Non-Polio Enterovirus Surveillance in the Ural Federal District and Western Siberia, 2022: Is There a Need for a Vaccine?
Abstract
:1. Introduction
2. Materials and Methods
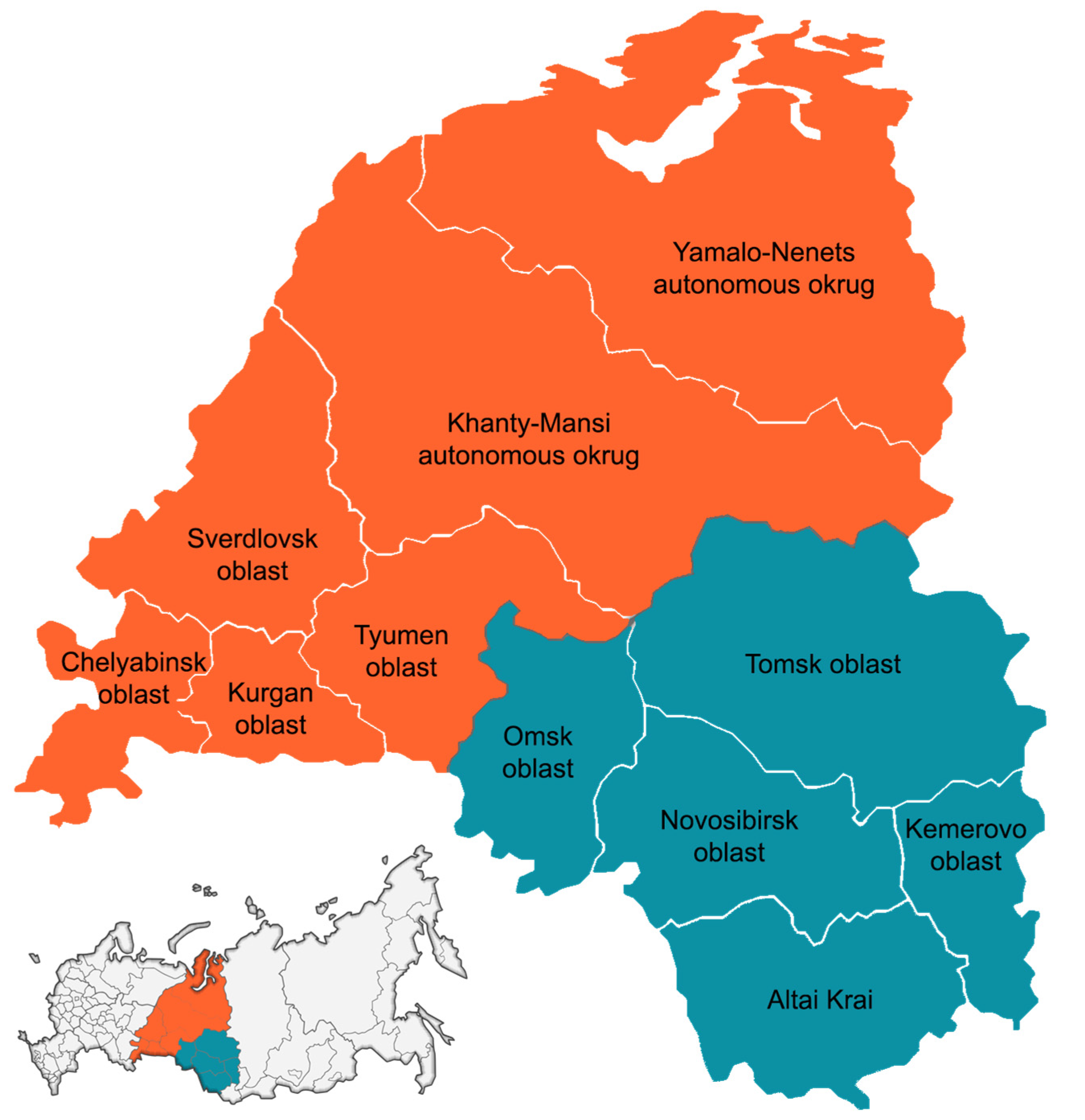
2.1. Data Collection and Samples
2.2. RNA Extraction and Molecular Detection
2.3. Sequencing of the VP1 Gene
2.4. Genotyping and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
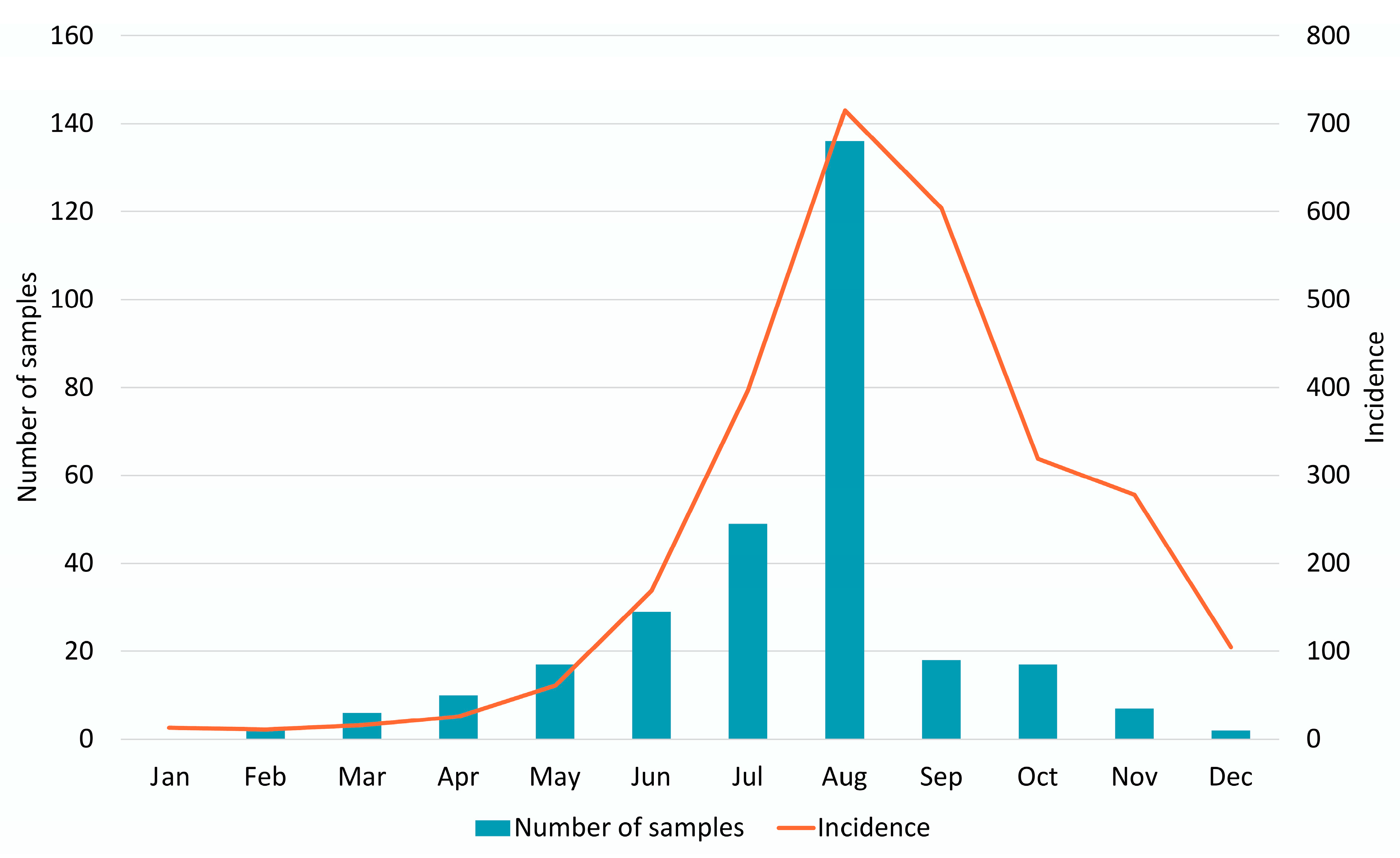
3.1. Epidemiology and Patient Demographics
3.2. Molecular Typing
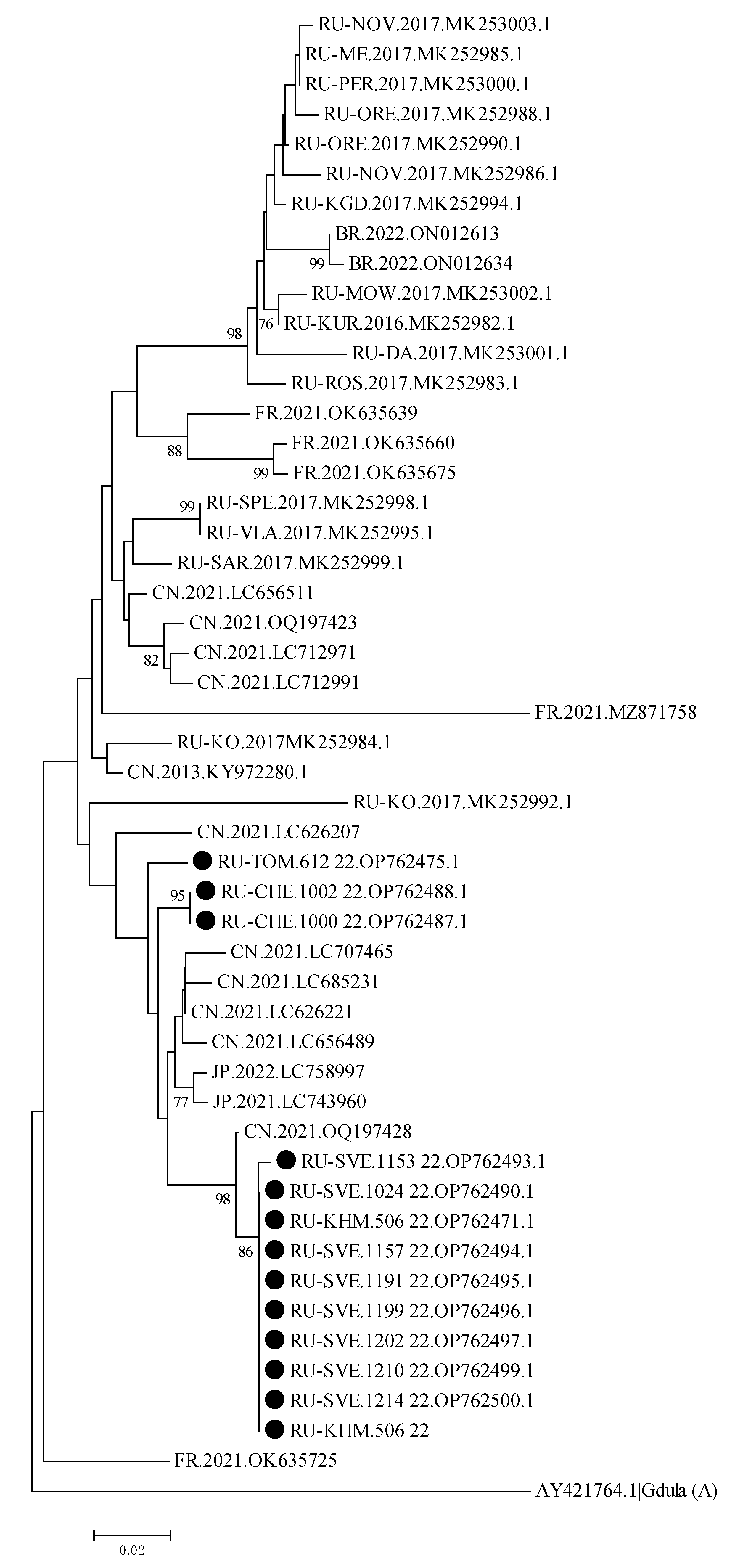
3.3. Sequence and Phylogenetic Analysis of Enterovirus Genotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef] [PubMed]
- Pallansch, M.A.; Oberste, M.S.; Whitton, L.J. Enteroviruses: Polioviruses, Coxsackieviruses, Echoviruses, and Newer Enteroviruses. In Fields’ Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1, pp. 490–530. ISBN 1-4511-0563-0. [Google Scholar]
- Pons-Salort, M.; Parker, E.P.K.; Grassly, N.C. The Epidemiology of Non-Polio Enteroviruses: Recent Advances and Outstanding Questions. Curr. Opin. Infect. Dis. 2015, 28, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, M.H. Enterovirus Infections: Diagnosis and Treatment. Semin. Pediatr. Infect. Dis. 2002, 13, 40–47. [Google Scholar] [CrossRef]
- Pichichero, M.E.; McLinn, S.; Rotbart, H.A.; Menegus, M.A.; Cascino, M.; Reidenberg, B.E. Clinical and Economic Impact of Enterovirus Illness in Private Pediatric Practice. Pediatrics 1998, 102, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.J. Enteroviruses and Parechoviruses. Microbiol. Spectr. 2016, 4, 22. [Google Scholar] [CrossRef]
- Pons-Salort, M.; Grassly, N.C. Serotype-Specific Immunity Explains the Incidence of Diseases Caused by Human Enteroviruses. Science 2018, 361, 800–803. [Google Scholar] [CrossRef]
- Rotbart, H.A. Viral Meningitis. Semin. Neurol. 2000, 20, 277–292. [Google Scholar] [CrossRef]
- Savolainen, C.; Hovi, T.; Mulders, M.N. Molecular Epidemiology of Echovirus 30 in Europe: Succession of Dominant Sublineages within a Single Major Genotype. Arch. Virol. 2001, 146, 521–537. [Google Scholar] [CrossRef]
- Chen, P.; Tao, Z.; Song, Y.; Liu, G.; Wang, H.; Liu, Y.; Song, L.; Li, Y.; Lin, X.; Cui, N.; et al. A Coxsackievirus B5-Associated Aseptic Meningitis Outbreak in Shandong Province, China in 2009. J. Med. Virol. 2013, 85, 483–489. [Google Scholar] [CrossRef]
- Fratty, I.S.; Kriger, O.; Weiss, L.; Vasserman, R.; Erster, O.; Mendelson, E.; Sofer, D.; Weil, M. Increased Detection of Echovirus 6-Associated Meningitis in Patients Hospitalized during the COVID-19 Pandemic, Israel 2021–2022. J. Clin. Virol. 2023, 162, 105425. [Google Scholar] [CrossRef]
- Richter, J.; Tryfonos, C.; Christodoulou, C. Molecular Epidemiology of Enteroviruses in Cyprus 2008–2017. PLoS ONE 2019, 14, e0220938. [Google Scholar] [CrossRef] [PubMed]
- Farshadpour, F.; Taherkhani, R. Molecular Epidemiology of Enteroviruses and Predominance of Echovirus 30 in an Iranian Population with Aseptic Meningitis. J. NeuroVirol. 2021, 27, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, L.; Moreni, G.; Wolthers, K.C.; Pajkrt, D. World-Wide Prevalence and Genotype Distribution of Enteroviruses. Viruses 2021, 13, 434. [Google Scholar] [CrossRef] [PubMed]
- Harvala, H.; Broberg, E.; Benschop, K.; Berginc, N.; Ladhani, S.; Susi, P.; Christiansen, C.; McKenna, J.; Allen, D.; Makiello, P.; et al. Recommendations for Enterovirus Diagnostics and Characterisation within and beyond Europe. J. Clin. Virol. 2018, 101, 11–17. [Google Scholar] [CrossRef]
- Iturriza-Gómara, M.; Megson, B.; Gray, J. Molecular Detection and Characterization of Human Enteroviruses Directly from Clinical Samples Using RT-PCR and DNA Sequencing. J. Med. Virol. 2006, 78, 243–253. [Google Scholar] [CrossRef]
- Abedi, G.R.; Watson, J.T.; Pham, H.; Nix, W.A.; Oberste, M.S.; Gerber, S.I. Enterovirus and Human Parechovirus Surveillance—United States, 2009–2013. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 940–943. [Google Scholar] [CrossRef]
- Abedi, G.R.; Watson, J.T.; Nix, W.A.; Oberste, M.S.; Gerber, S.I. Enterovirus and Parechovirus Surveillance—United States, 2014–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 515–518. [Google Scholar] [CrossRef]
- Nix, W.A.; Oberste, M.S.; Pallansch, M.A. Sensitive, Seminested PCR Amplification of VP1 Sequences for Direct Identification of All Enterovirus Serotypes from Original Clinical Specimens. J. Clin. Microbiol. 2006, 44, 2698–2704. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Khetsuriani, N.; Lamonte-Fowlkes, A.; Oberste, M.S.; Pallansch, M.A. Enterovirus Surveillance—United States, 1970–2005. MMWR Surveill. Summ. 2006, 55, 1–20. [Google Scholar]
- Peng, Q.; Xie, M.; Zhang, Y.; Liu, Q.; Li, W.; Li, S.; Ma, Q.; Lu, X.; Zhong, B. Molecular Epidemiology of the Enteroviruses Associated with Hand, Foot and Mouth Disease/Herpangina in Dongguan, China, 2015. Arch. Virol. 2016, 161, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Q.; Xie, G.-C.; Li, D.-D.; Pang, L.-L.; Xie, J.; Wang, P.; Chen, Y.; Yang, J.; Cheng, W.-X.; Zhang, Q.; et al. Prevalence of Coxsackievirus A6 and Enterovirus 71 in Hand, Foot and Mouth Disease in Nanjing, China in 2013. Pediatr. Infect. Dis. J. 2015, 34, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Deng, F.; Tian, H.; He, W.; Xiao, Y.; Sun, X. Estimation of the Reproduction Number and Identification of Periodicity for HFMD Infections in Northwest China. J. Theor. Biol. 2020, 484, 110027. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, Z.; Zheng, K.; Li, X.; Kong, J.; Duan, X.; Xiao, X.; Guo, B.; Luan, R.; Long, L. Epidemiology of Hand, Foot, and Mouth Disease before and after the Introduction of Enterovirus 71 Vaccines in Chengdu, China, 2009–2018. Pediatr. Infect. Dis. J. 2020, 39, 969–978. [Google Scholar] [CrossRef]
- Österback, R.; Vuorinen, T.; Linna, M.; Susi, P.; Hyypiä, T.; Waris, M. Coxsackievirus A6 and Hand, Foot, and Mouth Disease, Finland. Emerg. Infect. Dis. 2009, 15, 1485–1488. [Google Scholar] [CrossRef]
- Lo, S.-H.; Huang, Y.-C.; Huang, C.-G.; Tsao, K.-C.; Li, W.-C.; Hsieh, Y.-C.; Chiu, C.-H.; Lin, T.-Y. Clinical and Epidemiologic Features of Coxsackievirus A6 Infection in Children in Northern Taiwan between 2004 and 2009. J. Microbiol. Immunol. Infect. 2011, 44, 252–257. [Google Scholar] [CrossRef]
- Wu, Y.; Yeo, A.; Phoon, M.C.; Tan, E.L.; Poh, C.L.; Quak, S.H.; Chow, V.T.K. The Largest Outbreak of Hand; Foot and Mouth Disease in Singapore in 2008: The Role of Enterovirus 71 and Coxsackievirus A Strains. Int. J. Infect. Dis. 2010, 14, e1076–e1081. [Google Scholar] [CrossRef]
- Hoa-Tran, T.N.; Dao, A.T.H.; Nguyen, A.T.; Kataoka, C.; Takemura, T.; Pham, C.H.; Vu, H.M.; Hong, T.T.T.; Ha, N.T.V.; Duong, T.N.; et al. Coxsackieviruses A6 and A16 Associated with Hand, Foot, and Mouth Disease in Vietnam, 2008–2017: Essential Information for Rational Vaccine Design. Vaccine 2020, 38, 8273–8285. [Google Scholar] [CrossRef]
- Kang, H.J.; Yoon, Y.; Lee, Y.-P.; Kim, H.-J.; Lee, D.-Y.; Lee, J.-W.; Hyeon, J.-Y.; Yoo, J.S.; Lee, S.; Kang, C.; et al. A Different Epidemiology of Enterovirus A and Enterovirus B Co-Circulating in Korea, 2012–2019. J. Pediatr. Infect. Dis. Soc. 2021, 10, 398–407. [Google Scholar] [CrossRef]
- Ndiaye, N.; Thiaw, F.D.; Fall, A.; Kébé, O.; Diatta, K.L.; Dia, N.; Fall, M.; Sall, A.A.; Faye, M.; Faye, O. Molecular Epidemiology of Enterovirus A71 in Surveillance of Acute Flaccid Paralysis Cases in Senegal, 2013–2020. Vaccines 2022, 10, 843. [Google Scholar] [CrossRef]
- Romanenkova, N.I.; Nguyen, T.T.T.; Golitsyna, L.N.; Ponomareva, N.V.; Rozaeva, N.R.; Kanaeva, O.I.; Leonov, A.V.; Novikova, N.A.; Bichurina, M.A. Enterovirus 71-Associated Infection in South Vietnam: Vaccination Is a Real Solution. Vaccines 2023, 11, 931. [Google Scholar] [CrossRef] [PubMed]
- Keeren, K.; Böttcher, S.; Diedrich, S. Enterovirus Surveillance (EVSurv) in Germany. Microorganisms 2021, 9, 2005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dong, Z.; Li, J.; Carr, M.J.; Zhuang, D.; Wang, J.; Zhang, Y.; Ding, S.; Tong, Y.; Li, D.; et al. Protective Efficacies of Formaldehyde-Inactivated Whole-Virus Vaccine and Antivirals in a Murine Model of Coxsackievirus A10 Infection. J. Virol. 2017, 91, e00333-17. [Google Scholar] [CrossRef]
- Somasundaram, B.; Chang, C.; Fan, Y.Y.; Lim, P.-Y.; Cardosa, J.; Lua, L. Characterizing Enterovirus 71 and Coxsackievirus A16 Virus-like Particles Production in Insect Cells. Methods 2016, 95, 38–45. [Google Scholar] [CrossRef]
- Fan, S.; Liao, Y.; Jiang, G.; Jiang, L.; Wang, L.; Xu, X.; Feng, M.; Yang, E.; Zhang, Y.; Cui, W.; et al. Study of Integrated Protective Immunity Induced in Rhesus Macaques by the Intradermal Administration of a Bivalent EV71-CA16 Inactivated Vaccine. Vaccine 2020, 38, 2034–2044. [Google Scholar] [CrossRef]
- He, X.; Zhang, M.; Zhao, C.; Zheng, P.; Zhang, X.; Xu, J. From Monovalent to Multivalent Vaccines, the Exploration for Potential Preventive Strategies Against Hand, Foot, and Mouth Disease (HFMD). Virol. Sin. 2021, 36, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, W.; Zhang, C.; Zhou, Y.; Xiong, P.; Wang, S.; Ye, X.; Liu, Q.; Zhou, D.; Huang, Z. A Virus-like Particle-Based Tetravalent Vaccine for Hand, Foot, and Mouth Disease Elicits Broad and Balanced Protective Immunity. Emerg. Microbes Infect. 2018, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Clinical Features | Age Groups | ||||
|---|---|---|---|---|---|
| 0–2 Years | 3–6 Years | 7–17 Years | Adults | Total | |
| Meningitis/meningoencephalitis | 10 (3.8) | 19 (7.2) | 14 (5.3) | 5 (1.9) | 48 (18.1) |
| Hand-foot-and-mouth disease | 54 (20.4) | 36 (13.6) | 23 (8.7) | 1 (0.4) | 114 (43.0) |
| Herpangina | 16 (6.0) | 21 (7.9) | 5 (1.9) | - | 42 (15.8) |
| Gastrointestinal forms | 4 (1.5) | 2 (0.8) | 1 (0.4) | 1 (0.4) | 8 (3.0) |
| Exanthematous fever | 5 (1.9) | 3 (1.1) | - | 1 (0.4) | 9 (3.4) |
| Other infections | 7 (2.6) | 23 (8.7) | 10 (3.8) | 4 (1.5) | 44 (16.6) |
| Total | 96 (36.2) | 104 (39.2) | 53 (20.0) | 12 (4.5) | 265 (100) |
| Species/Types | Meningitis/Meningoencephalitis | Hand-Foot-and-Mouth Disease | Herpangina | Gastrointestinal Forms | Exanthematous Fever | Other Infections | Total, n (%) |
|---|---|---|---|---|---|---|---|
| Coxsackievirus A2 | - | 1 (0.5) | - | - | - | - | 1 (0.5) |
| Coxsackievirus A3 | - | 1 (0.5) | - | - | - | - | 1 (0.5) |
| Coxsackievirus A4 | - | 2 (1.0) | 2 (1.0) | - | - | - | 4 (1.9) |
| Coxsackievirus A5 | - | 1 (0.5) | - | - | - | - | 1 (0.5) |
| Coxsackievirus A6 | 9 (4.3) | 53 (25.4) | 9 (4.3) | - | 5 (2.4) | 9 (4.3) | 85 (40.7) |
| Coxsackievirus A8 | - | 3 (1.4) | 2 (1.0) | - | - | 1 (0.5) | 6 (2.9) |
| Coxsackievirus A10 | - | 1 (0.5) | 9 (4.3) | 1 (0.5) | 2 (1.0) | 3 (1.4) | 16 (7.7) |
| Coxsackievirus A16 | - | 8 (3.8) | 8 (3.8) | - | 1 (0.5) | 5 (2.4) | 22 (10.5) |
| Enterovirus A71 | - | 5 (2.4) | 1 (0.5) | - | 1 (0.5) | 1 (0.5) | 8 (3.8) |
| Enterovirus A | 9 (4.3) | 75 (35.9) | 31 (14.8) | 1 (0.5) | 9 (4.3) | 19 (9.1) | 144 (68.9) |
| Coxsackievirus A9 | 11 (5.3) | 5 (2.4) | 1 (0.5) | - | - | 3 (1.4) | 20 (9.6) |
| Coxsackievirus B2 | 8 (3.8) | 1 (0.5) | 1 (0.5) | - | - | - | 10 (4.8) |
| Coxsackievirus B5 | - | - | - | 1 (0.5) | - | 1 (0.5) | 2 (1.0) |
| Echovirus E5 | - | - | - | 1 (0.5) | - | - | 1 (0.5) |
| Echovirus E6 | 7 (3.3) | 4 (1.9) | - | - | - | - | 11 (5.3) |
| Echovirus E7 | - | 1 (0.5) | - | 1 (0.5) | - | - | 2 (1.0) |
| Echovirus E9 | 2 (1.0) | 1 (0.5) | - | - | - | 6 (2.9) | 9 (4.3) |
| Echovirus E11 | 2 (1.0) | - | - | - | - | 1 (0.5) | 3 (1.4) |
| Echovirus E25 | - | - | 2 (1.0) | - | - | 2 (1.0) | 4 (1.9) |
| Echovirus E30 | - | 1 (0.5) | - | - | - | - | 1 (0.5) |
| Enterovirus B | 30 (14.4) | 13 (6.2) | 4 (1.9) | 3 (1.4) | - | 13 (6.2) | 63 (30.1) |
| Coxsackievirus A1 | - | - | - | - | - | 1 (0.5) | 1 (0.5) |
| Coxsackievirus A22 | - | - | - | 1 (0.5) | - | - | 1 (0.5) |
| Enterovirus C | - | - | - | 1 (0.5) | - | 1 (0.5) | 2 (1.0) |
| Total, n (%) | 39 (18.1) | 88 (43.0) | 35 (15.8) | 5 (3.0) | 9 (3.4) | 33 (16.6) | 209 (100) |
| Species/Types | 0–2 Years | 3–6 Years | 7–17 Years | Adults | Total, n (%) |
|---|---|---|---|---|---|
| Coxsackievirus A2 | 1 (0.5) | - | - | - | 1 (0.5) |
| Coxsackievirus A3 | - | 1 (0.5) | - | - | 1 (0.5) |
| Coxsackievirus A4 | 1 (0.5) | 3 (1.4) | - | - | 4 (1.9) |
| Coxsackievirus A5 | 1 (0.5) | - | - | - | 1 (0.5) |
| Coxsackievirus A6 | 38 (18.2) | 32 (15.3) | 14 (6.7) | 1 (0.5) | 85 (40.7) |
| Coxsackievirus A8 | 3 (1.4) | 1 (0.5) | 2 (1) | - | 6 (2.9) |
| Coxsackievirus A10 | 7 (3.3) | 5 (2.4) | 3 (1.4) | 1 (0.5) | 16 (7.7) |
| Coxsackievirus A16 | 7 (3.3) | 14 (6.7) | 1 (0.5) | - | 22 (10.5) |
| Enterovirus A71 | 4 (1.9) | 2 (1) | 2 (1) | - | 8 (3.8) |
| Enterovirus A | 62 (29.7) | 58 (27.8) | 22 (10.5) | 2 (1) | 144 (68.9) |
| Coxsackievirus A9 | 4 (1.9) | 11 (5.3) | 5 (2.4) | - | 20 (9.6) |
| Coxsackievirus B2 | 3 (1.4) | 4 (1.9) | 1 (0.5) | 2 (1) | 10 (4.8) |
| Coxsackievirus B5 | 1 (0.5) | - | - | 1 (0.5) | 2 (1) |
| Echovirus E5 | 1 (0.5) | - | - | - | 1 (0.5) |
| Echovirus E6 | 2 (1) | 3 (1.4) | 5 (2.4) | 1 (0.5) | 11 (5.3) |
| Echovirus E7 | 1 (0.5) | 1 (0.5) | - | - | 2 (1) |
| Echovirus E9 | - | 1 (0.5) | 7 (3.3) | 1 (0.5) | 9 (4.3) |
| Echovirus E11 | - | 1 (0.5) | 2 (1) | - | 3 (1.4) |
| Echovirus E25 | - | 3 (1.4) | - | 1 (0.5) | 4 (1.9) |
| Echovirus E30 | - | - | 1 (0.5) | - | 1 (0.5) |
| Enterovirus B | 12 (5.7) | 24 (11.5) | 21 (10) | 6 (2.9) | 63 (30.1) |
| Coxsackievirus A1 | 1 (0.5) | - | - | - | 1 (0.5) |
| Coxsackievirus A22 | - | 1 (0.5) | - | - | 1 (0.5) |
| Enterovirus C | 1 (0.5) | 1 (0.5) | - | - | 2 (1) |
| Total, n (%) | 75 (36.2) | 83 (39.2) | 43 (20.0) | 8 (4.5) | 209 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itani, T.M.; Chalapa, V.I.; Slautin, V.N.; Bykov, R.O.; Imangaliev, B.S.; Starikova, P.K.; Sergeev, A.G.; Semenov, A.V. Non-Polio Enterovirus Surveillance in the Ural Federal District and Western Siberia, 2022: Is There a Need for a Vaccine? Vaccines 2023, 11, 1588. https://doi.org/10.3390/vaccines11101588
Itani TM, Chalapa VI, Slautin VN, Bykov RO, Imangaliev BS, Starikova PK, Sergeev AG, Semenov AV. Non-Polio Enterovirus Surveillance in the Ural Federal District and Western Siberia, 2022: Is There a Need for a Vaccine? Vaccines. 2023; 11(10):1588. https://doi.org/10.3390/vaccines11101588
Chicago/Turabian StyleItani, Tarek M., Vladislav I. Chalapa, Vasilii N. Slautin, Roman O. Bykov, Bolat S. Imangaliev, Polina K. Starikova, Aleksandr G. Sergeev, and Aleksandr V. Semenov. 2023. "Non-Polio Enterovirus Surveillance in the Ural Federal District and Western Siberia, 2022: Is There a Need for a Vaccine?" Vaccines 11, no. 10: 1588. https://doi.org/10.3390/vaccines11101588




