Dendritic Cell Vaccines Impact the Type 2 Innate Lymphoid Cell Population and Their Cytokine Generation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Dendritic Cell Cultures
2.3. Dendritic Cell Vaccine Preparation
2.4. Flow Cytometry Gating Strategy
2.5. Intracellular Cytokine Antibody Staining
2.6. Tissue Processing
2.7. DC Vaccine Migration to Lymph Node
2.8. Cytokine Response Assay
2.9. B16F10 Cell Culture, Tumor Challenge, and Tumor Nodule Quantification
2.10. Statistical Analysis
3. Results
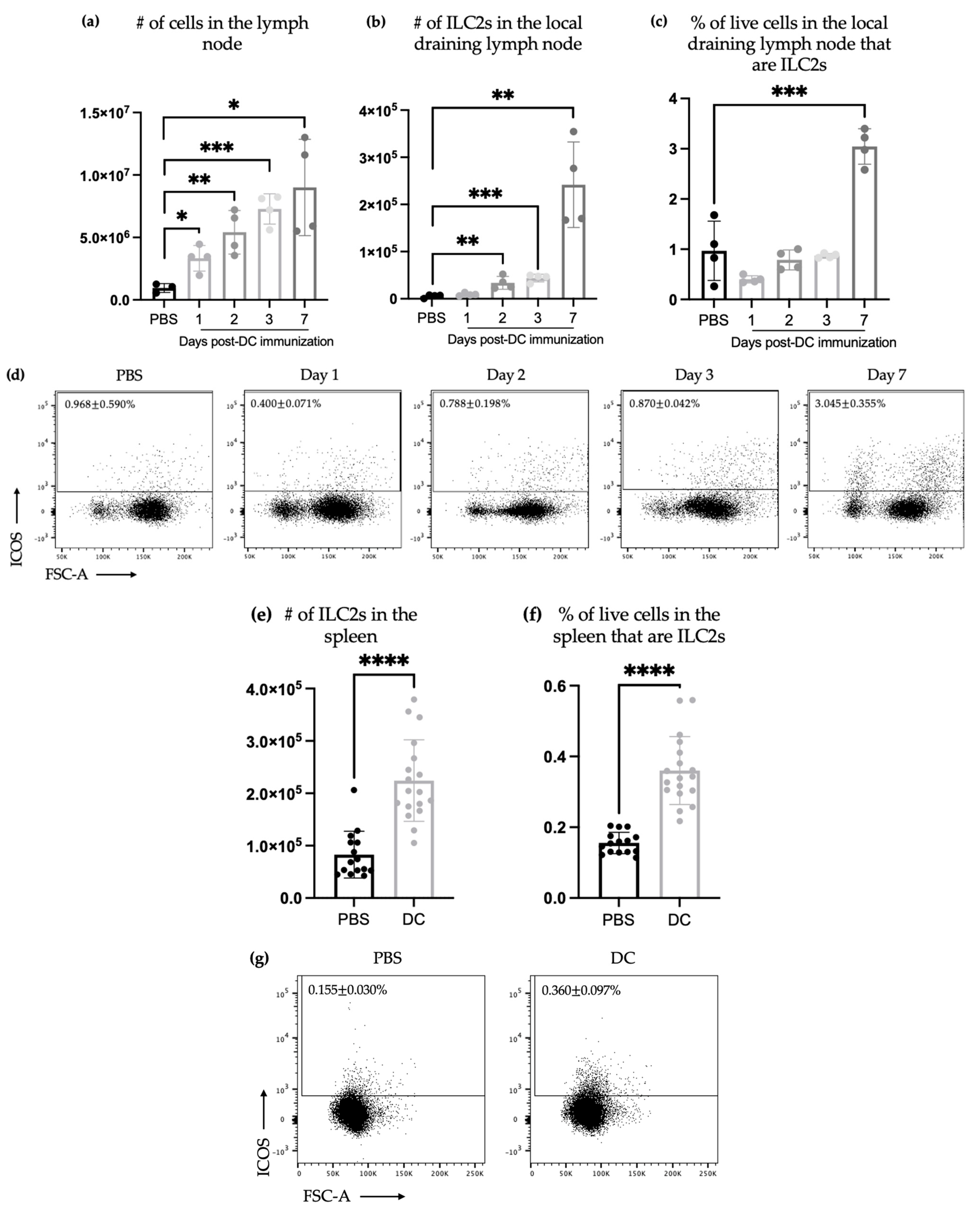
3.1. DC-Based Vaccines Efficiently Induced an Increase in the Number of ILC2s in Draining Lymph Nodes and Spleen
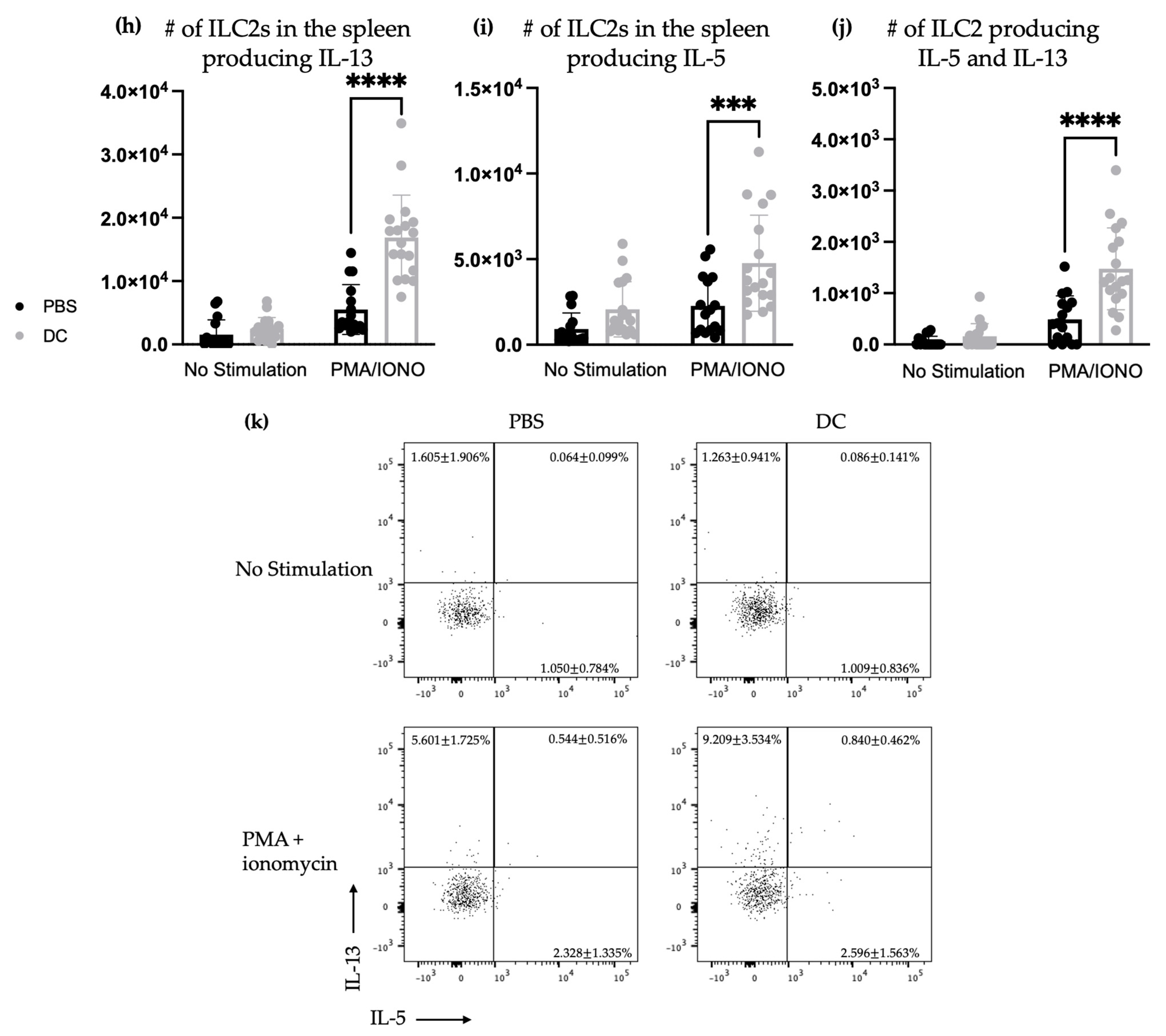
3.2. The DC Vaccine Elicited Cytokine Secretion via Splenic ILC2s after Mice Were Challenged Intravenously with B16F10 Melanoma Cells
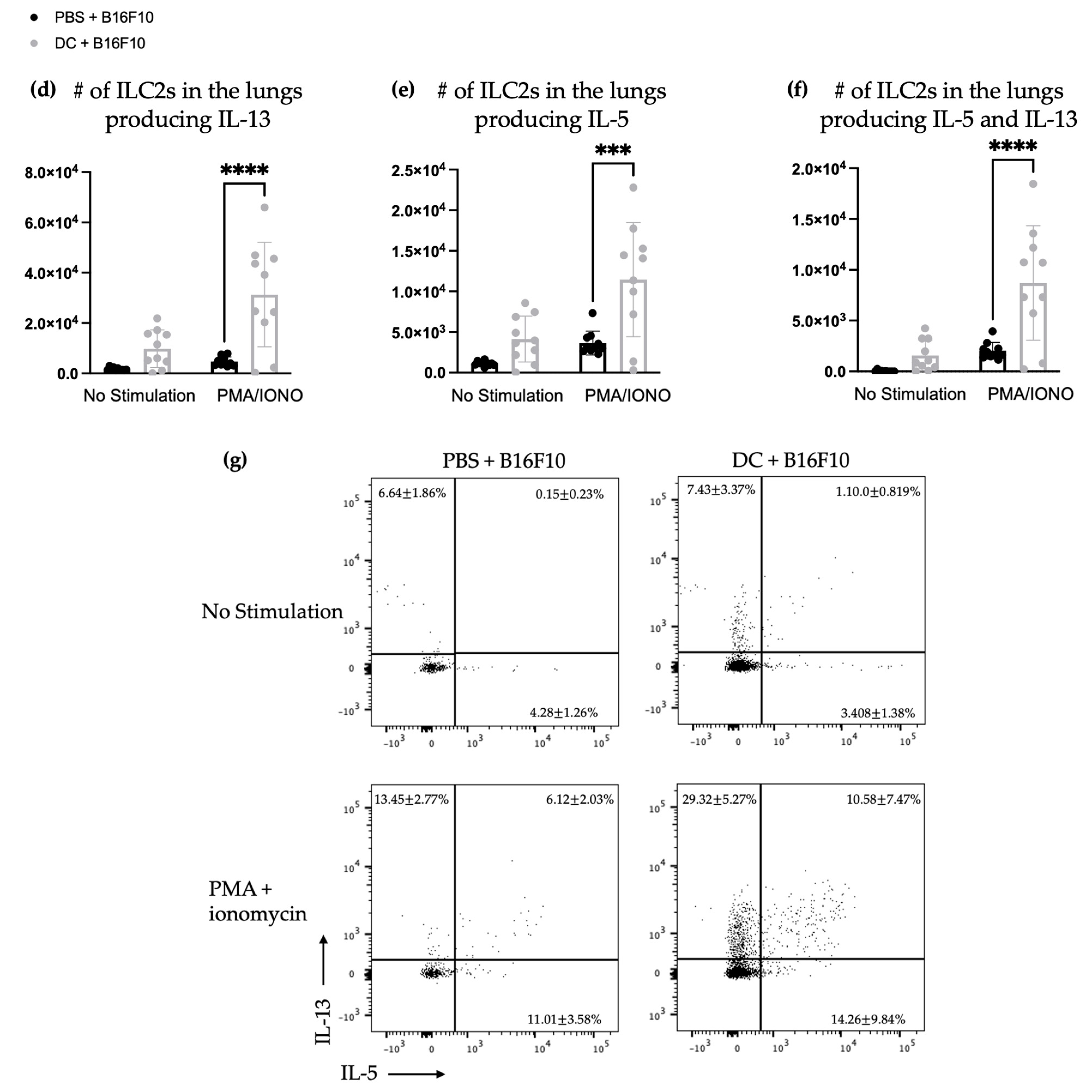
3.3. The Number and Proportion of Cytokine-Producing Pulmonary ILC2s Increased after DC Vaccination Followed by Intravenous Challenge with B16F10 Melanoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cording, S.; Medvedovic, J.; Aychek, T.; Eberl, G. Innate lymphoid cells in defense, immunopathology and immunotherapy. Nat. Immunol. 2016, 17, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Wagner, M.; Gao, Y.; Koyasu, S.; Smyth, M.J. Hide and seek: Plasticity of innate lymphoid cells in cancer. Semin. Immunol. 2019, 41, 101273. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.M.; Golebski, K.; Spits, H. Plasticity of innate lymphoid cell subsets. Nat. Rev. Immunol. 2020, 20, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Dammeijer, F.; Aerts, J.G.J.V.; Vroman, H. Current State of Dendritic Cell-Based Immunotherapy: Opportunities for. Front. Immunol. 2018, 9, 2804. [Google Scholar] [CrossRef]
- Li, X.; Dai, H.; Wang, H.; Han, W. Exploring innate immunity in cancer immunotherapy: Opportunities and challenges. Cell Mol. Immunol. 2021, 18, 1607–1609. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Boudreau, J.E.; Fraser, K.; Liu, H.; Delanghe, J.; Gauldie, J.; Xing, Z.; Bramson, J.L.; Wan, Y. Enhanced Antitumor Immunity Elicited by Dendritic Cell Vaccines Is a Result of Their Ability to Engage Both CTL and IFNγ-producing NK Cells. Mol. Ther. 2008, 16, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Bouwer, A.L.; Saunderson, S.C.; Caldwell, F.J.; Damani, T.T.; Pelham, S.J.; Dunn, A.C.; Jack, R.W.; Stoitzner, P.; McLellan, A.D. NK cells are required for dendritic cell-based immunotherapy at the time of tumor challenge. J. Immunol. 2014, 192, 2514–2521. [Google Scholar] [CrossRef]
- Pampena, M.B.; Levy, E.M. Natural killer cells as helper cells in dendritic cell cancer vaccines. Front. Immunol. 2015, 6, 13. [Google Scholar] [CrossRef]
- Jacquelot, N.; Ghaedi, M.; Warner, K.; Chung, D.C.; Crome, S.Q.; Ohashi, P.S. Immune Checkpoints and Innate Lymphoid Cells-New Avenues for Cancer Immunotherapy. Cancers 2021, 13, 5967. [Google Scholar] [CrossRef]
- Crinier, A.; Vivier, E.; Bléry, M. Helper-like innate lymphoid cells and cancer immunotherapy. Semin. Immunol. 2019, 41, 101274. [Google Scholar] [CrossRef]
- Guillerey, C. Roles of cytotoxic and helper innate lymphoid cells in cancer. Mamm. Genome 2018, 29, 777–789. [Google Scholar] [CrossRef]
- Herbert, D.R.; Douglas, B.; Zullo, K. Group 2 Innate Lymphoid Cells (ILC2): Type 2 Immunity and Helminth Immunity. Int. J. Mol. Sci. 2019, 20, 2276. [Google Scholar] [CrossRef]
- Trabanelli, S.; Chevalier, M.F.; Martinez-Usatorre, A.; Gomez-Cadena, A.; Salomé, B.; Lecciso, M.; Salvestrini, V.; Verdeil, G.; Racle, J.; Papayannidis, C.; et al. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 2017, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salomé, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.S.; et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Investig. 2017, 127, 2916–2929. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, I.P.; Pejnovic, N.N.; Radosavljevic, G.D.; Pantic, J.M.; Milovanovic, M.Z.; Arsenijevic, N.N.; Lukic, M.L. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int. J. Cancer 2014, 134, 1669–1682. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Png, S.; Richard, A.C.; Tsyben, A.; Hamm, G.; Stockis, J.; Garcia, C.; Pinaud, S.; Nicholls, A.; Ros, X.R.; et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat. Immunol. 2020, 21, 998–1009. [Google Scholar] [CrossRef]
- Salimi, M.; Wang, R.; Yao, X.; Li, X.; Wang, X.; Hu, Y.; Chang, X.; Fan, P.; Dong, T.; Ogg, G. Activated innate lymphoid cell populations accumulate in human tumour tissues. BMC Cancer 2018, 18, 341. [Google Scholar] [CrossRef]
- Bie, Q.; Zhang, P.; Su, Z.; Zheng, D.; Ying, X.; Wu, Y.; Yang, H.; Chen, D.; Wang, S.; Xu, H. Polarization of ILC2s in peripheral blood might contribute to immunosuppressive microenvironment in patients with gastric cancer. J. Immunol. Res. 2014, 2014, 923135. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jacquelot, N.; Preaudet, A.; Hediyeh-Zadeh, S.; Souza-Fonseca-Guimaraes, F.; McKenzie, A.N.J.; Hansbro, P.M.; Davis, M.J.; Mielke, L.A.; Putoczki, T.L.; et al. Type 2 Innate Lymphoid Cells Protect against Colorectal Cancer Progression and Predict Improved Patient Survival. Cancers 2021, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- Saranchova, I.; Han, J.; Zaman, R.; Arora, H.; Huang, H.; Fenninger, F.; Choi, K.B.; Munro, L.; Pfeifer, C.G.; Welch, I.; et al. Type 2 Innate Lymphocytes Actuate Immunity Against Tumours and Limit Cancer Metastasis. Sci. Rep. 2018, 8, 2924. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Moon, U.J.; Kim, H.J.; Choi, H.J.; Sin, J.I.; Park, N.H.; Cho, H.R.; Kwon, B. Intratumorally Establishing Type 2 Innate Lymphoid Cells Blocks Tumor Growth. J. Immunol. 2016, 196, 2410–2423. [Google Scholar] [CrossRef] [PubMed]
- Cortez, V.S.; Robinette, M.L.; Colonna, M. Innate lymphoid cells: New insights into function and development. Curr. Opin. Immunol. 2015, 32, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.; Steer, C.A.; Mathä, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.; Takei, F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef] [PubMed]
- van Beek, J.J.P.; Martens, A.W.J.; Bakdash, G.; de Vries, I.J.M. Innate Lymphoid Cells in Tumor Immunity. Biomedicines 2016, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Chan, L. Investigating Components of the Innate Immune System Following Administration of a Dendritic Cell-Based Vaccine. PhD Thesis, University of Guelph, Guelph, ON, Canada, May 2023. [Google Scholar]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef]
- Nadafi, R.; Koning, J.J.; Veninga, H.; Stachtea, X.N.; Konijn, T.; Zwiers, A.; Malmström, A.; den Haan, J.M.M.; Mebius, R.E.; Maccarana, M.; et al. Dendritic Cell Migration to Skin-Draining Lymph Nodes Is Controlled by Dermatan Sulfate and Determines Adaptive Immunity Magnitude. Front. Immunol. 2018, 9, 206. [Google Scholar] [CrossRef]
- Alvarez, D.; Vollmann, E.H.; von Andrian, U.H. Mechanisms and consequences of dendritic cell migration. Immunity 2008, 29, 325–342. [Google Scholar] [CrossRef]
- Shang, N.; Figini, M.; Shangguan, J.; Wang, B.; Sun, C.; Pan, L.; Ma, Q.; Zhang, Z. Dendritic cells based immunotherapy. Am. J. Cancer Res. 2017, 7, 2091–2102. [Google Scholar]
- Aliyu, M.; Zohora, F.; Saboor-Yaraghi, A.A. Spleen in innate and adaptive immunity regulation. AIMS Allergy Immunol. 2021, 5, 1–17. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef]
- Ishiguro, T.; Nakajima, M.; Naito, M.; Muto, T.; Tsuruo, T. Identification of genes differentially expressed in B16 murine melanoma sublines with different metastatic potentials. Cancer Res. 1996, 56, 875–879. [Google Scholar] [PubMed]
- Karimi, K.; Karimi, Y.; Chan, J.; Boudreau, J.E.; Basset, J.; Chew, M.V.; Reid, S.; Bramson, J.L.; Wan, Y.; Ashkar, A.A. Type I IFN signaling on dendritic cells is required for NK cell-mediated anti-tumor immunity. Innate Immun. 2015, 21, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Corral, D.; Charton, A.; Krauss, M.; Blanquart, E.; Levillain, F.; Lefrançais, E.; Sneperger, T.; Girard, J.-P.; Eberl, G.; Poquet, Y.; et al. Metabolic regulation of ILC2 differentiation into ILC1-like cells during Mycobacterium tuberculosis infection. bioRxiv 2021, 1, 427257. [Google Scholar] [CrossRef]
- Markov, O.V.; Mironova, N.L.; Sennikov, S.V.; Vlassov, V.V.; Zenkova, M.A. Prophylactic Dendritic Cell-Based Vaccines Efficiently Inhibit Metastases in Murine Metastatic Melanoma. PLoS ONE 2015, 10, e0136911. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koido, S.; Kashiwaba, M.; Chen, D.; Gendler, S.; Kufe, D.; Gong, J. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. J. Immunol. 2000, 165, 5713–5719. [Google Scholar] [CrossRef]
- Hong, X.; Dong, T.; Hu, J.; Yi, T.; Li, W.; Zhang, Z.; Lin, S.; Niu, W. Synergistical toll-like receptors activated dendritic cells induce antitumor effects against carcinoembryonic antigen-expressing colon cancer. Int. J. Color. Dis. 2013, 28, 25–33. [Google Scholar] [CrossRef]
- Dhakal, M.; Hardaway, J.C.; Guloglu, F.B.; Miller, M.M.; Hoeman, C.M.; Zaghouani, A.A.; Wan, X.; Rowland, L.M.; Cascio, J.A.; Sherman, M.P.; et al. IL-13Rα1 is a surface marker for M2 macrophages influencing their differentiation and function. Eur. J. Immunol. 2014, 44, 842–855. [Google Scholar] [CrossRef]
- Barderas, R.; Bartolomé, R.A.; Fernandez-Aceñero, M.J.; Torres, S.; Casal, J.I. High expression of IL-13 receptor α2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012, 72, 2780–2790. [Google Scholar] [CrossRef]
- Terabe, M.; Matsui, S.; Noben-Trauth, N.; Chen, H.; Watson, C.; Donaldson, D.D.; Carbone, D.P.; Paul, W.E.; Berzofsky, J.A. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 2000, 1, 515–520. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, L.; Mehrani, Y.; Minott, J.A.; Bridle, B.W.; Karimi, K. Dendritic Cell Vaccines Impact the Type 2 Innate Lymphoid Cell Population and Their Cytokine Generation in Mice. Vaccines 2023, 11, 1559. https://doi.org/10.3390/vaccines11101559
Chan L, Mehrani Y, Minott JA, Bridle BW, Karimi K. Dendritic Cell Vaccines Impact the Type 2 Innate Lymphoid Cell Population and Their Cytokine Generation in Mice. Vaccines. 2023; 11(10):1559. https://doi.org/10.3390/vaccines11101559
Chicago/Turabian StyleChan, Lily, Yeganeh Mehrani, Jessica A. Minott, Byram W. Bridle, and Khalil Karimi. 2023. "Dendritic Cell Vaccines Impact the Type 2 Innate Lymphoid Cell Population and Their Cytokine Generation in Mice" Vaccines 11, no. 10: 1559. https://doi.org/10.3390/vaccines11101559
APA StyleChan, L., Mehrani, Y., Minott, J. A., Bridle, B. W., & Karimi, K. (2023). Dendritic Cell Vaccines Impact the Type 2 Innate Lymphoid Cell Population and Their Cytokine Generation in Mice. Vaccines, 11(10), 1559. https://doi.org/10.3390/vaccines11101559








