Strategies for Prevention and Control of Vibriosis in Asian Fish Culture
Abstract
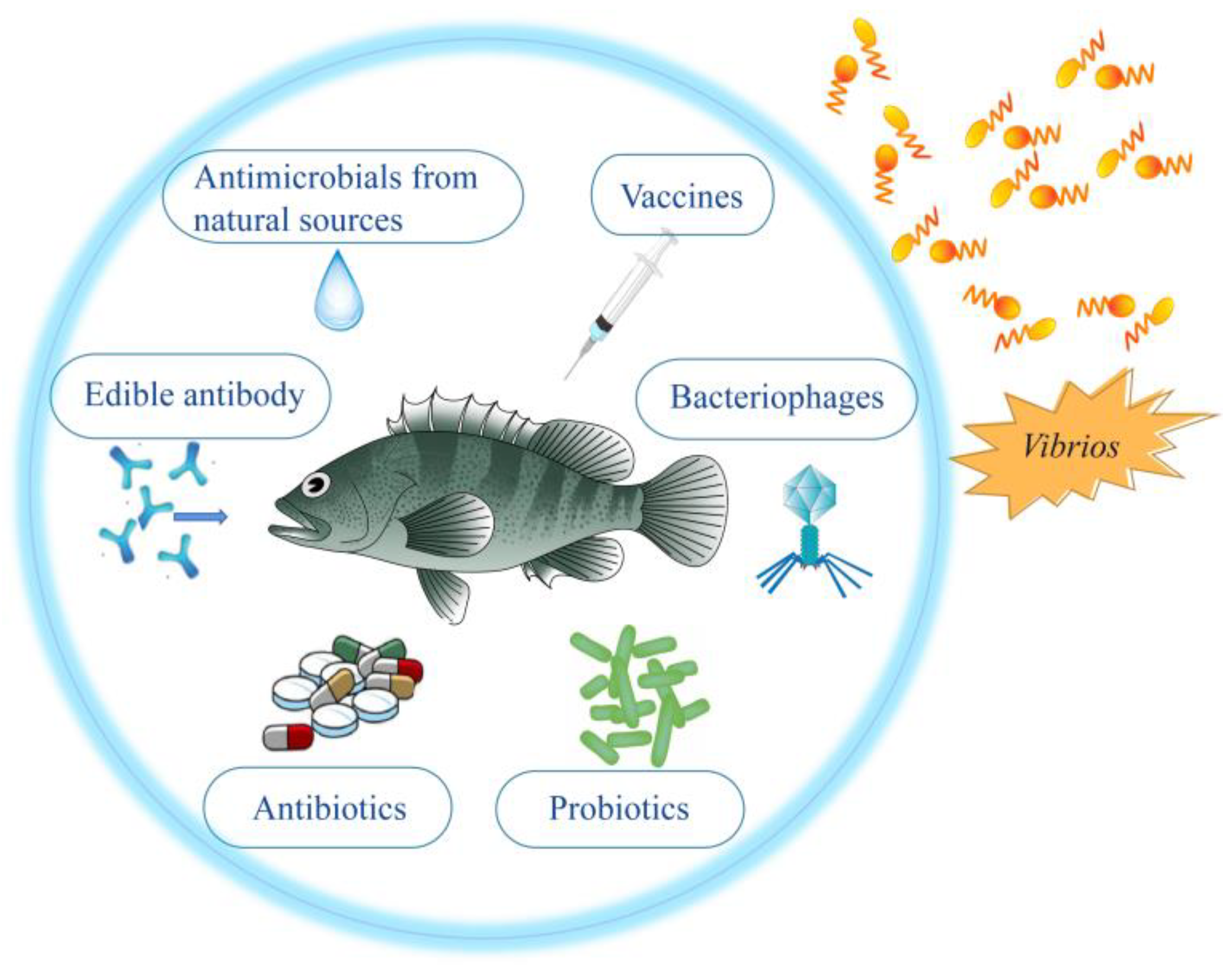
:1. Introduction
2. Control and Prevention Strategies of Vibrios
2.1. V. harveyi
2.1.1. Antibiotics
2.1.2. Bacteriophages
2.1.3. Vaccines
- Inactivated vaccines
- Attenuated live vaccines
- Subunit vaccines
- Anti-idiotypic vaccines
- DNA vaccines
- mRNA vaccines
| Pathogen | Prevention and Control Technology | Concrete Measure/ Vaccine Type | Host | Vaccine Antigen Components | Route of Infection | Ref. |
|---|---|---|---|---|---|---|
| V. harveyi | Antibiotics | Ceftriaxone, Doxycycline, Minocycline | Juvenile hybrid groupers | NR | Bath, Injection (IP) | [11] |
| Doxycycline, Tetracycline | Hippocampus | NR | Injection (IP) | [12] | ||
| Bacteriophages | Phage VhKM4 | Finfish | NR | [13] | ||
| Vaccines | Inactivated | Marine Red Hybrid Tilapia | V. harveyi strain Vh1 (Formalin-Inactivated) | Injection (IP) | [15] | |
| E. coioides | VICV | Injection (IP) | [16] | |||
| Orange-spotted grouper | V. Harveyi (formalin-killed, Adjuvant: ISA763 AVG) | Injection (IP) | [17] | |||
| Turbot | V. Harveyi (formalin-killed, Adjuvant: TMISA763 AVG) | Injection (IP) | [18] | |||
| Pearl gentian grouper | V. harveyi ZJ0603 (Formalin-killed, combine with β-glucanhas) | Injection (IP) | [20] | |||
| Attenuated | Grouper | Non-toxic V. harveyi | Bath, Injection (IP) | [22] | ||
| Japanese flounder | Attenuated mutant V. Harveyi T4DM | Bath, Injection (IP) | [24] | |||
| Subunit | Japanese flounder | Recombinant Vhp1 | Injection (IP) | [21] | ||
| Golden pompano | Antigen encoding TssJ | Injection (IP) | [25] | |||
| Orange-spotted grouper | VirB11 | Injection (IP) | [26] | |||
| Juvenile sea bass | Expressed r-OmpK of Vibrio | Injection (IP) | [27] | |||
| Anti-idiotypic | Grouper | Anti-Id IgG (Fab) | Injection (IP) | [28] | ||
| DNA | Japanese flounder | Plasmid pDV | Injection (IP, IM) | [29] | ||
| Turbot | Plasmid with OmpU | Injection (IP, IM) | [30] | |||
| mRNA | Fish | and B-cell epitopes in hemolysin protein | None | [32] |
2.2. V. vulnificus
Vaccines
- Inactivated vaccines
- Subunit vaccines
- Multivalent vaccines
2.3. V. parahaemolyticus
Vaccines
- Inactivated vaccines
- Recombinant vaccines
2.4. V. cholerae
2.4.1. Antibiotics
2.4.2. Vaccines
2.4.3. Edible Antibodies
2.4.4. Bacteriophages
2.5. V. anguillarum
2.5.1. Antibiotics
2.5.2. Vaccines
- Inactivated vaccines
- Attenuated live vaccines
- DNA and subunit vaccines
| Pathogen | Prevention and Control Technology | Concrete Measure/ Vaccine Type | Host | Vaccine Antigen Components | Route of Infection | Ref. |
|---|---|---|---|---|---|---|
| V. anguillarum | Vaccines | Inactivated | Fish | Inactivated strains of V. anguillarum serotypes O2 and O1 | Injection (IP) | [54] |
| Attenuated | Turbot, Oliver flounder, Zebrafish, Tiger puffer | Genetically engineered live V. anguillarum | Injection | [59,60,61] | ||
| Zebrafish | live attenuated V. anguillarum strain MVAV6203 | Injection (IP) | [62] | |||
| Subunit | Paralichthys olivaceus | Recombinant protein (rOmpK) | Injection | [69] | ||
| DNA | Japanese flounder | Zinc metalloprotease EmpA | Injection (IM) | [65] | ||
| Paralichthys olivaceus | Bicistronic plasmids (p-OmpK-CCL19 and p-OmpK-CCL4) | Injection (IM) | [66] | |||
| Flounder | DNA plasmid encoding the VAA of V. anguillarum | Injection (IP) | [67] | |||
| Paralichthys olivaceus | Plasmid OmpK from V. anguillarum plus cyclosporine A | Injection | [69] |
2.6. V. mimicus
2.6.1. Antibiotics
2.6.2. Vaccines
- DNA vaccines
- Attenuated live vaccines
2.7. V. alginolyticus
2.7.1. Antibiotics
2.7.2. Probiotics
2.7.3. Bacteriophages
2.7.4. Antimicrobials from Natural Sources
2.7.5. Vaccines
- Attenuated live vaccines
- Recombinant vaccines
- DNA vaccines
- BGs vaccines
| Pathogen | Prevention and Control Technology | Concrete Measure/ Vaccine Type | Host | Vaccine Antigen Components | Route of Infection | Ref. |
|---|---|---|---|---|---|---|
| V. alginolyticus | Antibiotics | Florfenicol, Enrofloxacin, Terramycin | Nile tilapia | NR | Injection (IP) | [92] |
| Doxycycline, Tetracycline | Hippocampus | NR | Injection (IP) | [12] | ||
| Probiotics | Pseudoalteromonas sp. IBRL PD4.8 | None | NR | None | [95] | |
| Antimicrobials from natural sources | Phage Endolysins Lysqdvp001 | None | NR | None | [100] | |
| Exogenous malic acid, Taurine | Zebrafish | NR | Injection (IP) | [101] | ||
| Astaxanthin | Fish | NR | Oral | [102] | ||
| Secondary metabolite (From Bacillus sp. strain JS04) | None | NR | None | [103] | ||
| Bacteriophages | Phage VP01, Phage VEN Phage HH109, Phage valsw3-3 | None | NR | None | [96,97,98,99] | |
| Vaccines | Inactivated | E. coioides | VICV | Injection (IP) | [16] | |
| Attenuated | Zebrafish | hfq mutant | Injection (IM) | [104] | ||
| Pearl gentian grouper | ∆acfA, HY9901∆hop, HY9901ΔvscB, ∆clpP mutant | Injection (IP) | [105,106,107,108] | |||
| Japanese flounder | Attenuated mutant V. harveyi T4DM | Injection (IP), Bath | [24] | |||
| Zebrafish | Attenuated V. alginolyticus T3SS | Injection (IM) | [109] | |||
| Zebrafish | Attenuated V. alginolyticus (Mg) | Injection (IM) | [110] | |||
| Recombinant | Juvenile sea bass | Expressed r-OmpK of Vibrio | Injection (IP) | [27] | ||
| DNA | Red snapper | Plasmid encoding flaA | Injection | [115] | ||
| Crimson snapper | pcDNA plasmid with OmpW | Injection (IM) | [116] | |||
| BGs | Large yellow croaker | V. alginolyticus bacterial ghosts | Injection (IP) | [118] |
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.D.; Holt, C.C. Global adoption of aquaculture to supply seafood. Environ. Res. Lett. 2022, 17, 041003. [Google Scholar] [CrossRef]
- Thompson, F.L.; Iida, T.; Swings, J. Biodiversity of Vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic Bacteria as Biological Control Agents in Aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zhu, X.; Li, Y.; Yang, P.; Wang, S.; Ning, K.; Chen, S. Intestinal microbiome-mediated resistance against vibriosis for Cynoglossus semilaevis. Microbiome 2022, 10, 153. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, B.; Xu, Z.; Jia, L.; Li, M.; He, X.; Bao, B. Detecting Cynoglossus semilaevis infected with Vibrio harveyi using micro RNAs from mucous exosomes. Mol. Immunol. 2020, 128, 268–276. [Google Scholar] [CrossRef]
- Miccoli, A.; Manni, M.; Picchietti, S.; Scapigliati, G. State-of-the-Art Vaccine Research for Aquaculture Use: The Case of Three Economically Relevant Fish Species. Vaccines 2021, 9, 140. [Google Scholar] [CrossRef]
- Zhang, X.-H.; He, X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef]
- Shen, G.M.; Shi, C.Y.; Fan, C.; Jia, D.; Wang, S.Q.; Xie, G.S.; Li, G.Y.; Mo, Z.L.; Huang, J. Isolation, identification and pathogenicity of Vibrio harveyi, the causal agent of skin ulcer disease in juvenile hybrid groupers Epinephelus fuscoguttatus × Epinephelus lanceolatus. J. Fish Dis. 2017, 40, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bu, L.; Jin, S.; Wang, X.; Zhao, Q.; Zhou, S.; Xu, Y. Outbreak of vibriosis caused by Vibrio harveyi and Vibrio alginolyticus in farmed seahorse Hippocampus kuda in China. Aquaculture 2020, 523, 735168. [Google Scholar] [CrossRef]
- Lal, T.M.; Sano, M.; Ransangan, J. Isolation and Characterization of Large Marine Bacteriophage (Myoviridae), VhKM4 Infecting Vibrio harveyi. J. Aquat. Anim. Health 2017, 29, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Thomas, J. A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Abu Nor, N.; Zamri-Saad, M.; Md Yasin, I.S.; Salleh, A.; Mustaffa-Kamal, F.; Matori, M.F.; Azmai, M.N.A. Efficacy of Whole Cell Inactivated Vibrio harveyi Vaccine against Vibriosis in a Marine Red Hybrid Tilapia (Oreochromis niloticus × O. mossambicus) Model. Vaccines 2020, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Tang, J.; Li, M.; Fu, Y.; Dong, C.; Zhong, J.F.; He, J. Immunological evaluation of Vibrio alginolyticus, Vibrio harveyi, Vibrio vulnificus and infectious spleen and kidney necrosis virus (ISKNV) combined-vaccine efficacy in Epinephelus coioides. Vet. Immunol. Immunopathol. 2012, 150, 61–68. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, T.T.T.; Tsai, M.-A.; Ya-Zhen, E.; Wang, P.-C.; Chen, S.-C. A formalin-inactivated vaccine provides good protection against Vibrio harveyi infection in orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 2017, 65, 118–126. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, C.; Bao, P.; Liu, Q.; Wang, P.; Zhang, R.; Liu, X.; Zhang, Y. Efficacy of Montanide™ ISA 763 A VG as aquatic adjuvant administrated with an inactivated Vibrio harveyi vaccine in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2018, 84, 56–61. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, J.-S.; Balasundaram, C.; Heo, M.-S. Vaccination effect of liposomes entrapped whole cell bacterial vaccine on immune response and disease protection in Epinephelus bruneus against Vibrio harveyi. Aquaculture 2012, 342–343, 69–74. [Google Scholar] [CrossRef]
- Wei, G.; Tan, H.; Ma, S.; Sun, G.; Zhang, Y.; Wu, Y.; Cai, S.; Huang, Y.; Jian, J. Protective effects of beta-glucan as adjuvant combined inactivated Vibrio harveyi vaccine in pearl gentian grouper. Fish Shellfish Immunol. 2020, 106, 1025–1030. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, W.-W.; Zhang, M.; Sun, L. Evaluation of the vaccine potential of a cytotoxic protease and a protective immunogen from a pathogenic Vibrio harveyi strain. Vaccine 2010, 28, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.-Y.; Long, H.; Cui, J.; Zhang, X.; Cai, X.-N.; Xie, Z.-Y. Characterization of a pathogenic Vibrio harveyi strain from diseased Epinephelus coioides and evaluation of different methods to control its infection. Aquaculture 2020, 526, 735371. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Cheng, S.; Zhang, M.; Sun, L. Construction and evaluation of a live vaccine against Edwardsiella tarda and Vibrio harveyi: Laboratory vs. mock field trial. Vaccine 2011, 29, 4081–4085. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-H.; Deng, T.; Sun, B.-G.; Sun, L. Development and efficacy of an attenuated Vibrio harveyi vaccine candidate with cross protectivity against Vibrio alginolyticus. Fish Shellfish Immunol. 2012, 32, 1155–1161. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, S.; He, M.; Liu, A.; Long, H.; Guo, W.; Cao, Z.; Xie, Z.; Zhou, Y. Construction and analysis of the immune effect of Vibrio harveyi subunit vaccine and DNA vaccine encoding TssJ antigen. Fish Shellfish Immunol. 2019, 98, 45–51. [Google Scholar] [CrossRef]
- Atujona, D.; Huang, Y.; Wang, Z.; Jian, J.; Cai, S. Vibrio harveyi (VirB11) recombinant vaccine development against vibriosis in orange-spotted grouper (Epinephelus coioides). Aquac. Res. 2019, 50, 2628–2634. [Google Scholar] [CrossRef]
- Silvaraj, S.; Md Yasin, I.S.; MMAK; Saad, M.Z. Elucidating the Efficacy of Vaccination against Vibriosis in Lates calcarifer Using Two Recombinant Protein Vaccines Containing the Outer Membrane Protein K (r-OmpK) of Vibrio alginolyticus and the DNA Chaperone J (r-DnaJ) of Vibrio harveyi. Vaccines 2020, 8, 660. [Google Scholar] [CrossRef]
- Huang, W.-L.; Chuang, S.-C.; Yang, C.-D. Anti-Idiotype Vaccine Provides Protective Immunity Against Vibrio Harveyi in Grouper (Epinephelus coioides). Vaccines 2019, 7, 210. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-H.; Sun, L. A bivalent Vibrio harveyi DNA vaccine induces strong protection in Japanese flounder (Paralichthys olivaceus). Vaccine 2011, 29, 4328–4333. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Liu, R.; Jia, J. Identification and evaluation of an outer membrane protein OmpU from a pathogenic Vibrio harveyi isolate as vaccine candidate in turbot (Scophthalmus maximus). Lett. Appl. Microbiol. 2011, 53, 22–29. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.I.; Mou, M.J.; Sanjida, S.; Tariq, M.; Nasir, S.; Mahfuj, S. Designing a novel mRNA vaccine against Vibrio harveyi infection in fish: An immunoinformatics approach. Genom. Inform. 2022, 20, e11. [Google Scholar] [CrossRef] [PubMed]
- Bisharat, N.; Agmon, V.; Finkelstein, R.; Raz, R.; Ben-Dror, G.; Lerner, L.; Soboh, S.; Colodner, R.; Cameron, D.N.; Wykstra, D.L.; et al. Clinical, epidemiological, and microbiological features of Vibrio vulnificus biogroup 3 causing outbreaks of wound infection and bacteraemia in Israel. Lancet 1999, 354, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, C.A.; Lafrentz, B.R.; Klesius, P.H. Vaccination of sex reversed hybrid tilapia (Oreochromis niloticus × O. aureus) with an inactivated Vibrio vulnificus vaccine. Biologicals 2011, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Lihua, D.; Jianjun, F.; Peng, L.; Songlin, G. Immunogenicity study of an expressed outer membrane protein U of Vibrio vulnificus in Japanese eel (Anguilla japonica). J. Appl. Microbiol. 2018. Online ahead of print. [Google Scholar] [CrossRef]
- Guo, S.; Hu, L.; Feng, J.; Lin, P.; He, L.; Yan, Q. Immunogenicity of a bivalent protein as a vaccine against Edwardsiella anguillarum and Vibrio vulnificus in Japanese eel (Anguilla japonica). Microbiologyopen 2018, 8, e00766. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Wu, L.; Lin, P.; Zhai, S.; Guo, S.; Xiao, Y.; Wan, Q. First expression and immunogenicity study of a novel trivalent outer membrane protein (OmpII-U-A) from Aeromonas hydrophila, Vibrio vulnificus and Edwardsiella anguillarum. Aquaculture 2020, 519, 734932. [Google Scholar] [CrossRef]
- Jun, J.W.; Kim, J.H.; Choresca, C.H., Jr.; Shin, S.P.; Han, J.E.; Han, S.Y.; Chai, J.Y.; Park, S.C. Isolation, molecular characterization, and antibiotic susceptibility of Vibrio parahaemolyticus in Korean seafood. Foodborne Pathog. Dis. 2012, 9, 224–231. [Google Scholar] [CrossRef]
- Lee, J.-K.; Jung, D.-W.; Eom, S.-Y.; Oh, S.-W.; Kim, Y.; Kwak, H.-S.; Kim, Y.-H. Occurrence of Vibrio parahaemolyticus in oysters from Korean retail outlets. Food Control. 2008, 19, 990–994. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, L. Virulence and Antibiotic and Heavy Metal Resistance of Vibrio parahaemolyticus Isolated from Crustaceans and Shellfish in Shanghai, China. J. Food Prot. 2016, 79, 1371–1377. [Google Scholar] [CrossRef]
- Kang, C.-H.; Shin, Y.; Jang, S.; Yu, H.; Kim, S.; An, S.; Park, K.; So, J.-S. Characterization of Vibrio parahaemolyticus isolated from oysters in Korea: Resistance to various antibiotics and prevalence of virulence genes. Mar. Pollut. Bull. 2017, 118, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibiotic-Resistant Bacteria: Prevalence in Food and Inactivation by Food-Compatible Compounds and Plant Extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Istiqomah, I.; Sukardi; Murwantoko; Isnansetyo, A. Review Vibriosis Management in Indonesian Marine Fish Farming. E3S Web Conf. 2020, 147, 01001. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Guo, S.; Gao, Y.; Liang, X.; Liu, L.; Pan, S. Comparative immunogenicity of outer membrane protein K and whole-cell antigens of Vibrio parahaemolyticus for diagnosis. Lett. Appl. Microbiol. 2021, 73, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Chukwu-Osazuwa, J.; Cao, T.; Vasquez, I.; Gnanagobal, H.; Hossain, A.; Machimbirike, V.I.; Santander, J. Comparative Reverse Vaccinology of Piscirickettsia salmonis, Aeromonas salmonicida, Yersinia ruckeri, Vibrio anguillarum and Moritella viscosa, Frequent Pathogens of Atlantic Salmon and Lumpfish Aquaculture. Vaccines 2022, 10, 473. [Google Scholar] [CrossRef]
- Liu, X.; Yang, M.-J.; Wang, S.-N.; Xu, D.; Li, H.; Peng, X.-X. Differential Antibody Responses to Outer Membrane Proteins Contribute to Differential Immune Protections between Live and Inactivated Vibrio parahemolyticus. J. Proteome Res. 2018, 17, 2987–2994. [Google Scholar] [CrossRef]
- Peterson, K.M.; Gellings, P.S. Multiple intraintestinal signals coordinate the regulation of Vibrio cholerae virulence determinants. Pathog. Dis. 2018, 76, ftx126. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.A.; El Bayomi, R.M.; Hussein, M.A.; Khedr, M.H.; Remela, E.M.A.; El-Ashram, A.M. Molecular characterization, antibiotic resistance pattern and biofilm formation of Vibrio parahaemolyticus and V. cholerae isolated from crustaceans and humans. Int. J. Food Microbiol. 2018, 274, 31–37. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Liu, Y.; Dong, J.; Xu, N.; Yang, Q.; Zhou, S.; Ai, X. Identification of Vibrio cholerae as a bacterial pathogen of bluegill sunfish. Aquac. Rep. 2022, 23, 101092. [Google Scholar] [CrossRef]
- Elmahdi, S.; DaSilva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef]
- Gao, X.; Chen, N.; Zhang, Y.; Zhang, X.; Bing, X. Non-O1 Vibrio cholerae pathogen from Cyprinus carpio and control with anti-non-O1 V. cholerae egg yolk powder (IgY). Aquaculture 2017, 479, 69–74. [Google Scholar] [CrossRef]
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2013, 5, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Plaza, N.; Castillo, D.; Pérez-Reytor, D.; Higuera, G.; García, K.; Bastías, R. Bacteriophages in the control of pathogenic vibrios. Electron. J. Biotechnol. 2018, 31, 24–33. [Google Scholar] [CrossRef]
- Frans, I.; Michiels, C.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish Dis. 2011, 34, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.E.; Lee, J.L. A comprehensive review of Vibrio (Listonella) anguillarum: Ecology, pathology and prevention. Rev. Aquac. 2017, 10, 585–610. [Google Scholar] [CrossRef]
- Weber, B.; Chen, C.; Milton, D.L. Colonization of fish skin is vital for Vibrio anguillarum to cause disease. Environ. Microbiol. Rep. 2010, 2, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.S.; Shaapan, R.M.; Mohamed, L.A.; Gayed, S.S. Recent biocontrol measures for fish bacterial diseases, in particular to probiotics, bio-encapsulated vaccines, and phage therapy. Open Vet. J. 2019, 9, 190–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Liu, C.-S.; Sun, L. A multivalent killed whole-cell vaccine induces effective protection against Edwardsiella tarda and Vibrio anguillarum. Fish Shellfish Immunol. 2011, 31, 595–599. [Google Scholar] [CrossRef]
- Bao, P.; Sun, X.; Liu, Q.; Zhang, Y.; Liu, X. Synergistic effect of a combined live Vibrio anguillarum and Edwardsiella piscicida vaccine in turbot. Fish Shellfish Immunol. 2019, 88, 84–90. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, C.; Ma, Y.; Wang, Q.; Zhang, Y. A live attenuated Vibrio anguillarum vaccine induces efficient immunoprotection in Tiger puffer (Takifugu rubripes). Vaccine 2018, 36, 1460–1466. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Q.; Cai, M.; Wang, Q.; Zhang, Y. Fermentation preparation of recombinant Vibrio anguillarum vaccine with heterogeneous antigen display. Prep. Biochem. Biotechnol. 2013, 43, 79–94. [Google Scholar] [CrossRef]
- Chu, T.; Guan, L.; Shang, P.; Wang, Q.; Xiao, J.; Liu, Q.; Zhang, Y. A controllable bacterial lysis system to enhance biological safety of live attenuated Vibrio anguillarum vaccine. Fish Shellfish Immunol. 2015, 45, 742–749. [Google Scholar] [CrossRef]
- Kwon, H.C.; Kang, Y.J. Effects of a subunit vaccine (FlaA) and immunostimulant (CpG-ODN 1668) against Vibrio anguillarum in tilapia (Oreochromis niloticus). Aquaculture 2016, 454, 125–129. [Google Scholar] [CrossRef]
- Jia, P.-P.; Hu, Y.-H.; Chi, H.; Sun, B.-G.; Yu, W.-G.; Sun, L. Comparative study of four flagellins of Vibrio anguillarum: Vaccine potential and adjuvanticity. Fish Shellfish Immunol. 2013, 34, 514–520. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Yang, G.; Zhang, X.-H.; Liu, R.; Xue, X. Protection of Japanese flounder (Paralichthys olivaceus) against Vibrio anguillarum with a DNA vaccine containing the mutated zinc-metalloprotease gene. Vaccine 2009, 27, 2150–2155. [Google Scholar] [CrossRef]
- Li, H.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Two bicistronic DNA vaccines against Vibrio anguillarum and the immune effects on flounder Paralichthys olivaceus. J. Oceanol. Limnol. 2022, 40, 786–804. [Google Scholar] [CrossRef]
- Xing, J.; Xu, H.; Tang, X.; Sheng, X.; Zhan, W. A DNA Vaccine Encoding the VAA Gene of Vibrio anguillarum Induces a Protective Immune Response in Flounder. Front. Immunol. 2019, 10, 499. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Xing, J.; Tang, X.; Zhan, W. Evaluation of bivalent vaccines candidates among VAA, OmpK and OmpR from Vibrio anguillarum in flounder (Paralichthys olivaceus). Dev. Comp. Immunol. 2018, 85, 1–9. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, Z.; Luo, K.; Tang, X.; Sheng, X.; Zhan, W. T and B lymphocytes immune responses in flounder (Paralichthys olivaceus) induced by two forms of outer membrane protein K from Vibrio anguillarum: Subunit vaccine and DNA vaccine. Mol. Immunol. 2019, 118, 40–51. [Google Scholar] [CrossRef]
- Li, J.; Tang, L.; Li, S.; Li, G.; Mo, Z. The efficacy and side-effects of oil-based adjuvants emulsified Vibrio anguillarum bivalent inactivated vaccine in turbot (Scophthalmus maximus) under production mode. Aquaculture 2020, 524, 735259. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Gao, Y.; Zhang, Y.; Wu, H.; Zhang, Y. Efficacy of chitosan oligosaccharide as aquatic adjuvant administrated with a formalin-inactivated Vibrio anguillarum vaccine. Fish Shellfish Immunol. 2015, 47, 855–860. [Google Scholar] [CrossRef]
- Sun, X.; Jin, P.; Liu, Q.; Wang, Q.; Zhang, Y.; Liu, X. A CpG-riched plasmid as vaccine adjuvant reduce antigen dose of an inactivated Vibrio anguillarum vaccine in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 98, 312–317. [Google Scholar] [CrossRef]
- Torres-Corral, Y.; Girons, A.; González-Barreiro, O.; Seoane, R.; Riaza, A.; Santos, Y. Effect of Bivalent Vaccines against Vibrio anguillarum and Aeromonas salmonicida Subspecie achromogenes on Health and Survival of Turbot. Vaccines 2021, 9, 906. [Google Scholar] [CrossRef]
- Liu, W.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Characterization of Co-Stimulatory Ligand CD80/86 and Its Effect as a Molecular Adjuvant on DNA Vaccine Against Vibrio anguillarum in Flounder (Paralichthys olivaceus). Front. Immunol. 2022, 13, 881753. [Google Scholar] [CrossRef]
- Xu, H.; Xing, J.; Tang, X.; Sheng, X.; Zhan, W. The effects of CCL3, CCL4, CCL19 and CCL21 as molecular adjuvants on the immune response to VAA DNA vaccine in flounder (Paralichthys olivaceus). Dev. Comp. Immunol. 2019, 103, 103492. [Google Scholar] [CrossRef]
- Lee, S.H.; Beck, B.R.; Hwang, S.-H.; Song, S.K. Feeding olive flounder (Paralichthys olivaceus) with Lactococcus lactis BFE920 expressing the fusion antigen of Vibrio OmpK and FlaB provides protection against multiple Vibrio pathogens: A universal vaccine effect. Fish Shellfish Immunol. 2021, 114, 253–262. [Google Scholar] [CrossRef]
- Chakraborty, S.; Cao, T.; Hossain, A.; Gnanagobal, H.; Vasquez, I.; Boyce, D.; Santander, J. Vibrogen-2 vaccine trial in lumpfish (Cyclopterus lumpus) against Vibrio anguillarum. J. Fish Dis. 2019, 42, 1057–1064. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Y.-H.; Sun, B.-G.; Chi, H.; Li, J.; Sun, L. Environmental isolates P1SW and V3SW as a bivalent vaccine induce effective cross-protection against Edwardsiella tarda and Vibrio anguillarum. Dis. Aquat. Org. 2013, 103, 45–53. [Google Scholar] [CrossRef]
- Yu, L.; Hu, Y.; Sun, B.; Sun, L. C312M: An attenuated Vibrio anguillarum strain that induces immunoprotection as an oral and immersion vaccine. Dis. Aquat. Org. 2012, 102, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Baliga, P.; Shekar, M.; Venugopal, M.N. Potential Outer Membrane Protein Candidates for Vaccine Development Against the Pathogen Vibrio anguillarum: A Reverse Vaccinology Based Identification. Curr. Microbiol. 2017, 75, 368–377. [Google Scholar] [CrossRef]
- Vieira, V.V.; Teixeira, L.F.; Vicente, A.C.; Momen, H.; Salles, C.A. Differentiation of environmental and clinical isolates of Vibrio mimicus from Vibrio cholerae by multilocus enzyme electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2360–2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, Y.-W.; Mo, Z.-Q.; Luo, X.-C.; Sun, H.-Y.; Liu, P.; Li, A.-X.; Zhou, S.-M.; Dan, X.-M. Outbreak of a novel disease associated with Vibrio mimicus infection in fresh water cultured yellow catfish, Pelteobagrus fulvidraco. Aquaculture 2014, 432, 119–124. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, D.; Han, S.; Zhou, Y.; Wang, K.; Huang, X.; Chen, D.; Peng, X.; Lai, W. Outbreaks of vibriosis associated with Vibrio mimicus in freshwater catfish in China. Aquaculture 2014, 433, 82–84. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, X.-C.; Liu, X.-Y.; Yuan, K.; Zhang, J.-J.; Gao, H.-H.; Li, J.-N. An oral double-targeted DNA vaccine induces systemic and intestinal mucosal immune responses and confers high protection against Vibrio mimicus in grass carps. Aquaculture 2019, 504, 248–259. [Google Scholar] [CrossRef]
- Cao, J.; Huang, A.-L.; Zhu, X.-C.; Li, L.; Li, J.-N. Construction of Vibrio mimicus ghosts as a novel inactivated vaccine candidate and its protective efficacy against ascites disease in grass carps (Ctenopharyngodon idella). Aquaculture 2018, 485, 147–153. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Zhang, T.-T.; Li, J.-N.; Liu, X.-L.; Li, L. Design and evaluation of a tandemly arranged outer membrane protein U (OmpU) multi-epitope as a potential vaccine antigen against Vibrio mimicus in grass carps (Ctenopharyngodon idella). Vet. Immunol. Immunopathol. 2014, 160, 61–69. [Google Scholar] [CrossRef]
- Li, J.-N.; Zhao, Y.-T.; Cao, S.-L.; Wang, H.; Zhang, J.-J. Integrated transcriptomic and proteomic analyses of grass carp intestines after vaccination with a double-targeted DNA vaccine of Vibrio mimicus. Fish Shellfish Immunol. 2019, 98, 641–652. [Google Scholar] [CrossRef]
- Cao, S.-L.; Guo, J.-J.; Zhao, W.-P.; Yang, W.-F.; Zhang, S.-L.; Tao, H.-Z.; Li, J.-N. Impacts of oral Vibrio mimicus double-targeted DNA vaccine on the gut microbiota in grass carps (Ctenopharyngodon idella) and correlations with intestinal mucosa innate immunity. Aquaculture 2020, 533, 736201. [Google Scholar] [CrossRef]
- Yu, Z.; Geng, Y.; Wang, K.; Chen, D.; Huang, X.; Ou, Y.; Peng, G. Complete genome sequence of Vibrio mimicus strain SCCF01 with potential application in fish vaccine development. Virulence 2017, 8, 1028–1030. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Wang, E.; Geng, Y.; Wang, K.; Chen, D.; Huang, X.; Ouyang, P.; Zuo, Z.; He, C.; Tang, L.; et al. Multiplex genome editing by natural transformation in Vibrio mimicus with potential application in attenuated vaccine development. Fish Shellfish Immunol. 2019, 92, 377–383. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, C.; Zhao, Z.; Huang, X.; Yang, Y.; Ding, X. Complete Genome Sequence of Vibrio alginolyticus ZJ-T. Genome Announc. 2016, 4, e00912-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gohary, M.; El Gamal, A.M.; Atia, A.; El-Dakrour, M. Treatment Trial of Nile Tilapia (Oreochromis niloticus) Experimentally Infected with Vibrio alginolyticus Isolated from Sea bass (Dicentrarchus labrax). Pak. J. Biol. Sci. 2020, 23, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.-G.; Son, K.-T.; Yu, H.; Lee, T.-S.; Lee, H.-J.; Shin, S.; Kwon, J.-Y.; Park, K.; Kim, J. Antimicrobial Resistance of Vibrio parahaemolyticus and Vibrio alginolyticus Strains Isolated from Farmed Fish in Korea from 2005 through 2007. J. Food Prot. 2011, 74, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Shin, Y.; Jang, S.; Jung, Y.; So, J.-S. Antimicrobial susceptibility of Vibrio alginolyticus isolated from oyster in Korea. Environ. Sci. Pollut. Res. 2016, 23, 21106–21112. [Google Scholar] [CrossRef] [PubMed]
- Supardy, N.A.; Ibrahim, D.; Nor, S.R.M.; Noordin, W.N. Bioactive Compounds of Pseudoalteromonas sp. IBRL PD4.8 Inhibit Growth of Fouling Bacteria and Attenuate Biofilms of Vibrio alginolyticus FB3. Pol. J. Microbiol. 2019, 68, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Sasikala, D.; Srinivasan, P. Characterization of potential lytic bacteriophage against Vibrio alginolyticus and its therapeutic implications on biofilm dispersal. Microb. Pathog. 2016, 101, 24–35. [Google Scholar] [CrossRef]
- Kokkari, C.; Sarropoulou, E.; Bastías, R.; Mandalakis, M.; Katharios, P. Isolation and characterization of a novel bacteriophage infecting Vibrio alginolyticus. Arch. Microbiol. 2018, 200, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Q.; Fan, J.; Yan, T.; Zhang, H.; Yang, J.; Deng, D.; Liu, C.; Wei, T.; Ma, Y. Characterization and Genomic Analysis of ValSw3-3, a New Siphoviridae Bacteriophage Infecting Vibrio alginolyticus. J. Virol. 2020, 94, e00066-20. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, C.; Wei, F.; Yu, F.; Zhao, Z. Bactericidal activity of a holin-endolysin system derived from Vibrio alginolyticus phage HH109. Microb. Pathog. 2021, 159, 105135. [Google Scholar] [CrossRef]
- Matamp, N.; Bhat, S.G. Phage Endolysins as Potential Antimicrobials against Multidrug Resistant Vibrio alginolyticus and Vibrio parahaemolyticus: Current Status of Research and Challenges Ahead. Microorganisms 2019, 7, 84. [Google Scholar] [CrossRef]
- Yang, M.-J.; Xu, D.; Yang, D.-X.; Li, L.; Peng, X.-X.; Chen, Z.-G.; Li, H. Malate enhances survival of zebrafish against Vibrio alginolyticus infection in the same manner as taurine. Virulence 2020, 11, 349–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Dietary astaxanthin augments disease resistance of Asian seabass, Lates calcarifer (Bloch, 1790), against Vibrio alginolyticus infection. Fish Shellfish Immunol. 2021, 114, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Setiaji, J.; Feliatra, F.; Teruna, H.Y.; Lukistyowati, I.; Suharman, I.; Muchlisin, Z.A.; Johan, T.I. Antibacterial activity in secondary metabolite extracts of heterotrophic bacteria against Vibrio alginolyticus, Aeromonas hydrophila, and Pseudomonas aeruginosa. F1000Research 2020, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Q.; Liu, Q.; Cao, X.; Shi, C.; Zhang, Y. Roles of Hfq in the stress adaptation and virulence in fish pathogen Vibrio alginolyticus and its potential application as a target for live attenuated vaccine. Appl. Microbiol. Biotechnol. 2011, 91, 353–364. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, S.; Jian, J. Protection against Vibrio alginolyticus in pearl gentian grouper (female symbol Epinephelus fuscoguttatus × male symbol Epinephelus lanceolatu) immunized with an acfA-deletion live attenuated vaccine. Fish Shellfish Immunol. 2019, 86, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Qiu, M.; Zhao, J.; Hoare, R.; Monaghan, S.J.; Song, D.; Chang, Y.; Jian, J. Construction of a Vibrio alginolyticus hopPmaJ (hop) mutant and evaluation of its potential as a live attenuated vaccine in orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 2018, 76, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.; Chang, Y.; Zheng, H.; Tan, H.; Zhou, S.; Zeng, F.; Hoare, R.; Monaghan, S.J.; Wang, N.; Ding, Y. A live attenuated strain of HY9901ΔvscB provides protection against Vibrio alginolyticus in pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatu). Aquaculture 2022, 546, 737353. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, F.; Wang, Z.; Tang, J.; Cai, S.; Jian, J. Construction and evaluation of Vibrio alginolyticus ΔclpP mutant, as a safe live attenuated vibriosis vaccine. Fish Shellfish Immunol. 2020, 98, 917–922. [Google Scholar] [CrossRef]
- Zhou, S.; Tu, X.; Pang, H.; Hoare, R.; Monaghan, S.J.; Luo, J.; Jian, J. A T3SS Regulator Mutant of Vibrio alginolyticus Affects Antibiotic Susceptibilities and Provides Significant Protection to Danio rerio as a Live Attenuated Vaccine. Front. Cell. Infect. Microbiol. 2020, 10, 183. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.-L.; Su, Y.-B.; Peng, X.-X.; Li, H. Activation of the TCA Cycle to Provide Immune Protection in Zebrafish Immunized by High Magnesium-Prepared Vibrio alginolyticus Vaccine. Front. Immunol. 2021, 12, 739591. [Google Scholar] [CrossRef]
- Cai, S.H.; Lu, Y.S.; Wu, Z.H.; Jian, J.C. Cloning, expression of Vibrio alginolyticus outer membrane protein-OmpU gene and its potential application as vaccine in crimson snapper, Lutjanus erythropterus Bloch. J. Fish Dis. 2013, 36, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Nehlah, R.; Firdaus-Nawi, M.; Nik-Haiha, N.Y.; Karim, M.; Zamri-Saad, M.; Ina-Salwany, M.Y. Recombinant vaccine protects juvenile hybrid grouper, Epinephelus fuscoguttatus × Epinephelus lanceolatus, against infection by Vibrio alginolyticus. Aquac. Int. 2017, 25, 2047–2059. [Google Scholar] [CrossRef]
- Chen, C.; Kang, C.; Rong, N.; Wu, N.; Chen, C.; Wu, S.; Zhang, X.; Liu, X. Evaluation of Immunogenicity, Protective Immunity on Aquaculture Pathogenic Vibrio and Fermentation of Vibrio alginolyticus Flagellin FlaC Protein. Iran. J. Biotechnol. 2019, 17, e2628-42. [Google Scholar]
- Cheng, Z.-X.; Chu, X.; Wang, S.-N.; Peng, X.-X.; Li, H. Six genes of ompA family shuffling for development of polyvalent vaccines against Vibrio alginolyticus and Edwardsiella tarda. Fish Shellfish Immunol. 2018, 75, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wu, Z.-H.; Jian, J.-C.; Huang, Y. Protection of red snapper (Lutjanus sanguineus) against Vibrio alginolyticus with a DNA vaccine containing flagellin flaA gene. Lett. Appl. Microbiol. 2010, 52, 156–161. [Google Scholar] [CrossRef]
- Cai, S.-H.; Lu, Y.-S.; Jian, J.-C.; Wang, B.; Huang, Y.-C.; Tang, J.-F.; Ding, Y.; Wu, Z.-H. Protection against Vibrio alginolyticus in crimson snapper Lutjanus erythropterus immunized with a DNA vaccine containing the ompW gene. Dis. Aquat. Org. 2013, 106, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.-M.; Tao, J.-J.; Kuang, S.-F.; Jiang, M.; Peng, X.-X.; Li, H. Identification of Polyvalent Vaccine Candidates From Extracellular Secretory Proteins in Vibrio alginolyticus. Front. Immunol. 2021, 12, 736360. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, J.; Ma, L.; Li, L.; Zhang, W.; Li, J. Identification of fish source Vibrio alginolyticus and evaluation of its bacterial ghosts vaccine immune effects. Microbiologyopen 2018, 7, e00576. [Google Scholar] [CrossRef] [Green Version]
- Ji, Q.; Wang, S.; Ma, J.; Liu, Q. A review: Progress in the development of fish Vibrio spp. vaccines. Immunol. Lett. 2020, 226, 46–54. [Google Scholar] [CrossRef]
- Dadar, M.; Dhama, K.; Vakharia, V.N.; Hoseinifar, S.H.; Karthik, K.; Tiwari, R.; Khandia, R.; Munjal, A.; Salgado-Miranda, C.; Joshi, S.K. Advances in Aquaculture Vaccines Against Fish Pathogens: Global Status and Current Trends. Rev. Fish. Sci. Aquac. 2016, 25, 184–217. [Google Scholar] [CrossRef]
| Pathogen | Prevention and Control Technology | Concrete Measure/ Vaccine Type | Host | Vaccine Antigen Components | Route of Infection | Ref. |
|---|---|---|---|---|---|---|
| V. vulnificus | Vaccines | Inactivated | Tilapia (Sex reversed hybrid) | Atypical V. vulnificus (Formalin killed cells) | Injection (IP) | [34] |
| E. coioides | Vh + Vv + Va inactive vaccine and ISKNV whole cell inactive vaccine | Injection (IP) | [16] | |||
| Subunit | Japanese eel | Expressed OmpU of V. vulnificus | Injection (IP) | [35] | ||
| Multivalent | Japanese eel | Recombinant Omp containing both OmpA and OmpU | Injection (IP) | [36] | ||
| European eel | Trivalent outer membrane protein (OmpⅡ-U-A) | Injection (IP) | [37] |
| Pathogen | Prevention and Control Technology | Concrete Measure/ Vaccine Type | Host | Vaccine Antigen Components | Route of Infection | Ref. |
|---|---|---|---|---|---|---|
| V. parahaemolyticus | Vaccines | Inactivated | Tiger grouper | Vaksin polivalen Vibrio (formalin killed cells) | Injection, Bath | [43] |
| Recombinant | Juvenile sea bass | Expressed r-OmpK of Vibrio | Injection (IP) | [27] |
| Pathogen | Prevention and Control Technology | Concrete Measure/ Vaccine Type | Host | Route of Infection | Ref. |
|---|---|---|---|---|---|
| V. cholerae | Antibiotics | Neomycin, Doxycycline | Bluegill sunfish | Injection (IP) | [49] |
| Edible antibody | Anti-non-O1 V. cholerae egg yolk powder | Carp | Injection | [51] | |
| Vaccines | None | None | None | None |
| Pathogen | Prevention and Control Technology | Concrete Measure/ Vaccine Type | Host | Vaccine Antigen Components | Route of Infection | Ref. |
|---|---|---|---|---|---|---|
| V. mimicus | Antibiotics | Florfenicol, Gentamicin, Lomefloxacin | Southern catfish | NR | Injection (IP) | [83] |
| Vaccines | DNA | Grass carp | BGS and ICLP | Injection (IP), Oral | [84] | |
| Attenuated | Yellow catfish | V. mimicus SCCF01 (attenuated) | Bath | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Wang, Y.; Yang, W.; Cai, H.; Zhang, Y.; Huang, L. Strategies for Prevention and Control of Vibriosis in Asian Fish Culture. Vaccines 2023, 11, 98. https://doi.org/10.3390/vaccines11010098
Xu K, Wang Y, Yang W, Cai H, Zhang Y, Huang L. Strategies for Prevention and Control of Vibriosis in Asian Fish Culture. Vaccines. 2023; 11(1):98. https://doi.org/10.3390/vaccines11010098
Chicago/Turabian StyleXu, Kangping, Yushu Wang, Wangxiaohan Yang, Hongyan Cai, Youyu Zhang, and Lixing Huang. 2023. "Strategies for Prevention and Control of Vibriosis in Asian Fish Culture" Vaccines 11, no. 1: 98. https://doi.org/10.3390/vaccines11010098
APA StyleXu, K., Wang, Y., Yang, W., Cai, H., Zhang, Y., & Huang, L. (2023). Strategies for Prevention and Control of Vibriosis in Asian Fish Culture. Vaccines, 11(1), 98. https://doi.org/10.3390/vaccines11010098




