Immunogenicity of Co-Administered Omicron BA.4/BA.5 Bivalent COVID-19 and Quadrivalent Seasonal Influenza Vaccines in Israel during the 2022–2023 Winter Season
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Hemagglutination Inhibition and Microneutralization Assays
2.3. Statistical Analyses
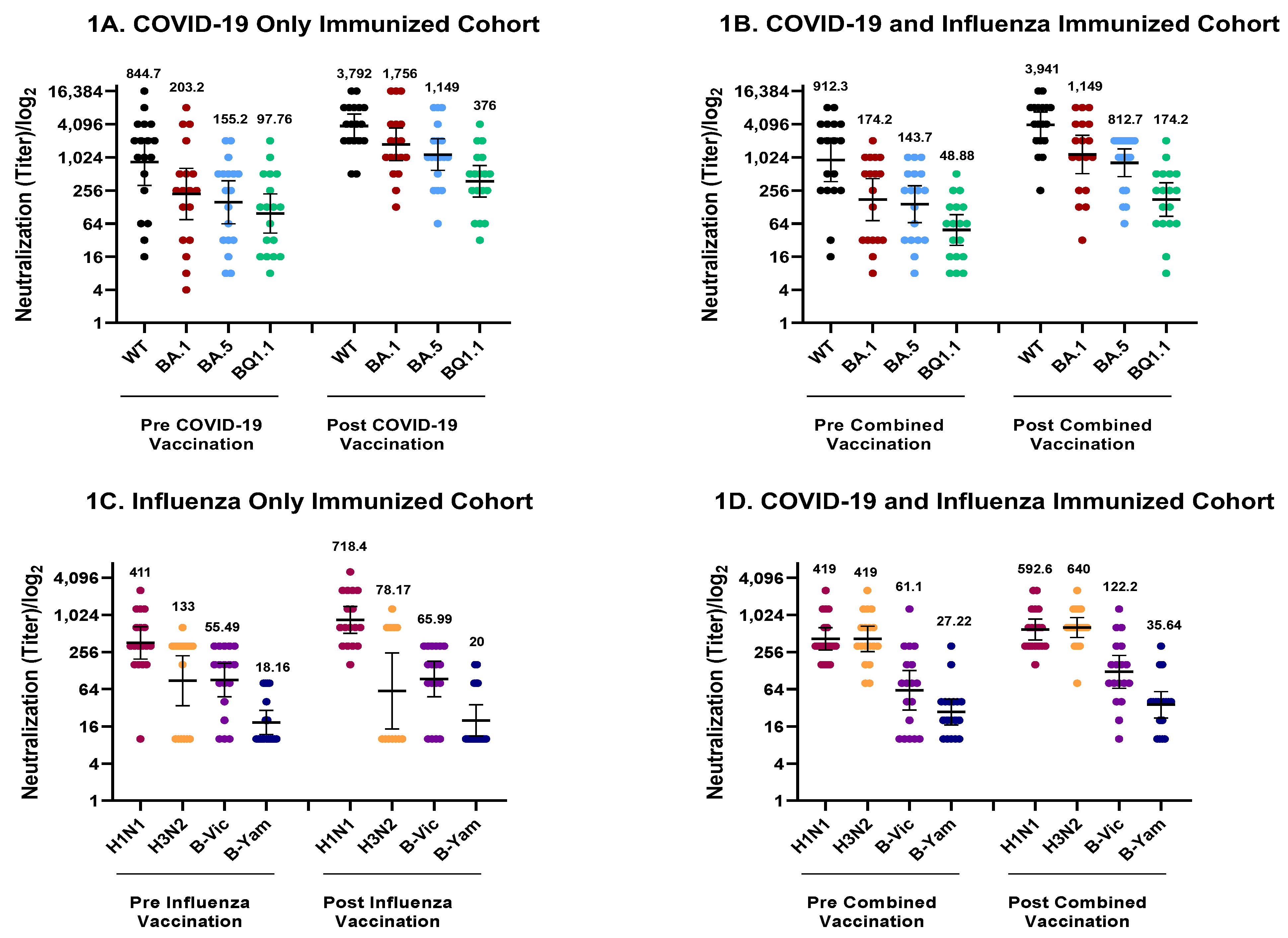
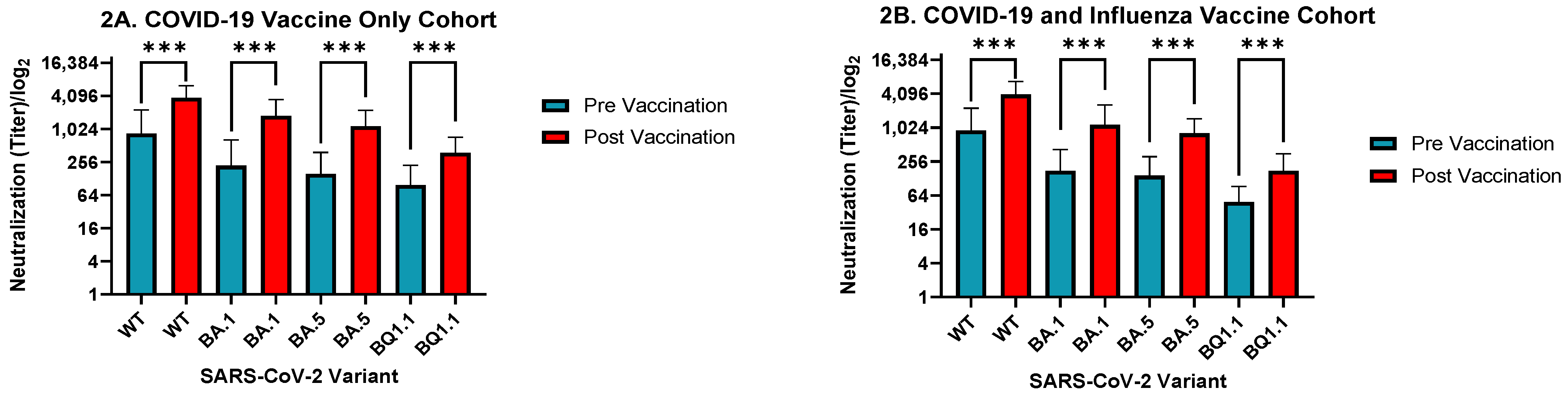
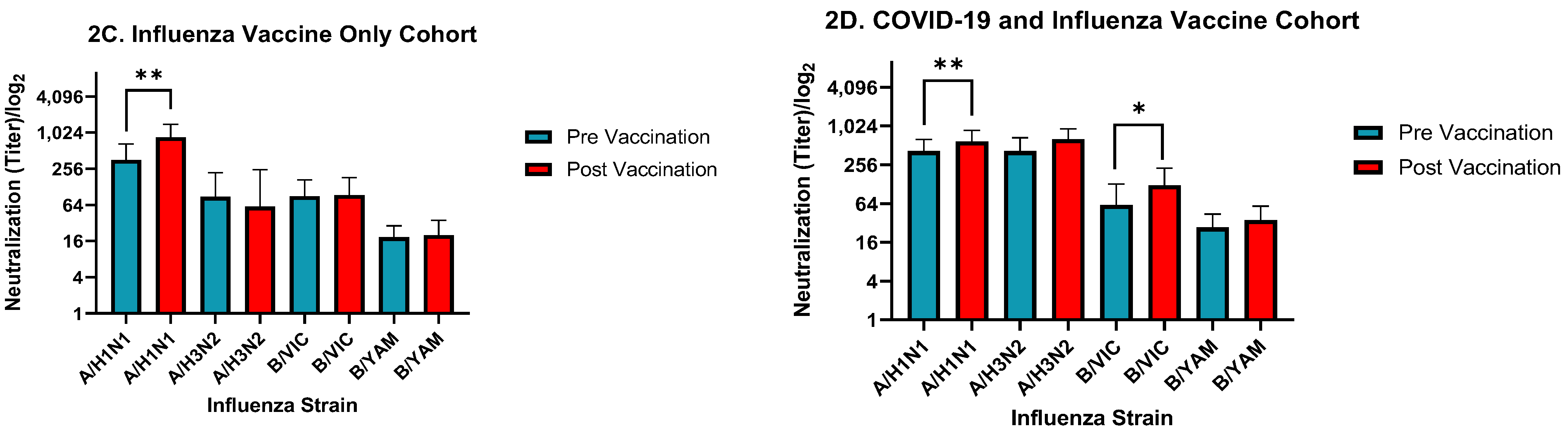
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers. 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Piroth, L.; Cottenet, J.; Mariet, A.-S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021, 9, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Pierse, N.; Huang, Q.S.; Prasad, N.; Duque, J.; Newbern, E.C.; Baker, M.G.; Turner, N.; McArthur, C. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine 2018, 36, 5916–5925. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Calabrò, G.E.; Pappalardo, C.; D’Ambrosio, F.; Vece, M.; Lupi, C.; Lontano, A.; Di Russo, M.; Ricciardi, R.; de Waure, C. The Impact of Vaccination on COVID-19 Burden of Disease in the Adult and Elderly Population: A Systematic Review of Italian Evidence. Vaccines 2023, 11, 1011. [Google Scholar] [CrossRef]
- Grewal, R.; Nguyen, L.; Buchan, S.A.; Wilson, S.E.; Nasreen, S.; Austin, P.C.; Brown, K.A.; Fell, D.B.; Gubbay, J.B.; Schwartz, K.L.; et al. Effectiveness of mRNA COVID-19 vaccine booster doses against Omicron severe outcomes. Nat. Commun. 2023, 14, 1273. [Google Scholar] [CrossRef]
- Tan, C.Y.; Chiew, C.J.; Pang, D.; Lee, V.J.; Ong, B.; Wang, L.F.; Ren, E.C.; Lye, D.C.; Tan, K.B. Effectiveness of bivalent mRNA vaccines against medically attended symptomatic SARS-CoV-2 infection and COVID-19-related hospital admission among SARS-CoV-2-naive and previously infected individuals: A retrospective cohort study. Lancet Infect. Dis. 2023. [Google Scholar] [CrossRef]
- Lazarus, R.; Baos, S.; Cappel-Porter, H.; Carson-Stevens, A.; Clout, M.; Culliford, L.; Emmett, S.R.; Garstang, J.; Gbadamoshi, L.; Hallis, B.; et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): A multicentre, randomised, controlled, phase 4 trial. Lancet 2021, 398, 2277–2287. [Google Scholar] [CrossRef]
- Toback, S.; Galiza, E.; Cosgrove, C.; Galloway, J.; Goodman, A.L.; Swift, P.A.; Rajaram, S.; Graves-Jones, A.; Edelman, J.; Burns, F.; et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: An exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2022, 10, 167–179. [Google Scholar] [CrossRef]
- Izikson, R.; Brune, D.; Bolduc, J.S.; Bourron, P.; Fournier, M.; Moore, T.M.; Pandey, A.; Perez, L.; Sater, N.; Shrestha, A.; et al. Safety and immunogenicity of a high-dose quadrivalent influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine in adults aged ≥65 years: A phase 2, randomised, open-label study. Lancet Respir. Med. 2022, 10, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Radner, H.; Sieghart, D.; Jorda, A.; Fedrizzi, C.; Hasenöhrl, T.; Zdravkovic, A.; Redlberger-Fritz, M.; Puchammer-Stoeckl, E.; Anderle, K.; Bergmann, F.; et al. Reduced immunogenicity of BNT162b2 booster vaccination in combination with a tetravalent influenza vaccination: Results of a prospective cohort study in 838 health workers. Clin. Microbiol. Infect. 2023, 29, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Wagenhäuser, I.; Reusch, J.; Gabel, A.; Höhn, A.; Lâm, T.-T.; Almanzar, G.; Prelog, M.; Krone, L.B.; Frey, A.; Schubert-Unkmeir, A.; et al. Immunogenicity and safety of coadministration of COVID-19 and influenza vaccination. Eur. Respir. J. 2023, 61, 2201390. [Google Scholar] [CrossRef]
- Dulfer, E.A.; Geckin, B.; Taks, E.J.; GeurtsvanKessel, C.H.; Dijkstra, H.; van Emst, L.; van der Gaast–de, C.E.; van Mourik, D.; Koopmans, P.C.; Domínguez-Andrés, J.; et al. Timing and sequence of vaccination against COVID-19 and influenza (TACTIC): A single-blind, placebo-controlled randomized clinical trial. Lancet Reg. Health-Eur. 2023, 29, 100628. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Barda, N.; Asraf, K.; Joseph, G.; Weiss-Ottolenghi, Y.; Doolman, R.; Kreiss, Y.; Lustig, Y.; Regev-Yochay, G. Immunogenicity and Reactogenicity of Coadministration of COVID-19 and Influenza Vaccines. JAMA Netw. Open 2023, 6, e2332813. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, L.; Quan, K.; Baber, J.A.; Ho, A.W.; Zhang, Y.; Xu, X.; Lu, C.; Cooper, D.; Koury, K.; Lockhart, S.P.; et al. Safety and Immunogenicity of the BNT162b2 Vaccine Coadministered with Seasonal Inactivated Influenza Vaccine in Adults. Infect. Dis. Ther. 2023, 12, 2241–2258. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Weil, M.; Shohat, T.; Bromberg, M.; Bassal, R.; Dichtiar, R.; Mandelboim, M.; Sofer, D.; Cohen, D.; Mendelson, E. The dynamics of infection and the persistence of immunity to A(H1N1)pdm09 virus in Israel. Influenza Other Respir. Viruses 2013, 7, 838–846. [Google Scholar] [CrossRef]
- Allen, J.D.; Ross, T.M. H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation. Hum. Vaccines Immunother. 2018, 14, 1840–1847. [Google Scholar] [CrossRef]
- Kliker, L.; Zuckerman, N.; Atari, N.; Barda, N.; Gilboa, M.; Nemet, I.; Elkader, B.A.; Fratty, I.S.; Jaber, H.; Mendelson, E.; et al. COVID-19 vaccination and BA.1 breakthrough infection induce neutralising antibodies which are less efficient against BA.4 and BA.5 Omicron variants, Israel, March to June 2022. Eurosurveillance 2022, 27, 2200559. [Google Scholar] [CrossRef]
- Tzenios, N.; Tazanios, M.E.; Chahine, M. Combining Influenza and COVID-19 Booster Vaccination Strategy to improve vaccination uptake necessary for managing the health pandemic: A Systematic Review and Meta-Analysis. Vaccines 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, X.; Zhang, L.; Wan, S.; Zhang, L.; Zhou, F. SARS-CoV-2 Omicron variant: Recent progress and future perspectives. Signal Transduct. Target. Therapy 2022, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Rössler, A.; Riepler, L.; Bante, D.; von Laer, D.; Kimpel, J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N. Engl. J. Med. 2022, 386, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.B.; Foster, C.; Rawlinson, W.; Tedla, N.; Bull, R.A. Evolution of the SARS-CoV-2 omicron variants BA. 1 to BA. 5: Implications for immune escape and transmission. Rev. Med. Virol. 2022, 32, e2381. [Google Scholar] [CrossRef]
- Wen, S.; Wu, Z.; Zhong, S.; Li, M.; Shu, Y. Factors influencing the immunogenicity of influenza vaccines. Hum. Vaccines Immunother. 2021, 17, 2706–2718. [Google Scholar] [CrossRef]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef]
| All | Received Influenza Vaccine Only | Received COVID-19 Vaccine Only | Received Both Vaccines | p-Value | ||
|---|---|---|---|---|---|---|
| n = 54 | n = 18 | n = 18 | n = 18 | |||
| Age (years) | 65.20 (9.74) | 64.22 (9.81) | 65.94 (9.31) | 65.44 (10.55) | 0.8661 | |
| Gender (female) | 79.63% | 77.78% | 83.33% | 77.78% | 0.8921 | |
| Immunosuppression status | None | 90.74% | 100% | 77.78% | 94.44% | 0.0570 |
| Unknown | 9.26% | 0 | 22.22% | 5.56% | ||
| Days between vaccine and pre-sample (Influenza) | 5.28 (7.72) | 6.83 (7.98) | NA | 3.72 (7.34) | 0.2320 | |
| Days between vaccine and pre-sample (COVID-19) | 3.86 (6.72) | NA | 4.00 (6.35) | 3.72 (7.34) | 0.2800 | |
| Days between vaccine and post-sample (Influenza) | 36.39 (5.21) | 38.00 (5.14) | NA | 34.78 (4.88) | 0.0623 | |
| Days between vaccine and post-sample (COVID-19) | 35.94 (4.71) | NA | 37.11 (4.36) | 34.78 (4.88) | 0.1401 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moss, S.; Jurkowicz, M.; Nemet, I.; Atari, N.; Kliker, L.; Abd-Elkader, B.; Gonen, T.; Martin, E.T.; Lustig, Y.; Regev-Yochay, G.; et al. Immunogenicity of Co-Administered Omicron BA.4/BA.5 Bivalent COVID-19 and Quadrivalent Seasonal Influenza Vaccines in Israel during the 2022–2023 Winter Season. Vaccines 2023, 11, 1624. https://doi.org/10.3390/vaccines11101624
Moss S, Jurkowicz M, Nemet I, Atari N, Kliker L, Abd-Elkader B, Gonen T, Martin ET, Lustig Y, Regev-Yochay G, et al. Immunogenicity of Co-Administered Omicron BA.4/BA.5 Bivalent COVID-19 and Quadrivalent Seasonal Influenza Vaccines in Israel during the 2022–2023 Winter Season. Vaccines. 2023; 11(10):1624. https://doi.org/10.3390/vaccines11101624
Chicago/Turabian StyleMoss, Stephen, Menucha Jurkowicz, Ital Nemet, Nofar Atari, Limor Kliker, Bayan Abd-Elkader, Tal Gonen, Emily Toth Martin, Yaniv Lustig, Gili Regev-Yochay, and et al. 2023. "Immunogenicity of Co-Administered Omicron BA.4/BA.5 Bivalent COVID-19 and Quadrivalent Seasonal Influenza Vaccines in Israel during the 2022–2023 Winter Season" Vaccines 11, no. 10: 1624. https://doi.org/10.3390/vaccines11101624
APA StyleMoss, S., Jurkowicz, M., Nemet, I., Atari, N., Kliker, L., Abd-Elkader, B., Gonen, T., Martin, E. T., Lustig, Y., Regev-Yochay, G., & Mandelboim, M. (2023). Immunogenicity of Co-Administered Omicron BA.4/BA.5 Bivalent COVID-19 and Quadrivalent Seasonal Influenza Vaccines in Israel during the 2022–2023 Winter Season. Vaccines, 11(10), 1624. https://doi.org/10.3390/vaccines11101624






