Attenuating RNA Viruses with Expanded Genetic Codes to Evoke Adjustable Immune Response in PylRS-
Abstract
1. Introduction
2. Results
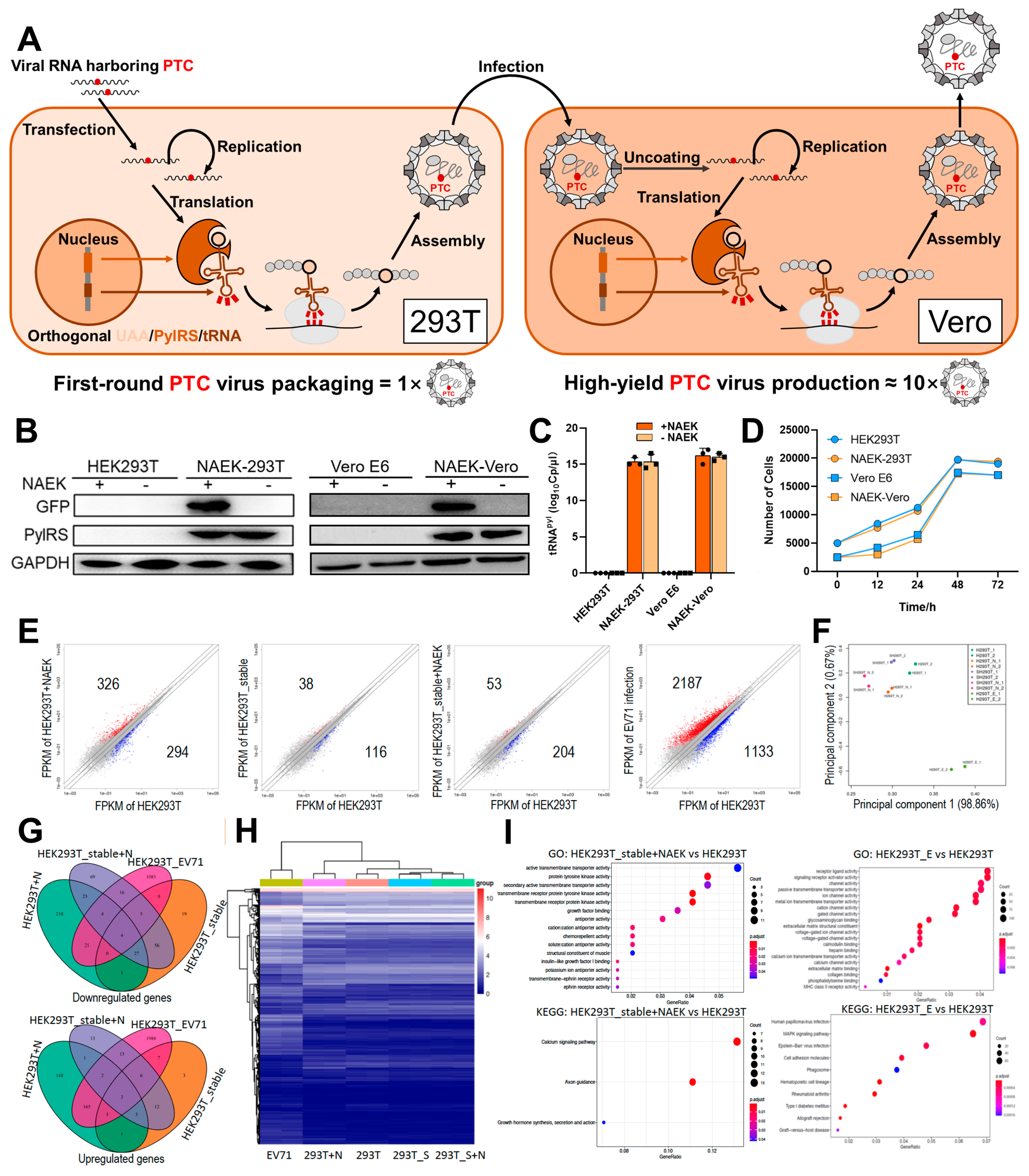
2.1. In Vitro UAA-Controllable RNA Virus-Packaging and -Production System
2.2. Parallel Screening Revealed Rules for NAEK Incorporation
2.3. Prediction and Validation of UAA-Controllable Virus Design
2.4. Efficacy, Safety, and Protective Evaluation of EV71-3D-E105NAEK as a Vaccine Candidate In Vivo
2.5. In Vivo UAA-Controllable RNA Virus Evoked Adjustable Immune Response in PylRS-–Transgenic Mice
3. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baltimore, D. Expression of Animal Virus Genomes. Bacteriol. Rev. 1971, 35, 235–241. [Google Scholar] [CrossRef]
- Koff, W.C.; Burton, D.R.; Johnson, P.R.; Walker, B.D.; King, C.R.; Nabel, G.J.; Ahmed, R.; Bhan, M.K.; Plotkin, S.A. Accelerating Next-Generation Vaccine Development for Global Disease Prevention. Science 2013, 340, 1232910. [Google Scholar] [CrossRef] [PubMed]
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 Vaccine Development Landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Ketzer, P.; Kaufmann, J.K.; Engelhardt, S.; Bossow, S.; von Kalle, C.; Hartig, J.S.; Ungerechts, G.; Nettelbeck, D.M. Artificial Riboswitches for Gene Expression and Replication Control of DNA and RNA Viruses. Proc. Natl. Acad. Sci. USA 2014, 111, E554–E562. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.K.; Jacobs, C.L.; Huo, Y.; Yang, J.; Krumm, S.A.; Plemper, R.K.; Tsien, R.Y.; Lin, M.Z. Tunable and Reversible Drug Control of Protein Production via a Self-Excising Degron. Nat. Chem. Biol. 2015, 11, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Takishima, Y.; Miyamoto, S.; Nakatsu, Y.; Someya, K.; Sato, M.; Tani, K.; Takeda, M. Photocontrollable Mononegaviruses. Proc. Natl. Acad. Sci. USA 2019, 116, 11587–11589. [Google Scholar] [CrossRef]
- Heilmann, E.; Kimpel, J.; Hofer, B.; Rössler, A.; Blaas, I.; Egerer, L.; Nolden, T.; Urbiola, C.; Kräusslich, H.G.; Wollmann, G.; et al. Chemogenetic ON and OFF Switches for RNA Virus Replication. Nat. Commun. 2021, 12, 1362. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological Considerations for COVID-19 Vaccine Strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Excler, J.-L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine Development for Emerging Infectious Diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef]
- Thi Nhu Thao, T.; Labroussaa, F.; Ebert, N.; V’kovski, P.; Stalder, H.; Portmann, J.; Kelly, J.; Steiner, S.; Holwerda, M.; Kratzel, A.; et al. Rapid Reconstruction of SARS-CoV-2 Using a Synthetic Genomics Platform. Nature 2020, 582, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Muruato, A.; Lokugamage, K.G.; Narayanan, K.; Zhang, X.; Zou, J.; Liu, J.; Schindewolf, C.; Bopp, N.E.; Aguilar, P.V.; et al. An Infectious CDNA Clone of SARS-CoV-2. Cell Host Microbe 2020, 27, 841–848.e3. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems Vaccinology of the BNT162b2 MRNA Vaccine in Humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D.; Wang, Z.; Finkin, S.; Gaebler, C.; Ramos, V.; Cipolla, M.; Mendoza, P.; Agudelo, M.; et al. Anti-SARS-CoV-2 Receptor Binding Domain Antibody Evolution after MRNA Vaccination. Nature 2021, 600, 517–522. [Google Scholar] [CrossRef]
- Chin, J.W.; Cropp, T.A.; Anderson, J.C.; Mukherji, M.; Zhang, Z.; Schultz, P.G. An Expanded Eukaryotic Genetic Code. Science 2003, 301, 964–967. [Google Scholar] [CrossRef]
- Wang, L.; Brock, A.; Herberich, B.; Schultz, P.G. Expanding the Genetic Code of Escherichia Coli. Science 2001, 292, 498–500. [Google Scholar] [CrossRef]
- Wang, N.; Li, Y.; Niu, W.; Sun, M.; Cerny, R.; Li, Q.; Guo, J. Construction of a Live-Attenuated HIV-1 Vaccine through Genetic Code Expansion. Angew. Chem. Int. Ed. 2014, 53, 4867–4871. [Google Scholar] [CrossRef]
- Si, L.; Xu, H.; Zhou, X.; Zhang, Z.; Tian, Z.; Wang, Y.; Wu, Y.; Zhang, B.; Niu, Z.; Zhang, C.; et al. Generation of Influenza A Viruses as Live but Replication-Incompetent Virus Vaccines. Science 2016, 354, 1170–1173. [Google Scholar] [CrossRef]
- Shi, N.; Yang, Q.; Zhang, H.; Lu, J.; Lin, H.; Yang, X.; Abulimiti, A.; Cheng, J.; Wang, Y.; Tong, L.; et al. Restoration of Dystrophin Expression in Mice by Suppressing a Nonsense Mutation through the Incorporation of Unnatural Amino Acids. Nat. Biomed. Eng. 2021, 6, 195–206. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, Y.; Wang, N.; Yuan, Z.; Niu, W.; Li, Q.; Guo, J. Controlling the Replication of a Genomically Recoded HIV-1 with a Functional Quadruplet Codon in Mammalian Cells. ACS Synth. Biol. 2018, 7, 1612–1617. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Liu, J.; Bailey, A.L.; Plante, K.S.; Plante, J.A.; Zou, J.; Xia, H.; Bopp, N.E.; Aguilar, P.V.; et al. A Trans-Complementation System for SARS-CoV-2 Recapitulates Authentic Viral Replication without Virulence. Cell 2021, 184, 2229–2238.e13. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Lax, I.; Luna, J.M.; Thao, T.T.N.; Le Pen, J.; Yu, Y.; Hoffmann, H.-H.; Schneider, W.M.; Razooky, B.S.; Fernandez-Martinez, J.; Schmidt, F.; et al. Replication and Single-Cycle Delivery of SARS-CoV-2 Replicons. Science 2021, 374, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.H.; Wong, S.C.; Lewthwaite, P.; Cardosa, M.J.; Solomon, T. Clinical Features, Diagnosis, and Management of Enterovirus 71. Lancet Neurol. 2010, 9, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, Epidemiology, Pathogenesis, and Control of Enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Hao, R.; Ma, K.; Ru, Y.; Li, D.; Song, G.; Lu, B.; Liu, H.; Li, Y.; Zhang, J.; Wu, C.; et al. Amber Codon Is Genetically Unstable in Generation of Premature Termination Codon (PTC)-Harbouring Foot-and-Mouth Disease Virus (FMDV) via Genetic Code Expansion. RNA Biol. 2021, 18, 2330–2341. [Google Scholar] [CrossRef]
- Roehl, H.H.; Parsley, T.B.; Ho, T.V.; Semler, B.L. Processing of a Cellular Polypeptide by 3CD Proteinase Is Required for Poliovirus Ribonucleoprotein Complex Formation. J. Virol. 1997, 71, 578–585. [Google Scholar] [CrossRef]
- Brown, W.; Liu, J.; Deiters, A. Genetic Code Expansion in Animals. ACS Chem. Biol. 2018, 13, 2375–2386. [Google Scholar] [CrossRef]
- Chin, J.W. Expanding and Reprogramming the Genetic Code of Cells and Animals. Annu. Rev. Biochem. 2014, 83, 379–408. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Lu, W.; Tian, M.; Thauvin, M.; Yuan, C.; Volovitch, M.; Wang, Q.; Holst, J.; Liu, M.; et al. Heritable Expansion of the Genetic Code in Mouse and Zebrafish. Cell Res. 2017, 27, 294–297. [Google Scholar] [CrossRef]
- ElSohly, A.M.; MacDonald, J.I.; Hentzen, N.B.; Aanei, I.L.; El Muslemany, K.M.; Francis, M.B. Ortho-Methoxyphenols as Convenient Oxidative Bioconjugation Reagents with Application to Site-Selective Heterobifunctional Cross-Linkers. J. Am. Chem. Soc. 2017, 139, 3767–3773. [Google Scholar] [CrossRef] [PubMed]
- Behrens, C.R.; Hooker, J.M.; Obermeyer, A.C.; Romanini, D.W.; Katz, E.M.; Francis, M.B. Rapid Chemoselective Bioconjugation through Oxidative Coupling of Anilines and Aminophenols. J. Am. Chem. Soc. 2011, 133, 16398–16401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Erickson, S.B.; Mukherjee, R.; Kelemen, R.E.; Wrobel, C.J.J.; Cao, X.; Chatterjee, A. Precise Photoremovable Perturbation of a Virus–Host Interaction. Angew. Chem. Int. Ed. 2017, 56, 4234–4237. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, F.; Wu, Y.; Si, L.; Xu, H.; Zhang, C.; Xia, Q.; Xiao, S.; Wang, Q.; He, Q.; et al. Broadening the Versatility of Lentiviral Vectors as a Tool in Nucleic Acid Research via Genetic Code Expansion. Nucleic Acids Res. 2015, 43, e73. [Google Scholar] [CrossRef]
- Sakin, V.; Hanne, J.; Dunder, J.; Anders-Össwein, M.; Laketa, V.; Nikić, I.; Kräusslich, H.-G.; Lemke, E.A.; Müller, B. A Versatile Tool for Live-Cell Imaging and Super-Resolution Nanoscopy Studies of HIV-1 Env Distribution and Mobility. Cell Chem. Biol. 2017, 24, 635–645.e5. [Google Scholar] [CrossRef]
- Nikić, I.; Plass, T.; Schraidt, O.; Szymański, J.; Briggs, J.A.G.; Schultz, C.; Lemke, E.A. Minimal Tags for Rapid Dual-Color Live-Cell Labeling and Super-Resolution Microscopy. Angew. Chem. Int. Ed. 2014, 53, 2245–2249. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, P.; Cao, M.; Yu, T.; Lane, S.T.; Zhao, H. Directed Evolution: Methodologies and Applications. Chem. Rev. 2021, 121, 12384–12444. [Google Scholar] [CrossRef]
- Kiesslich, S.; Kamen, A.A. Vero Cell Upstream Bioprocess Development for the Production of Viral Vectors and Vaccines. Biotechnol. Adv. 2020, 44, 107608. [Google Scholar] [CrossRef]
- Young, D.D.; Schultz, P.G. Playing with the Molecules of Life. ACS Chem. Biol. 2018, 13, 854–870. [Google Scholar] [CrossRef]
- Kelemen, R.E.; Mukherjee, R.; Cao, X.; Erickson, S.B.; Zheng, Y.; Chatterjee, A. A Precise Chemical Strategy To Alter the Receptor Specificity of the Adeno-Associated Virus. Angew. Chem. Int. Ed. 2016, 55, 10645–10649. [Google Scholar] [CrossRef]
- Yao, T.; Zhou, X.; Zhang, C.; Yu, X.; Tian, Z.; Zhang, L.; Zhou, D. Site-Specific PEGylated Adeno-Associated Viruses with Increased Serum Stability and Reduced Immunogenicity. Molecules 2017, 22, 1155. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. MRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Lo, N.C.; Arinaminpathy, N.; Frost, I.; Laxminarayan, R. Childhood Vaccines and Antibiotic Use in Low- and Middle-Income Countries. Nature 2020, 581, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II Study of COVID-19 RNA Vaccine BNT162b1 in Adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Biron-Shental, T.; Makov-Assif, M.; Key, C.; Kohane, I.S.; Hernán, M.A.; Lipsitch, M.; Hernandez-Diaz, S.; Reis, B.Y.; et al. Effectiveness of the BNT162b2 MRNA COVID-19 Vaccine in Pregnancy. Nat. Med. 2021, 27, 1693–1695. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, A.; Guo, T.; Sokolovska, A.; Miller, P.F.; Collins, J.J.; Lu, T.K.; Lora, J.M. Engineering Living Therapeutics with Synthetic Biology. Nat. Rev. Drug Discov. 2021, 20, 941–960. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Wu, X.; Wang, Y.; Yang, X.; Chen, H.; Shen, Y.; Yang, Y.; Xia, Q.
Attenuating RNA Viruses with Expanded Genetic Codes to Evoke Adjustable Immune Response in PylRS-
Zheng Z, Wu X, Wang Y, Yang X, Chen H, Shen Y, Yang Y, Xia Q.
Attenuating RNA Viruses with Expanded Genetic Codes to Evoke Adjustable Immune Response in PylRS-
Zheng, Zhetao, Xuesheng Wu, Yu Wang, Xu Yang, Hongmin Chen, Yuxuan Shen, Yuelin Yang, and Qing Xia.
2023. "Attenuating RNA Viruses with Expanded Genetic Codes to Evoke Adjustable Immune Response in PylRS-
Zheng, Z., Wu, X., Wang, Y., Yang, X., Chen, H., Shen, Y., Yang, Y., & Xia, Q.
(2023). Attenuating RNA Viruses with Expanded Genetic Codes to Evoke Adjustable Immune Response in PylRS-





