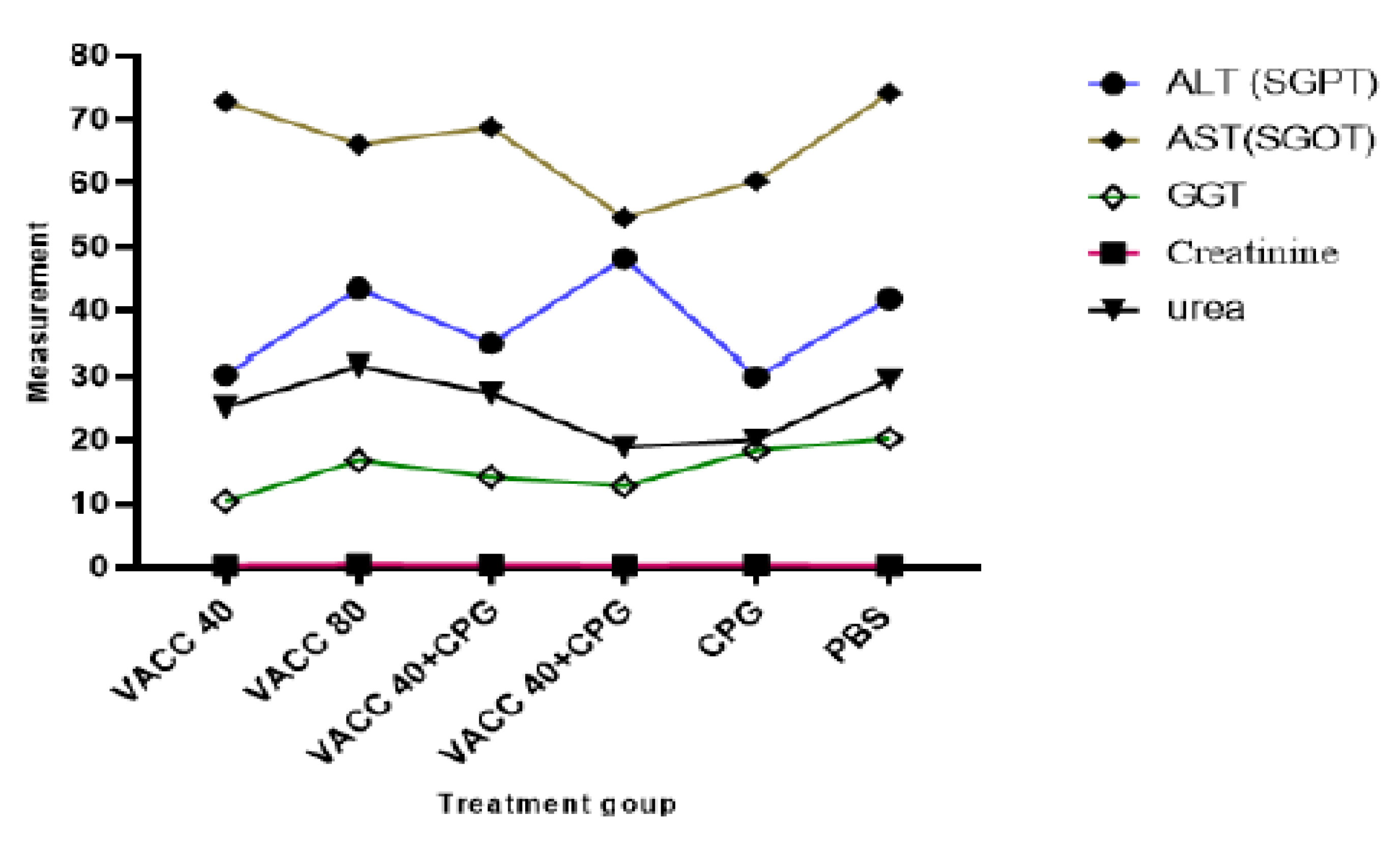
Biochemical analyses of female BALB/c mice immunized by 4 × 109 VP and 8 × 109 VP of vaccine concentration only (group 1 and group 2), 4 × 109 VP and 8 × 109 VP of vaccine with CpG ODNs (group 3 and group 4), CpG ODNs (group 5), and PBS (group 6) are presented in Figure 5. There was no statistically significant difference in biochemical parameters (ALT, AST and GGT, Creatinine, and urea) between the CPG ODNs-treated and non-treated groups.
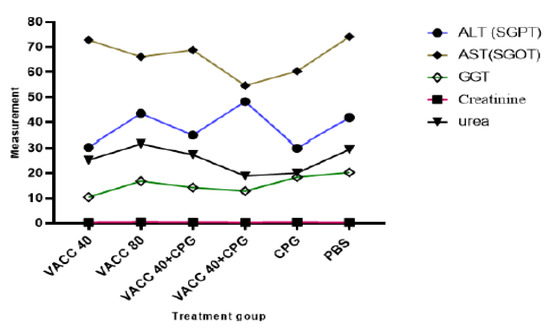
Figure 5.
Results of biochemical analysis. VACC 40 and VACC 80: groups treated with 4 × 109 VP and 8 × 109 VP of vaccine concentration, respectively, VACC 40 +CpG and VACC 80 + CpG: groups treated with 4 × 109 VP and 8 × 109 VP of vaccine plus CpG ODNs, CpG: group treated with CpG ODNs and PBS: group treated with PBS.
4. Discussion
The findings of this study demonstrate the potential improved efficacy of a viral vector-based vaccine against SARS-CoV-2 upon supplementation with CpG ODNs. To the best of our knowledge, this is one of the first studies to evaluate the potential of CPG ODNs to improve viral vector-based vaccines for a SARS-CoV-2 immune response. Other studies have demonstrated the use of CPG-adjuvanted anthrax vaccine-adsorbed (AVA) [20] hepatitis B vaccine [21]. In all such studies, CpG ODN demonstrated an ability to stimulate plasmacytoid dendritic cells (pDCs) and B cells which are capable of activating both innate and adaptive immune responses [22]. For instance, CpG ODN combined with AVA increased the serum IgG anti-Bacillus anthracis (BA) response, resulting in serum anti-BA titers that were 10-fold higher and significantly more protective by day 10 (p≤ 0.05) [20]. Other studies have also demonstrated that CpG ODNs have the potential to produce a Th1-biased immunological environment and enhance CD8+ T-cell responses, making them potential adjuvants for cancer vaccines [23].
In this study, we have demonstrated that both low-dose and high-dose JNJ-78436735 Ad26.COV2-S recombinants plus CpG ODNs produced high levels of specific IgG antibodies in comparison to vaccines only, and the revealed long-term stability of high titers of neutralizing IgG antibodies indicated that the CPG ODNs-supplemented vaccine has potential for long-lasting immune capabilities. The reliability of the CPG ODNs-supplemented vaccines was demonstrated for both concentrations of the viral particles (VP) at both 40µL and the 80µL, hence the potential stability in efficacy. The anti-SARS-CoV-2 IgG antibody is a sign of COVID-19 infection or immunization and aids in the monitoring and management of COVID-19 transmission. Neutralizing antibody titers are thus an essential evaluation index that cannot be replaced when assessing the efficiency of vaccines [24].
TNF is an important factor in the inflammatory response. This cytokine initiates the activation of several other cytokines and growth factors, as well as the recruitment of particular immune cells. Compared to other proinflammatory cytokines, TNF has been shown to be released more quickly [25]. The IFN response is triggered when host cell PRRs recognize viral PAMPs. The IFN-I response both directly and indirectly reduces viral replication [26]. Studies have demonstrated that IFN is the main agent inducing macrophage activation in lymphoid cells, an essential effector part of the adaptive immune response [27]. Multiple innate immune effector pathways, such as the release of cytokines that are often pro-inflammatory such as TNF and IL-12, and improved antigen presentation are all activated by IFN [28]. The IFN-γ is essential for controlling the adaptive immune response [29]. It is made by a wide range of lymphocytes, including regulatory T (Treg), CD4+, CD8+, B-cells, and NK cells. It strongly influences the immune system’s ability to fight off viruses and bacteria by promoting macrophage activation and antigen presentation [30]. In this study we have reported that the formulation JNJ-78436735 Ad26.COV2-S, recombinant vaccine with CpG-ODN has an improved ability to induce cellular immunity compared to the vaccine only (p < 0.001), as demonstrated by the higher titers of both TNF and IFN-γ for the vaccine supplemented with CPG ODNs. Both innate and adaptive immunity depend on TNF and IFN-γ, which also serve as major activators of macrophages and stimulate neutrophils and natural killer cells [29].
One main concern for DNA adjuvants, as with all adjuvants, is the problem for toxicity. Regarding ODN adjuvants, mice repeatedly treated with high doses of CpG ODN experience splenomegaly that is dose-dependent as well as additional damage from excessive immunological stimulation, including mortality [31]. Additional studies [19] have shown no apparent systemic toxicity or change in feeding, physical activity, or behavior. The findings of the hematological evaluation for all groups did not differ (p ≤ 0.05) for any of the parameters examined, indicating that the CpG ODNs did not affect the hematological parameters in BALB/c mice. Additionally, all groups’ values for RBC, Hematocrit, Hemoglobin, MCV, and CHCM have been shown to fall within the normal range as already published [19] for female Balb/c mice. With regard to the leukocyte differential count, the monocytes, eosinophils, and basophils had the lowest values in all groups (Table 6), showing no difference (p ≤ 0.05) between the groups compared to the untreated female Balb/c mice, despite their typically low levels.
Blood biochemical profiles serve as a highly valuable diagnostic tool by reflecting the physiological state of the animal [32]. Therefore, the renal biochemical profile was assessed based on the measures of urea and creatinine in order to determine if the surgical procedure affected the function of the transplant recipient organ. Additionally, the biochemical profile was assessed based on the results of liver enzymes including ALT, AST, and GGT to the animals’ overall metabolic health. The biochemical profile must be evaluated and interpreted with greater accuracy because the enzymatic profile is one of the blood parameters with the greatest variability [33]. For each group, there was no statistically significant difference compared to untreated female balb/c mice (p ≤ 0.05). Moreover, the amount of ALT enzyme is thought to be a preferable signal for determining the integrity of liver cells compared to AST [34], most likely due to its predominance in this organ, specifically in hepatocyte mitochondria, and longer half-life [35]. Additionally, ALT is regarded as a sign of general health in addition to being a marker of liver disorders [36]. It is thought that considerable elevations in AST levels alone are not typically indicative of liver damage [35].
5. Conclusions
Our study found that the co-administration of the CPG ODNs adjuvant and the Johnson and Johnson vaccine in Balb/C mice has the potential to boost humoral and cellular immune responses. The findings could contribute to the search for innovative approaches to improve the efficacy of viral vector-based COVID-19 vaccines amidst challenges such as mutations. However, further work has to be completed on the subject to be able to make scientific conclusions. Some limitations of this study include a small sample size. Even though the study produced intriguing results, the number of observations that could be made for distinct parameters was constrained by using only three female BALB/c mice per experimental unit. There is a need to conduct the study again with a larger sample size because the sample size is crucial in drawing scientific findings. Furthermore, quantitative ELISA experiments, more protective cytokines, and neutralizing antibodies could be explored in further studies.
Author Contributions
Conceptualization, E.O.O. and A.K.M.; formal analysis, D.A.C.; investigation, D.A.C.; resources, J.K. and J.W.K.; data curation, D.A.C. and E.O.O.; writing—original draft preparation, D.A.C., E.O.O. and A.K.M.; writing—review and editing, D.A.C., E.O.O. and A.K.M.; supervision, E.O.O. and A.K.M.; funding acquisition, D.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by African Union, Pan African University Institute for Basic Sciences, Technology and Innovation (PAUSTI).
Institutional Review Board Statement
Approval for animal use was obtained from the Mount Kenya University review committee. Approval number 1386.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The African Union, Pan African University Institute for Basic Sciences, Technology and Innovation (PAUSTI), Kenya Medical Research Institute (KEMRI).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Rahman, T.; Hossain, J.; Basiouni, S.; Khafaga, A.F.; Shehata, A.A.; Hafez, H.M. Poultry Production and Sustainability in Developing Countries under the COVID-19 Crisis: Lessons Learned. Animals 2022, 12, 644. [Google Scholar] [CrossRef]
- Frederiksen, L.S.F.; Zhang, Y.; Foged, C.; Thakur, A. The Long Road toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Front. Immunol. 2020, 11, 1817. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Moreira, J.N.; Lobo, J.M.S. Current Applications of Pharmaceutical Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Shehata, A.A.; Parvin, R.; Nagy, A.; Wang, Y.; Azhar, T.M.; Attia, Y.A.; Azhar, E.I.; Paul, A.K.; Rahmatullah, M. An overview of the ongoing challenges in SARS-CoV-2 global control. Ger. J. Microbiol. 2021, 1, 1–18. [Google Scholar] [CrossRef]
- Rahman, T.; Islam, S.; Shehata, A.A.; Basiouni, S.; Hafez, H.M.; Azhar, E.I.; Khafaga, A.F.; Bovera, F.; Attia, Y.A. Influence of COVID-19 on the sustainability of livestock performance and welfare on a global scale. Trop. Anim. Health Prod. 2022, 54, 1–10. [Google Scholar] [CrossRef]
- Ndwandwe, D.; Wiysonge, C.S. COVID-19 vaccines. Curr. Opin. Immunol. 2021, 71, 111–116. [Google Scholar] [CrossRef]
- Collignon, C.; Bol, V.; Chalon, A.; Surendran, N.; Morel, S.; van den Berg, R.A.; Capone, S.; Bechtold, V.; Temmerman, S.T. Innate Immune Responses to Chimpanzee Adenovirus Vector 155 Vaccination in Mice and Monkeys. Front. Immunol. 2020, 11, 579872. [Google Scholar] [CrossRef]
- Gyebi, G.A.; Adegunloye, A.P.; Ibrahim, I.M.; Ogunyemi, O.M.; Afolabi, S.O.; Ogunro, O.B. Prevention of SARS-CoV-2 cell entry: Insight from in silico interaction of drug-like alkaloids with spike glycoprotein, human ACE2, and TMPRSS2. J. Biomol. Struct. Dyn. 2020, 40, 2121–2145. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Foster, C.; Rawlinson, W.; Tedla, N.; Bull, R.A. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission. Rev. Med. Virol. 2022, 32, e2381. [Google Scholar] [CrossRef]
- Hackett, C.J.; Donald, A.H. (Eds.) Vaccine Adjuvants. 2006. Available online: https://link.springer.com/book/10.1007/978-1-59259-970-7 (accessed on 4 November 2022).
- DeJong, M.A.; Wolf, M.A.; Bitzer, G.J.; Hall, J.M.; Sen-Kilic, E.; Blake, J.M.; Petty, J.E.; Wong, T.Y.; Barbier, M.; Campbell, J.D.; et al. CpG 1018® adjuvant enhances Tdap immune responses against Bordetella pertussis in mice. Vaccine 2022, 40, 5229–5240. [Google Scholar] [CrossRef]
- Alignani, D.; Maletto, B.; Liscovsky, M.; Rópolo, A.; Morón, G.; Pistoresi-Palencia, M.C. Orally administered OVA/CpG-ODN induces specific mucosal and systemic immune response in young and aged mice. J. Leukoc. Biol. 2005, 77, 898–905. [Google Scholar] [CrossRef]
- Suriano, C.M.; Verpeut, J.L.; Kumar, N.; Ma, J.; Jung, C.; Boulanger, L.M. Adeno-associated virus (AAV) reduces cortical dendritic complexity in a TLR9-dependent manner. bioRxiv 2021. [Google Scholar] [CrossRef]
- Clair, J.B.S.; Detanico, T.; Aviszus, K.; Kirchenbaum, G.A.; Christie, M.; Carpenter, J.F.; Wysocki, L.J. Immunogenicity of Isogenic IgG in Aggregates and Immune Complexes. PLoS ONE 2017, 12, e0170556. [Google Scholar] [CrossRef]
- Medrano, G.; Guan, P.; Barlow-Anacker, A.J.; Gosain, A. Comprehensive selection of reference genes for quantitative RT-PCR analysis of murine extramedullary hematopoiesis during development. PLoS ONE 2017, 12, e0181881. [Google Scholar] [CrossRef]
- Odukoya, O.A.; Ajjan, R.; Lim, K.; Watson, P.F.; Weetman, A.P.; Cooke, I.D. The pattern of cytokine mRNA expression in ovarian endometriomata. Mol. Hum. Reprod. 1997, 3, 393–397. [Google Scholar] [CrossRef]
- Silva-Santana, G.; Bax, J.C.; Fernandes, D.C.S.; Bacellar, D.T.L.; Hooper, C.; Dias, A.A.S.O.; Silva, C.B.; de Souza, A.M.; Ramos, S.; Santos, R.A.; et al. Clinical hematological and biochemical parameters in Swiss, BALB/c, C57BL/6 and B6D2F1 Mus musculus. Anim. Model. Exp. Med. 2020, 3, 304–315. [Google Scholar] [CrossRef]
- Klinman, D.M.; Ylt, A.; Beaucaget, S.L.; Conover, J.; Kriegt, A.M. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon y. Proc. Natl. Acad. Sci. USA 1996, 93, 2879–2883. [Google Scholar] [CrossRef]
- Kuo, T.-Y.; Lin, M.-Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.-J.; Liu, L.T.-C.; Cheng, J.; Wu, Y.-C.; Wu, C.-C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef]
- Kumagai, Y.; Takeuchi, O.; Akira, S. TLR9 as a key receptor for the recognition of DNA. Adv. Drug Deliv. Rev. 2008, 60, 795–804. [Google Scholar] [CrossRef]
- Jeong, S.; Choi, Y.; Kim, K. Engineering Therapeutic Strategies in Cancer Immunotherapy via Exogenous Delivery of Toll-like Receptor Agonists. Pharmaceutics 2021, 13, 1374. [Google Scholar] [CrossRef] [PubMed]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P. Abstract Immunotoxicity Testing Methods and Protocols; Human Press: Totowa, NJ, USA, 2018; Volume 1803, ISBN 978-1-60761-400-5. [Google Scholar]
- Liu, C.; Tang, J. Expression levels of tumor necrosis factor-α and the corresponding receptors are correlated with trauma severity. Oncol. Lett. 2014, 8, 2747–2751. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, B.E.; González-Rojas, J.A.; Salazar, M.I.; Torres-Torres, C.; Castrejón-Jiménez, N.S. Taming the Autophagy as a Strategy for Treating COVID-19. Cells 2020, 9, 2679. [Google Scholar] [CrossRef] [PubMed]
- Farhang-Sardroodi, S.; Korosec, C.S.; Gholami, S.; Craig, M.; Moyles, I.R.; Ghaemi, M.S.; Ooi, H.K.; Heffernan, J.M. Analysis of Host Immunological Response of Adenovirus-Based COVID-19 Vaccines. Vaccines 2021, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Briesemeister, D.; Sommermeyer, D.; Loddenkemper, C.; Loew, R.; Uckert, W.; Blankenstein, T.; Kammertoens, T. Tumor rejection by local interferon gamma induction in established tumors is associated with blood vessel destruction and necrosis. Int. J. Cancer 2010, 128, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.R. Interferon Gamma in Autoimmunity: A Complicated Player on a Complex Stage; Department of Medicine, Uniformed Services University of the Health Sciences: Bethesda, MD, USA, 2014. [Google Scholar]
- Kumar, S. Immunopharmacological evaluation of adjuvant efficacy of Monophosphoryl lipid-A and CpG ODN with SARS-CoV-2 RBD antigen. bioRxiv 2022. [Google Scholar] [CrossRef]
- Pfattheicher, S.; Petersen, M.B.; Böhm, R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022, 41, 85–93. [Google Scholar] [CrossRef]
- Ružić-Muslić, D.; Cekić, B.; Ćosić, I.; Pavlović, I.; Maksimović, N.; Petrović, V.C.; Bijelić, Z. Hematological and Biochemical Blood Parameters of Pirot Pramenka-Endangered Sheep Population. In Proceedings of the 13th International Symposium “Modern Trends in Livestock Production”, Belgrade, Serbia, 6–8 October 2021; Available online: http://r.istocar.bg.ac.rs/handle/123456789/751 (accessed on 4 November 2022).
- Abbès, S.; Salah-Abbès, J.B.; Ouanes, Z.; Houas, Z.; Othman, O.; Bacha, H.; Abdel-Wahhab, M.A.; Oueslati, R. Preventive role of phyllosilicate clay on the Immunological and Biochemical toxicity of zearalenone in Balb/c mice. Int. Immunopharmacol. 2006, 6, 1251–1258. [Google Scholar] [CrossRef]
- Fernandes, D.P.; Pimentel, M.M.; Dos Santos, F.A.; Praxedes, A.; De Brito, P.D.; Lima, M.A.; Lelis, I.C.; De Macedo, M.F.; Bezerra, M.B. Hematological and biochemical profile of BALB/c nude and C57BL/6 SCID female mice after ovarian xenograft. An. Acad. Bras. Ciências 2018, 90, 3941–3948. [Google Scholar] [CrossRef]
- Botros, M.; Sikaris, K.A. The De Ritis Ratio: The Test of Time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38 (Suppl. S1), 2–6. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).