Vaccine Effectiveness of Cell-Based Quadrivalent Influenza Vaccine in Children: A Narrative Review
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Identification of Relevant Publications
2.3. Evaluation of Outcomes
3. Results
3.1. QIVc in Pediatric Populations
3.2. Laboratory-Confirmed or Any Influenza
3.2.1. VE
3.2.2. rVE
3.3. Influenza-Related Medical Encounters
3.4. Influenza-Related Hospitalizations/ER, and Other Hospitalizations
3.5. Cost-Effectiveness of QIVc in Pediatric Populations
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Disease Burden of Flu. 2022. Available online: https://www.cdc.gov/flu/about/burden/index.html (accessed on 5 June 2023).
- Centers for Disease Control and Prevention (CDC). Summary: ‘Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2022–2023’. 2023. Available online: https://www.cdc.gov/flu/professionals/acip/summary/summary-recommendations.htm (accessed on 19 June 2023).
- Office of Disease Prevention and Health Promotion. Healthy People 2030: Increase the Proportion of People Who Get the Flu Vaccine Every Year—IID-09. Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-people-who-get-flu-vaccine-every-year-iid-09 (accessed on 17 August 2023).
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- World Health Organization. Recommendations Announced for Influenza Vaccine Composition for the 2022–2023 Northern Hemisphere Influenza Season. 2022. Available online: https://www.who.int/news/item/25-02-2022-recommendations-announced-for-influenza-vaccine-composition-for-the-2022-2023-northern-hemisphere-influenza-season (accessed on 19 June 2023).
- Zanobini, P.; Bonaccorsi, G.; Lorini, C.; Haag, M.; McGovern, I.; Paget, J.; Caini, S. Global patterns of seasonal influenza activity, duration of activity and virus (sub)type circulation from 2010 to 2020. Influenza Other Respir. Viruses 2022, 16, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Martin, E.T.; Cheng, C.; Petrie, J.G.; Alyanak, E.; Gaglani, M.; Middleton, D.B.; Ghamande, S.; Silveira, F.P.; Murthy, K.; Zimmerman, R.K.; et al. Low Influenza Vaccine Effectiveness Against A(H3N2)-Associated Hospitalizations in 2016-2017 and 2017-2018 of the Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J. Infect. Dis. 2021, 223, 2062–2071. [Google Scholar] [CrossRef]
- Bellino, S.; Bella, A.; Puzelli, S.; Di Martino, A.; Facchini, M.; Punzo, O.; Pezzotti, P.; Castrucci, M.R.; The InfluNet Study Group. Moderate influenza vaccine effectiveness against A(H1N1)pdm09 virus, and low effectiveness against A(H3N2) subtype, 2018/19 season in Italy. Expert Rev. Vaccines 2019, 18, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Zost, S.J.; Thompson, A.J.; Oyen, D.; Nycholat, C.M.; McBride, R.; Paulson, J.C.; Hensley, S.E.; Wilson, I.A. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017, 13, e1006682. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Diaz Perez, S.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Bridges, C.B.; Cox, N.J.; Fukuda, K. Influenza-associated hospitalizations in the United States. JAMA 2004, 292, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Welch, V.L.; Moran, M.M.; Cane, A.; Lopez, S.M.C.; Srivastava, A.; Enstone, A.L.; Sears, A.; Markus, K.J.; Heuser, M.; et al. High Clinical Burden of Influenza Disease in Adults Aged ≥ 65 Years: Can We Do Better? A Systematic Literature Review. Adv. Ther. 2023, 40, 1601–1627. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, Y.; Zhao, X.; Wei, H.; Tan, M.; Li, X.; Zhu, W.; Huang, W.; Chen, W.; Liu, J.; et al. Mutations associated with egg adaptation of influenza A(H1N1)pdm09 virus in laboratory based surveillance in China, 2009–2016. Biosaf. Health 2019, 1, 41–45. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Cell-Based Flu Vaccines. 2022. Available online: https://www.cdc.gov/flu/prevent/cell-based.htm (accessed on 19 June 2023).
- Rajaram, S.; Boikos, C.; Gelone, D.K.; Gandhi, A. Influenza vaccines: The potential benefits of cell-culture isolation and manufacturing. Ther. Adv. Vaccines Immunother. 2020, 8, 2515135520908121. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Flucelvax Quadrivalent—Package Insert. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/flucelvax-quadrivalent (accessed on 17 September 2023).
- Hartvickson, R.; Cruz, M.; Ervin, J.; Brandon, D.; Forleo-Neto, E.; Dagnew, A.F.; Chandra, R.; Lindert, K.; Mateen, A.A. Non-inferiority of mammalian cell-derived quadrivalent subunit influenza virus vaccines compared to trivalent subunit influenza virus vaccines in healthy children: A phase III randomized, multicenter, double-blind clinical trial. Int. J. Infect. Dis. 2015, 41, 65–72. [Google Scholar] [CrossRef]
- Bart, S.; Cannon, K.; Herrington, D.; Mills, R.; Forleo-Neto, E.; Lindert, K.; Abdul Mateen, A. Immunogenicity and safety of a cell culture-based quadrivalent influenza vaccine in adults: A Phase III, double-blind, multicenter, randomized, non-inferiority study. Hum. Vaccin. Immunother. 2016, 12, 2278–2288. [Google Scholar] [CrossRef]
- Nolan, T.; Fortanier, A.C.; Leav, B.; Põder, A.; Bravo, L.C.; Szymański, H.T.; Heeringa, M.; Vermeulen, W.; Matassa, V.; Smolenov, I.; et al. Efficacy of a Cell-Culture-Derived Quadrivalent Influenza Vaccine in Children. N. Engl. J. Med. 2021, 385, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.S.; Naleway, A.L.; Flannery, B.; Levine, M.Z.; Murthy, K.; Sambhara, S.; Gangappa, S.; Edwards, L.; Ball, S.; Grant, L.; et al. Comparison of the Immunogenicity of Cell Culture-Based and Recombinant Quadrivalent Influenza Vaccines to Conventional Egg-Based Quadrivalent Influenza Vaccines Among Healthcare Personnel Aged 18–64 Years: A Randomized Open-Label Trial. Clin. Infect. Dis. 2021, 73, 1973–1981. [Google Scholar] [CrossRef]
- Essink, B.J.; Heeringa, M.; Jeanfreau, R.J.; Finn, D.; Matassa, V.; Edelman, J.; Hohenboken, M.; Molrine, D. Safety and Immunogenicity of Cell-Based Quadrivalent Influenza Vaccine: A Randomized Trial. Pediatrics 2022, 150, e2022057509. [Google Scholar] [CrossRef]
- Moehling, K.K.; Zimmerman, R.K.; Nowalk, M.P.; Jeng Lin, C.; Martin, J.M.; Alcorn, J.F.; Susick, M.; Burroughs, A.; Holiday, C.; Flannery, B.; et al. A randomized controlled trial of antibody response to 2018-19 cell-based vs. egg-based quadrivalent inactivated influenza vaccine in children. Vaccine 2020, 38, 5171–5177. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). People at Higher Risk of Flu Complications. 2021. Available online: https://www.cdc.gov/flu/highrisk/index.htm (accessed on 16 June 2022).
- Piedra, P.A.; Gaglani, M.J.; Kozinetz, C.A.; Herschler, G.; Riggs, M.; Griffith, M.; Fewlass, C.; Watts, M.; Hessel, C.; Cordova, J.; et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine 2005, 23, 1540–1548. [Google Scholar] [CrossRef]
- Reichert, T.A.; Sugaya, N.; Fedson, D.S.; Glezen, W.P.; Simonsen, L.; Tashiro, M. The Japanese Experience with Vaccinating Schoolchildren against Influenza. N. Engl. J. Med. 2001, 344, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Cobey, S.; Hensley, S.E. Immune history and influenza virus susceptibility. Curr. Opin. Virol. 2017, 22, 105–111. [Google Scholar] [CrossRef]
- Zhang, A.; Stacey, H.D.; Mullarkey, C.E.; Miller, M.S. Original Antigenic Sin: How First Exposure Shapes Lifelong Anti–Influenza Virus Immune Responses. J. Immunol. 2019, 202, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gross, F.L.; Jefferson, S.N.; Holiday, C.; Bai, Y.; Wang, L.; Zhou, B.; Levine, M.Z. Age-specific effects of vaccine egg adaptation and immune priming on A(H3N2) antibody responses following influenza vaccination. J. Clin. Investig. 2021, 131, e146138. [Google Scholar] [CrossRef] [PubMed]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Sylvester, G.C.; Mansi, J.A. Relative Effectiveness of the Cell-derived Inactivated Quadrivalent Influenza Vaccine Versus Egg-derived Inactivated Quadrivalent Influenza Vaccines in Preventing Influenza-related Medical Encounters During the 2018–2019 Influenza Season in the United States. Clin. Infect. Dis. 2021, 73, e692–e698. [Google Scholar] [PubMed]
- Boikos, C.; Imran, M.; Nguyen, V.H.; Ducruet, T.; Sylvester, G.C.; Mansi, J.A. Effectiveness of the Cell-Derived Inactivated Quadrivalent Influenza Vaccine in Individuals at High Risk of Influenza Complications in the 2018–2019 United States Influenza Season. Open Forum. Infect. Dis. 2021, 8, ofab167. [Google Scholar] [CrossRef]
- Boikos, C.; McGovern, I.; Molrine, D.; Ortiz, J.R.; Puig-Barberà, J.; Haag, M. Review of Analyses Estimating Relative Vaccine Effectiveness of Cell-Based Quadrivalent Influenza Vaccine in Three Consecutive US Influenza Seasons. Vaccines 2022, 10, 896. [Google Scholar] [CrossRef] [PubMed]
- Boikos, C.; Sylvester, G.C.; Sampalis, J.S.; Mansi, J.A. Relative Effectiveness of the Cell-Cultured Quadrivalent Influenza Vaccine Compared to Standard, Egg-derived Quadrivalent Influenza Vaccines in Preventing Influenza-like Illness in 2017–2018. Clin. Infect. Dis. 2020, 71, e665–e671. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Luo, Y.; Ackerson, B.; Tanenbaum, H.C.; Sy, L.S.; Gandhi, A.; Tseng, H.F. Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017–2018. Vaccine 2019, 37, 5807–5811. [Google Scholar] [CrossRef]
- DeMarcus, L.; Shoubaki, L.; Federinko, S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine 2019, 37, 4015–4021. [Google Scholar] [CrossRef]
- Divino, V.; Krishnarajah, G.; Pelton, S.I.; Mould-Quevedo, J.; Anupindi, V.R.; DeKoven, M.; Postma, M.J. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017-18 influenza season. Vaccine 2020, 38, 6334–6343. [Google Scholar] [CrossRef]
- Divino, V.; Ruthwik Anupindi, V.; DeKoven, M.; Mould-Quevedo, J.; Pelton, S.I.; Postma, M.J.; Levin, M.J. A Real-World Clinical and Economic Analysis of Cell-Derived Quadrivalent Influenza Vaccine Compared to Standard Egg-Derived Quadrivalent Influenza Vaccines During the 2019–2020 Influenza Season in the United States. Open Forum. Infect. Dis. 2022, 9, ofab604. [Google Scholar] [CrossRef]
- Hanson, K.E.I.J.; Meece, J.K.; Ambrose, K.; Slyvester, G.; McLean, H.Q. Effectiveness of Cell Culture-Based Inactivated Influenza Vaccine against Medically-Attended, Laboratory-Confirmed Influenza in Northcentral Wisconsin 2018–2019. In Proceedings of the Annual Conference on Vaccinology Research, Virutal, 18–19 June 2020. [Google Scholar]
- Imran, M.; Ortiz, J.R.; McLean, H.Q.; Fisher, L.; O’Brien, D.; Bonafede, M.; Mansi, J.A.; Boikos, C. Relative Effectiveness of Cell-based Versus Egg-based Quadrivalent Influenza Vaccines in Children and Adolescents in the United States During the 2019–2020 Influenza Season. Pediatr. Infect. Dis. J. 2022, 41, 769–774. [Google Scholar] [CrossRef]
- Klein, N.P.; Fireman, B.; Goddard, K.; Zerbo, O.; Asher, J.; Zhou, J.; King, J.; Lewis, N. Vaccine effectiveness of cell-culture relative to egg-based inactivated influenza vaccine during the 2017-18 influenza season. PLoS ONE 2020, 15, e0229279. [Google Scholar] [CrossRef]
- Krishnarajah, G.; Divino, V.; Postma, M.J.; Pelton, S.I.; Anupindi, V.R.; DeKoven, M.; Mould-Quevedo, J. Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018–2019 Influenza Season in the United States. Vaccines 2021, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.Q.; Petrie, J.G.; Hanson, K.E.; Meece, J.K.; Rolfes, M.A.; Sylvester, G.C.; Neumann, G.; Kawaoka, Y.; Belongia, E.A. Interim Estimates of 2022-23 Seasonal Influenza Vaccine Effectiveness—Wisconsin, October 2022–February 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.N.; Mills, C.; McGovern, I.; Dean, A.; Bogdanov, A.; Sullivan, S.; Haag, M. Superior Effectiveness of Cell-Based Versus Egg-Based Quadrivalent Influenza Vaccines Against Outpatient Test-Confirmed Influenza Over Three Consecutive Seasons in the United States (V130_67RWE). In Proceedings of the 9th ESWI Influenza Conference, Valencia, Spain, 17–20 September 2023. [Google Scholar]
- Stuurman, A.L.; Biccler, J.; Carmona, A.; Descamps, A.; Díez-Domingo, J.; Muñoz Quiles, C.; Nohynek, H.; Rizzo, C.; Riera-Montes, M. Brand-specific influenza vaccine effectiveness estimates during 2019/20 season in Europe—Results from the DRIVE EU study platform. Vaccine 2021, 39, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Urueña, A.; Micone, P.; Magneres, M.C.; McGovern, I.; Mould-Quevedo, J.; Sarmento, T.T.R.; Giglio, N. Cost-Effectiveness Analysis of Cell Versus Egg-Based Seasonal Influenza Vaccination in Children and Adults in Argentina. Vaccines 2022, 10, 1627. [Google Scholar] [CrossRef]
- Chi, C.-Y.; Cheng, M.-F.; Ko, K.; Mould-Quevedo, J.; Chen, C.-J.; Huang, Y.-C.; Lee, P.-I. Cost-effectiveness analysis of cell-based versus egg-based seasonal influenza vaccination in the pediatric population in Taiwan. In Proceedings of the ESPID, Lisbon, Portugal, 8–12 May 2023. [Google Scholar]
- Rizzo, C.; Trentini, F.; Capri, S.; Merler, S. Economic value of the introduction of a new quadrivalent cell culture derived influenza vaccine (Flucelvax(R) tetra) in Italian healthcare [in Italian]. Ital. J. Public Health 2019, 8, 113–143. [Google Scholar]
- Mould-Quevedo, J.; Nguyen, V.H. The Value of the Influenza Cell-Based Vaccine in the Pediatric Population. A Dynamic Transmission Modelling Approach in the U.S. In Proceedings of the ID Week 2023, Boston, MA, USA, 11–15 October 2023. [Google Scholar]
- Ballalai, I.; Toniolo, J.; Kfouri, R.; Vespa, G.; Magneres, C.; Mould-Quevedo, J.; Pires, B.; Angerami, R. Cost-effectiveness of cell-based quadrivalent versus egg-based trivalent influenza vaccination in the Brazilian National Immunization Program. J. Bras. Econ. Saude 2021, 13, 136–144. [Google Scholar]
- Cai, R.; Gerlier, L.; Eichner, M.; Schwehm, M.; Rajaram, S.; Mould-Quevedo, J.; Lamotte, M. Cost-effectiveness of the cell-based quadrivalent versus the standard egg-based quadrivalent influenza vaccine in Germany. J. Med. Econ. 2021, 24, 490–501. [Google Scholar] [CrossRef]
- Ruiz-Aragón, J.; Gani, R.; Márquez, S.; Alvarez, P. Estimated cost-effectiveness and burden of disease associated with quadrivalent cell-based and egg-based influenza vaccines in Spain. Hum. Vaccin. Immunother. 2020, 16, 2238–2244. [Google Scholar] [CrossRef]
- Eick-Cost, A.; Hu, Z. Relative Effectiveness of Cell-Based Influenza Vaccines Compared with Egg-Based Influenza Vaccines, Active Component, U.S. Service Members, 2017–2018 Season. In Proceedings of the ICEID, Atlanta, GA, USA, 26–29 August 2018. [Google Scholar]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Wernecke, M.; MaCurdy, T.; et al. Relative Effectiveness of Influenza Vaccines Among the United States Elderly, 2018–2019. J. Infect. Dis. 2020, 222, 278–287. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Lu, M.; Kelman, J.; Lu, Y.; Lindaas, A.; Loc, J.; Pratt, D.; Wei, Y.; Chillarige, Y.; Wernecke, M.; et al. Comparative Effectiveness of Influenza Vaccines Among US Medicare Beneficiaries Ages 65 Years and Older During the 2019–2020 Season. Clin. Infect. Dis. 2021, 73, e4251–e4259. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Chu, S.; Wernecke, M.; et al. Relative Effectiveness of Cell-Cultured and Egg-Based Influenza Vaccines Among Elderly Persons in the United States, 2017–2018. J. Infect. Dis. 2019, 220, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Suphaphiphat, P.; van Boxmeer, J.; Haag, M.; Leav, B.; Iheanacho, I.; Kistler, K.; Ortiz de Lejarazu, R. Retrospective Assessment of the Antigenic Similarity of Egg-Propagated and Cell Culture-Propagated Reference Influenza Viruses as Compared with Circulating Viruses across Influenza Seasons 2002–2003 to 2017–2018. Int. J. Environ. Res. Public Health 2020, 17, 5423. [Google Scholar] [CrossRef] [PubMed]
- Worby, C.J.; Chaves, S.S.; Wallinga, J.; Lipsitch, M.; Finelli, L.; Goldstein, E. On the relative role of different age groups in influenza epidemics. Epidemics 2015, 13, 10–16. [Google Scholar] [CrossRef] [PubMed]
| Study | Season | Age Group | Type of Study | Location | Outcomes | N Total Study Population | n Pediatric Population (<18 Years) * | rVE Study Population | VE Study Population | rVE Pediatric Population | VE Pediatric Population |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boikos et al., 2020 [33] | 2017–2018 | ≥4 years | Retrospective cohort | U.S. | Influenza-like illness | QIVc: 92,187 QIVe: 1,261,675 | QIVc: 7465 QIVe: 404,510 | 36.2% (26.1–44.1%) | – | – | – |
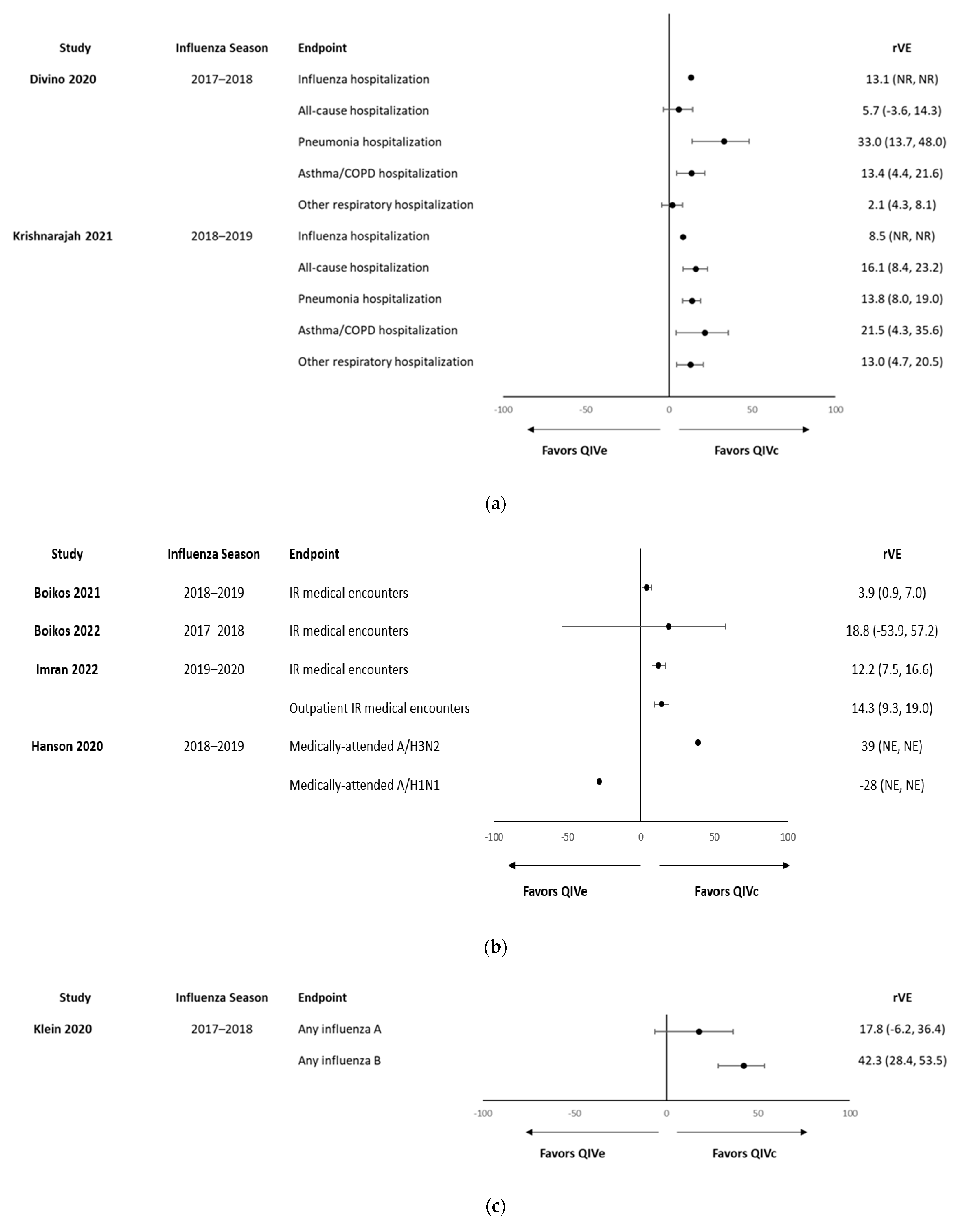
| Boikos et al., 2021 [30] | 2018–2019 | ≥4 years | Retrospective cohort | U.S. | Influenza-related medical encounters | QIVc: 2,125,430 QIVe: 8,000,903 | QIVc: 78,602 QIVe: 1,628,038 | 7.6% (95% CI, 6.5–8.6) | – | 4–17 years, 3.9% (95% CI, 0.9–7.0) | – |
| Boikos et al., 2021 [31] | 2018–2019 | ≥4 years with underlying medical conditions | Retrospective cohort | U.S. | Influenza-related medical encounters | QIVc: 471,301 QIVe: 1,641,915 | n/a | 13.4% (95% CI, 11.4–15.4%) | – | – | – |
| Boikos et al., 2022 [32] | 2017–2018, 2018–2019, and 2019–2020 | ≥4 years | 3 × retrospective cohort studies | U.S. | Influenza-related medical encounters | 2017–2018: QIVc: 92,187; QIVe: 1,261,675 2018–2019: QIVc: 2,125,430; QIVe: 8,000,903 2019–2020: QIVc: 1,499,215; QIVe: 4,126,263 | 2017–2018: QIVc: 7465; QIVe: 404,510 2018–2019: QIVc: 78,602; QIVe: 1,628,038 2019–2020: QIVc: 60,480; QIVe: 1,240,990 | 2017–2018: 19.3% (9.5% to 28.0%) 2018–2019: 7.6% (6.5% to 8.6%) 2019–2020: 17.2% (15.8% to 18.6%) | – | 4–17 years 2017–2018: 18.8% (−53.9% to 57.2%) 2018–2019: 3.9% (0.9% to 7.0%) 2019–2020: 12.2% (7.5% to 16.6%) | – |
| Bruxvoort et al., 2019 [34] | 2017–2018 | ≥4 years | Test-negative case–control | U.S. | Hospitalization | 1186 cases 6946 controls | Hospitalized cases: QIVc: 8 QIVe: 229 | rVE < 65 years: 6% (−46 to 39%) rVE ≥ 65 years 43% (−45 to 77%) | – | – | – |
| De Marcus et al., 2019 [35] | 2017–2018 | ≥6 months | Test-negative case–control | U.S. | Laboratory-confirmed influenza infections | 1757 cases 2280 controls | QIVc: 187 QIVe: 703 Unvaccinated: 1383 | – | QIVc: 46% (33% to 56%) QIVe: 53% (45% to 60%) | – | QIVc: 36% (12% to 54%) VE QIVe: 55% (45% to 64%) |
| Divino et al., 2020 [36] | 2017–2018 | ≥4 years | Retrospective cohort | U.S. | Influenza-related hospitalizations/ER visits, all-cause hospitalizations, and serious respiratory hospitalizations/ER visits | QIVc: 555,538 QIVe: 2,528,524 | QIVc: ~15,000 QIVe: ~70,000 | Influenza-related hospitalizations/ER visits: 14.4% All-cause hospitalizations: 11.8% Pneumonia hospitalization: 4.2% Asthma/COPD/bronchial hospitalizations: 8.3% Other respiratory hospitalizations: 6.9% | – | 4–17 years Influenza-related hospitalizations/ER visits: 13.1% All-cause hospitalizations: 5.7% Pneumonia hospitalizations: 33.0% Asthma/COPD/bronchial hospitalizations: 13.4% Other respiratory hospitalizations: 2.1% | – |
| Divino et al., 2022 [37] | 2019–2020 | 4–64 years | Retrospective claims-based | U.S. | Influenza-related hospitalizations, any respiratory hospitalizations, pneumonia hospitalizations, and ER visits | QIVc: 1,150,134 QIVe: 3,924,819 | QIVc: ~28,300 QIVe: ~92,200 | Influenza-related hospitalizations: 5.3% Any respiratory hospitalizations: 8.2% Pneumonia hospitalizations: 6.7% Asthma/COPD/bronchial hospitalizations: 7.6% | – | – | – |
| Imran et al., 2022 [39] | 2019–2020 | 4–17 years | Retrospective cohort | U.S. | Influenza-related medical encounters | QIVc: 60,480 QIVe: 1,240,990 | n/a | Any influenza-related medical encounters: 12.2% (7.5–16.6%) Outpatient encounters: 14.3% (9.3–19.0) | – | 4–17 years Any influenza-related medical encounters: 12.2% (7.5–16.6%) Outpatient encounters: 14.3% (9.3–19.0) | – |
| Klein et al., 2020 [40] | 2017–2018 | 4–64 years | Retrospective cohort | U.S. | Any influenza | QIVc: 84,420 TIVe/QIVe: 932,525 | QIVc: 43,735 TIVe/QIVe: 40,685 | Influenza A: 8.0% (95% CI: –10, 23) Influenza B: 39.6% (CI: 27.9, 49.3) | – | 4–18 years Influenza A: 17.8% (−6.2% to 36.4%) Influenza B: 42.3% (28.4% to 53.5%) | – |
| Krishnarajah et al., 2021 [41] | 2018–2019 | 4–64 years | Retrospective claims-based | U.S. | Hospitalizations/ER visits related to influenza; all-cause hospitalizations; and hospitalizations/ER related to any respiratory condition | QIVc: 669,030 QIVe: 3,062,797 | QIVc: ~169,200QIVe: 741,200 | Influenza-related hospital/ER visits: 6.5% (0.1–12.5%), All-cause hospitalizations: 7.9% (6.6–9.1%) Hospitalizations/ER visits related to any respiratory event: 7.7% (6.1–9.4%) | – | 4–17 years: Influenza-related hospital/ER visits: 8.5% All-cause hospitalizations: 16.1% Hospitalizations/ER visits related to any respiratory event: 13.8% Pneumonia hospitalizations/ER visits: 21.5% Asthma/COPD/bronchial: 13.0% | – |
| McLean et al., 2023 [42] | 2022–2023 | 6 months–64 years | Test negative case–control and community cohort study | U.S. | Influenza infection | 186 vaccinated, 545 total. 84% received cell-based vaccine | 223 vaccinated. Breakdown QIVc vs. QIVe not stated | – | Influenza A: 54% (23–73%) A/H3N2: 60% (25–79%) | – | 1–17 years: Influenza A: 71% (31–90%) |
| Nolan et al., 2021 [20] | 2017–2018 and 2018–2019 | 2–17 years | RCT | Australia, the Philippines, Thailand, Estonia, Finland, Lithuania, Poland, and Spain | Laboratory-confirmed influenza | QIVc: 2258 Comparator (MenACWY): 2256 | QIVc: 2258 | – | VE: 54.6% (45.7% to 62.1%) A/H1N1: 80.7% (69.2 to 87.9) against influenza A/H1N1 A/H3N2: 42.1% (95% CI, 20.3 to 57.9) B: 47.6% (95% CI, 31.4 to 60.0) | – | 3 to <18 years: 54.0 (44.8–61.7%) 2 to< 4 years: 62.7 (38.1–77.5%) 4 to <18 years: 53.3% (43.4–61.5%) |
| Stuurman et al., 2021 [44] | 2019–2020 | ≥6 months, although varies across studies included | Test-negative and population-based cohort study (13 studies in total) | Finland, France, Italy, Romania, Spain, Austria, U.K., and Italy | Primary care and hospitalized influenza | Not stated | n/a | – | – | – | 6 months to 17 years: 72% (−100% to 96%) |
| Hanson et al., 2020 [38] | 2018–2019 | ≥4 years | n/a | U.S. | Medically attended influenza | QIVc: 760 QIVe: 208 | n/a | A/H3N2: VE difference 51% (95% CI −6%, 125%) A/H1N1pdm09: VE difference: −7% (95% CI −33%, 22%) | – | 4 to 17 years: H3N2: VE difference 39% (NS) A/H1N1pdm09: VE difference: −28% (NS) | – |
| Stein et al., 2023 [43] | 2017–2018, 2018–2019, and 2019–2020 | 4–64 years | Test-negative case–control claims-based study | U.S. | Outpatient lab-confirmed influenza | QIVc: 31,824 (2017–2018), 33,388 (2018–2019), and 34,398 (2019–2020) QIVe: 28,709 (2017–2018), 29,962 (2018–2019), and 30,508 (2019–2020) | n/a | 2017–2018: 14.8% (7.0% to 22.0%) 2018–2019: 12.5% (4.7% to 19.6%) 2019–2020: 10.0% (2.7% to 16.7%) | – | – | – |
| Study | Age Group | Location | Study Model Type | ICER/QALY | Cost-Effectiveness Threshold | Conclusions |
|---|---|---|---|---|---|---|
| Rizzo et al., 2019 [47] | ≥6 months | Italy | Dynamic age-structured SEIR model combined with static decision-tree model | Payer: EUR 8021 Societal: EUR 1428 By age group: 6 months to 8 years: cost-saving 9 to 17 years: cost-saving | EUR 30,000/QALY | Cost-effective overall and cost-saving in children |
| Ruiz-Aragon et al., 2020 [51] | 9 to 64 years | Spain | Static decision-tree model | Payer: EUR 12,852 Societal: cost-saving | EUR 22,000–EUR 25,000/QALY | Cost-effective |
| Ballalai et al., 2021 [49] | ≥6 months | Brazil | Static decision-tree model | Payer: BRL 17,293 Societal: BRL 16,669 | BRL 103,599/QALY | Cost-effective |
| Cai et al., 2021 [50] | ≥9 years | Germany | Dynamic age-structured SEIR model combined with static decision-tree model | Payer: cost-saving Societal: cost-saving | EUR 30,000/QALY | Cost-saving |
| Urueña et al., 2022 [45] | 6 months to 64 years (2 to 64 years, high-risk only) | Argentina | Static decision-tree model | Payer: USD 12,214 Societal: cost-saving | USD 29,736.84/QALY | Cost-effective |
| Chi et al., 2023 [46] | 6 months to 17 years | Taiwan | Age-structured static model | Payer: USD 68,306 Societal: USD 40,090 | USD 99,177/QALY | Cost-effective |
| Mould-Quevedo et al. [48] | 6 months to 17 years | U.S. | Dynamic age-structured SEIR model | NR | USD 50,000/QALY | Cost-saving |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mould-Quevedo, J.F.; Pelton, S.I.; Nguyen, V.H. Vaccine Effectiveness of Cell-Based Quadrivalent Influenza Vaccine in Children: A Narrative Review. Vaccines 2023, 11, 1594. https://doi.org/10.3390/vaccines11101594
Mould-Quevedo JF, Pelton SI, Nguyen VH. Vaccine Effectiveness of Cell-Based Quadrivalent Influenza Vaccine in Children: A Narrative Review. Vaccines. 2023; 11(10):1594. https://doi.org/10.3390/vaccines11101594
Chicago/Turabian StyleMould-Quevedo, Joaquin F., Stephen I. Pelton, and Van Hung Nguyen. 2023. "Vaccine Effectiveness of Cell-Based Quadrivalent Influenza Vaccine in Children: A Narrative Review" Vaccines 11, no. 10: 1594. https://doi.org/10.3390/vaccines11101594
APA StyleMould-Quevedo, J. F., Pelton, S. I., & Nguyen, V. H. (2023). Vaccine Effectiveness of Cell-Based Quadrivalent Influenza Vaccine in Children: A Narrative Review. Vaccines, 11(10), 1594. https://doi.org/10.3390/vaccines11101594






