Spatial Optimization to Improve COVID-19 Vaccine Allocation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Measures
Statistical Analysis and Optimization
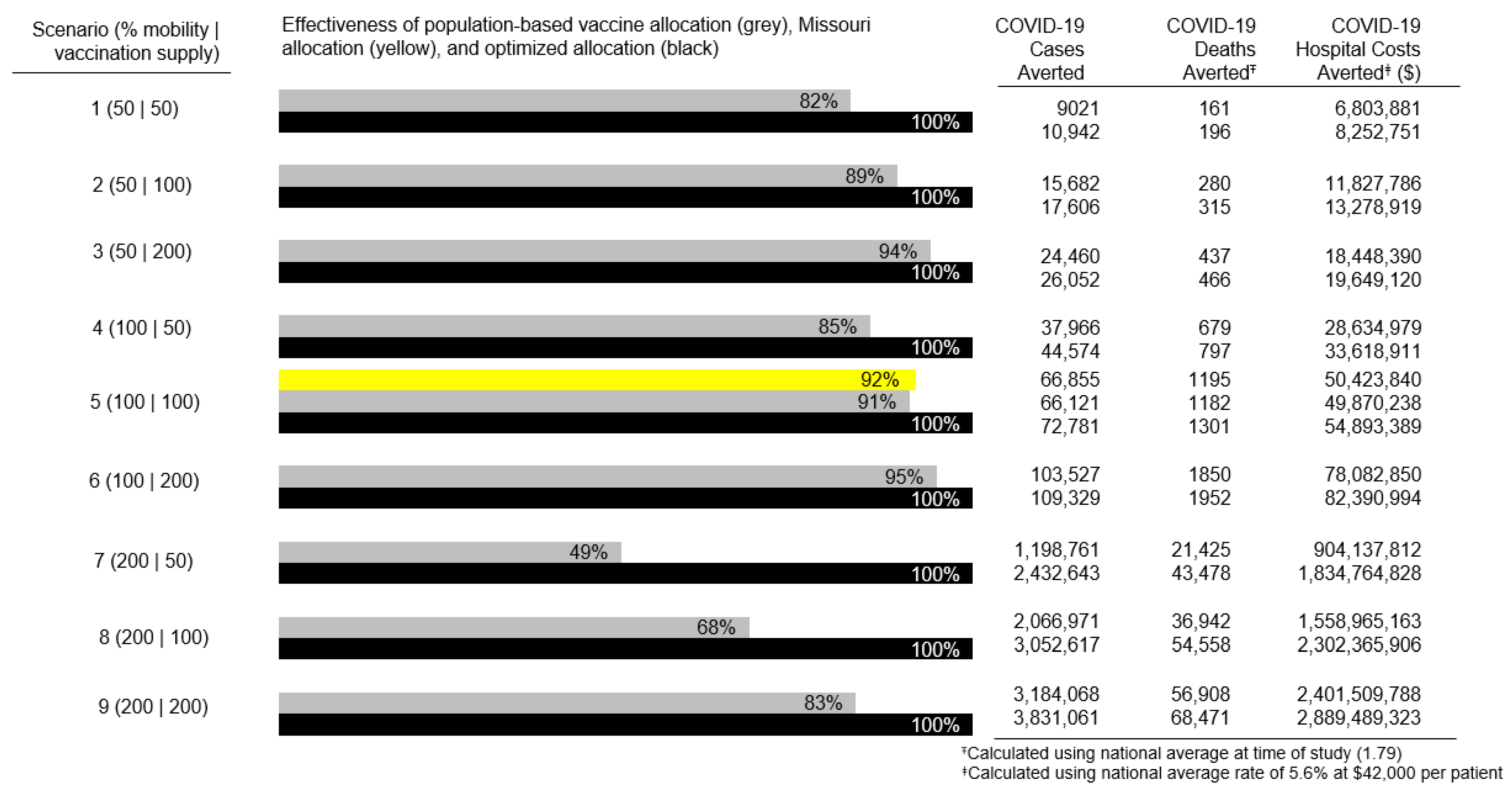
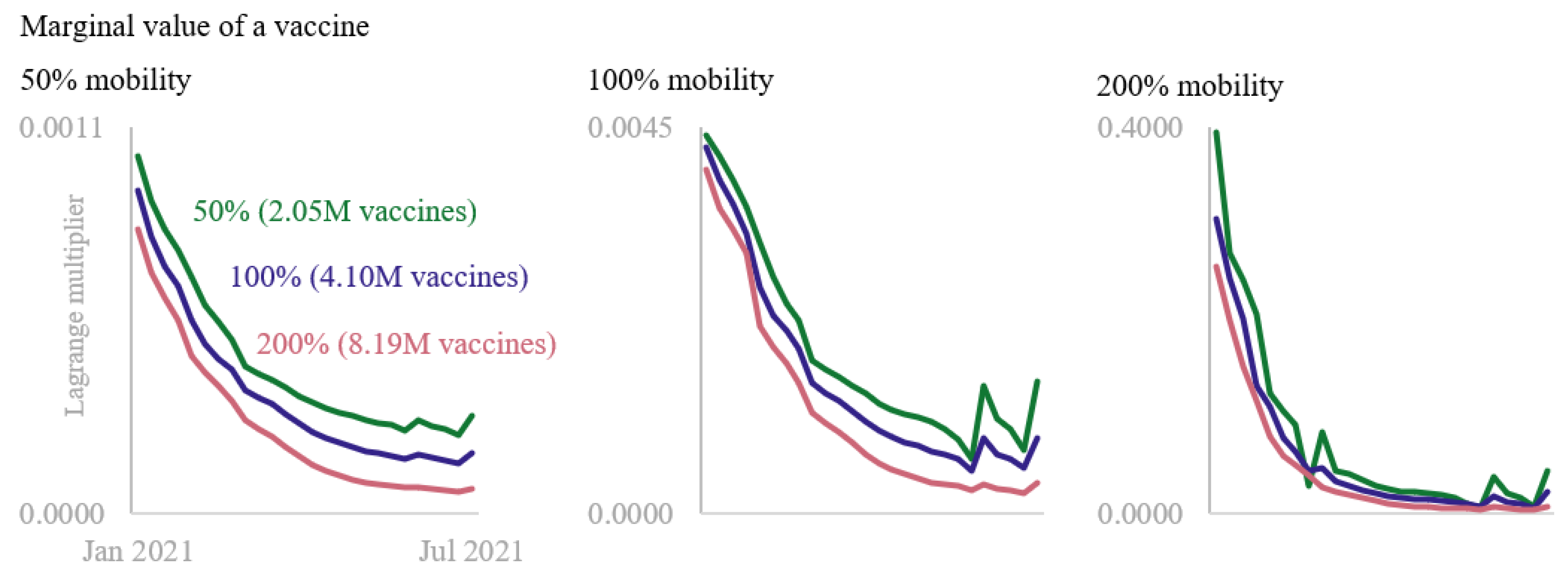
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. COVID Data Tracker. 2022. Available online: https://covid.cdc.gov/covid-data-tracker/ (accessed on 15 December 2021).
- French, G.; Hulse, M.; Nguyen, D.; Sobotka, K.; Webster, K.; Corman, J.; Aboagye-Nyame, B.; Dion, M.; Johnson, M.; Zalinger, B.; et al. Impact of hospital strain on excess deaths during the covid-19 pandemic—United States, July 2020–July 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Boutros, M.; Moujaess, E.; Kourie, H.R. Cancer management during the COVID-19 pandemic: Choosing between the devil and the deep blue sea. Crit. Rev. Oncol./Hematol. 2021, 167, 103271. [Google Scholar] [CrossRef]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing COVID-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Jean-Jacques, M.; Bauchner, H. Vaccine Distribution—Equity Left Behind? JAMA 2021, 325, 829–830. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. How COVID-19 Vaccines Get to You. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/distributing.html (accessed on 29 March 2022).
- Missouri Department of Health and Senior Services. Stronger Together. 2022. Available online: https://covidvaccine.mo.gov/ (accessed on 1 March 2022).
- Jensen, P.A.; Bard, J.F. Operations Research: Models and Methods; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Chang, M.-C.; Kahn, R.; Li, Y.-A.; Lee, C.-S.; Buckee, C.O.; Chang, H.-H. Variation in human mobility and its impact on the risk of future COVID-19 outbreaks in Taiwan. BMC Public Health 2021, 21, 226. [Google Scholar] [CrossRef]
- Sobolik, J.S.; Sajewski, E.T.; Jaykus, L.-A.; Cooper, D.K.; Lopman, B.A.; Kraay, A.N.M.; Ryan, P.B.; Leon, J.S. Controlling risk of SARS-CoV-2 infection in essential workers of enclosed food manufacturing facilities. Food Control. 2022, 133, 108632. [Google Scholar] [CrossRef]
- Senger, E. Infectious risks in family doctors’ offices. CMAJ 2011, 183, 175–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, F.; O’Clery, N. Modelling COVID-19 transmission in supermarkets using an agent-based model. PLoS ONE 2021, 16, e0249821. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Eagle, L.; Phillips, C.; Hedges, D.S.; Bodenhamer, C.; Brown, R.; Wheeler, J.G.; Kirking, H. High COVID-19 Attack Rate Among Attendees at Events at a Church—Arkansas, March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Missouri Department of Health and Senior Services. COVID-19 Activity Report. 2022. Available online: https://health.mo.gov/living/healthcondiseases/communicable/novel-coronavirus/data/public-health/ (accessed on 5 January 2022).
- Missouri Department of Health and Senior Services. Vaccines, Statewide. 2022. Available online: https://health.mo.gov/living/healthcondiseases/communicable/novel-coronavirus/data/public-health/vaccine.php/ (accessed on 5 January 2022).
- Safegraph, L. Patterns GIS Location Data. 2022. Available online: https://www.safegraph.com/products/patterns (accessed on 5 January 2022).
- Ilin, C.; Annan-Phan, S.; Tai, X.H.; Mehra, S.; Hsiang, S.; Blumenstock, J.E. Public mobility data enables COVID-19 forecasting and management at local and global scales. Sci Rep. 2021, 11, 13531. [Google Scholar] [CrossRef] [PubMed]
- Klise, K.; Beyeler, W.; Finley, P.; Makvandi, M. Analysis of mobility data to build contact networks for COVID-19. PLoS ONE 2021, 16, e0249726. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.-P.; Conti, R.M.; Becker, N.V. Assessment of Out-of-Pocket Spending for COVID-19 Hospitalizations in the US in 2020. JAMA Netw. Open 2021, 4, e2129894. [Google Scholar] [CrossRef] [PubMed]
- Knitro, Version 13.2; Artelys: Paris, France, 2022.
- Buckner, J.H.; Chowell, G.; Springborn, M.R. Dynamic prioritization of COVID-19 vaccines when social distancing is limited for essential workers. Proc. Natl. Acad. Sci. USA 2021, 118, e2025786118. [Google Scholar] [CrossRef] [PubMed]
- Mak, H.; Dai, T.; Tang, C. Managing two-dose COVID-19 vaccine rollouts with limited supply: Operations strategies for distributing time-sensitive resources. Prod. Oper. Manag. 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, L.; Eaton, J.; Leung, T.; Dimitrov, D.; Schiffer, J.T.; Swan, D.A. Optimizing vaccine allocation for COVID-19 vaccines shows the potential role of single-dose vaccination. Nat. Commun. 2021, 12, 3449. [Google Scholar] [CrossRef] [PubMed]
- Wrigley-Field, E.; Kiang, M.V.; Riley, A.R.; Barbieri, A.; Chen, Y.-H.; Duchowny, K.A.; Matthay, E.C.; Van Riper, D.; Jegathesan, K.; Bibbins-Domingo, K.; et al. Geographically targeted COVID-19 vaccination is more equitable and averts more deaths than age-based thresholds alone. Sci. Adv. 2021, 7, eabj2099. [Google Scholar] [CrossRef] [PubMed]
- Peikes, K. Mass Vaccination Clinics in Iowa Often Have Leftover Doses, but Counties Make Sure They Don’t Go to Waste. 2021. Available online: https://www.iowapublicradio.org/ipr-news/2021-03-11/mass-vaccination-clinics-in-iowa-often-have-leftover-doses-but-counties-make-sure-they-dont-go-to-waste (accessed on 1 February 2022).
- McDermott, J. As Vaccine Demand Falls, States Are Left with Huge Stockpile. 2022. Available online: https://www.usnews.com/news/health-news/articles/2022-03-03/as-vaccine-demand-falls-states-are-left-with-huge-stockpile (accessed on 22 March 2022).
- Jones, B. The Changing Political Geography of COVID-19 Over the Last Two Years. 2022. Available online: https://www.pewresearch.org/politics/2022/03/03/the-changing-political-geography-of-covid-19-over-the-last-two-years/ (accessed on 22 March 2022).
- Poorolajal, J. Geographical Distribution of COVID-19 Cases and Deaths Worldwide. J. Res. Health Sci. 2020, 20, e00483. [Google Scholar] [CrossRef] [PubMed]

| Coefficient Estimate † | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| % Vaccinated | −2.488 | −2.552, −2.423 | <0.001 |
| Time spent at grocery/food stores (min) | 0.001 | 0.000, 0.001 | <0.001 |
| Time spent at restaurants/bars (min) | 0.014 | 0.013, 0.015 | <0.001 |
| Time spent at retail locations (min) | 0.016 | 0.015, 0.016 | <0.001 |
| Time spent at healthcare locations (min) | 0.004 | 0.003, 0.004 | <0.001 |
| Time spent at education locations (min) | 0.001 | 0.011, 0.012 | <0.001 |
| Time spent at senior living facility (min) | 0.004 | 0.004, 0.004 | <0.001 |
| Distance traveled from residence (km) | 0.371 | 0.356, 0.385 | <0.001 |
| Population ‡ | 0.912 | 0.709, 1.114 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scroggins, S.; Goodson, J.; Afroze, T.; Shacham, E. Spatial Optimization to Improve COVID-19 Vaccine Allocation. Vaccines 2023, 11, 64. https://doi.org/10.3390/vaccines11010064
Scroggins S, Goodson J, Afroze T, Shacham E. Spatial Optimization to Improve COVID-19 Vaccine Allocation. Vaccines. 2023; 11(1):64. https://doi.org/10.3390/vaccines11010064
Chicago/Turabian StyleScroggins, Stephen, Justin Goodson, Tasnova Afroze, and Enbal Shacham. 2023. "Spatial Optimization to Improve COVID-19 Vaccine Allocation" Vaccines 11, no. 1: 64. https://doi.org/10.3390/vaccines11010064
APA StyleScroggins, S., Goodson, J., Afroze, T., & Shacham, E. (2023). Spatial Optimization to Improve COVID-19 Vaccine Allocation. Vaccines, 11(1), 64. https://doi.org/10.3390/vaccines11010064





