Ocular Inflammation Post-Vaccination
Abstract
:1. Introduction
2. Methods of Literature Search
3. Results
3.1. General Analysis Regarding Cases of Uveitis Post-Vaccination
3.2. Detailed Analysis by Vaccine
3.2.1. Human Papillomavirus (HPV) Vaccine
3.2.2. Hepatitis B Virus (HBV) Vaccine
3.2.3. Influenza Virus Vaccine
3.2.4. Measles–Mumps–Rubella (MMR) Vaccine
3.2.5. Varicella Zoster Virus (VZV) Vaccine
3.2.6. Yellow Fever Vaccine/Hepatitis A Virus (HAV) Vaccine/Co-Administration
3.3. Different Types of Uveitis Induced by Antiviral Vaccines
3.3.1. Acute Posterior Multifocal Placoid Pigment Epitheliopathy (AMPEE) and Multiple Evanescent White Dot Syndrome (MEWDS)
3.3.2. Vogt–Koyanagi–Harada (VKH)
3.3.3. Acute Retinal Necrosis (ARN)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maldonado, Y.A. Current Controversies in Vaccination: Vaccine Safety. JAMA 2002, 288, 3155–3158. [Google Scholar] [CrossRef] [PubMed]
- Vaccines and Immunization. Available online: https://www.who.int/health-topics/vaccines-and-immunization (accessed on 28 July 2023).
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D. Live Attenuated Vaccines: Historical Successes and Current Challenges. Virology 2015, 479–480, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Pérez, O.; Romeu, B.; Cabrera, O.; González, E.; Batista-Duharte, A.; Labrada, A.; Pérez, R.; Reyes, L.M.; Ramírez, W.; Sifontes, S.; et al. Adjuvants Are Key Factors for the Development of Future Vaccines: Lessons from the Finlay Adjuvant Platform. Front. Immunol. 2013, 4, 407. [Google Scholar] [CrossRef]
- Smith, J.; Lipsitch, M.; Almond, J.W. Vaccine Production, Distribution, Access, and Uptake. Lancet 2011, 378, 428–438. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Agarwal, M.; Majumder, P.D.; Babu, K.; Konana, V.K.; Goyal, M.; Touhami, S.; Stanescu-Segall, D.; Bodaghi, B. Drug-Induced Uveitis: A Review. Indian J. Ophthalmol. 2020, 68, 1799–1807. [Google Scholar] [CrossRef]
- Kuniyoshi, K.; Hatsukawa, Y.; Kimura, S.; Fujino, T.; Ohguro, H.; Nakai, R.; Sunami, K.; Mishima, S.; Sato, T.; Kusaka, S.; et al. Acute Bilateral Photoreceptor Degeneration in an Infant After Vaccination Against Measles and Rubella. JAMA Ophthalmol. 2017, 135, 478–482. [Google Scholar] [CrossRef]
- Standardization of Uveitis Nomenclature (SUN) Working Group. Classification Criteria for Multiple Evanescent White Dot Syndrome. Am. J. Ophthalmol. 2021, 228, 198–204. [Google Scholar] [CrossRef]
- Ronday, M.J.; Stilma, J.S.; Barbe, R.F.; Kijlstra, A.; Rothova, A. Blindness from Uveitis in a Hospital Population in Sierra Leone. Br. J. Ophthalmol. 1994, 78, 690–693. [Google Scholar] [CrossRef]
- Gritz, D.C.; Wong, I.G. Incidence and Prevalence of Uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 2004, 111, 491–500; discussion 500. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Shimizu, V.; Zhang, S.-Y.; Abel, L.; Tardieu, M.; Rozenberg, F.; Jouanguy, E.; Casanova, J.-L. Genetic Susceptibility to Herpes Simplex Virus 1 Encephalitis in Mice and Humans. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 495. [Google Scholar] [CrossRef] [PubMed]
- Kinchington, P.; Leger, A.; Guedon, J.; Hendricks, R. Herpes Simplex Virus and Varicella Zoster Virus, the House Guests Who Never Leave. Herpesviridae 2012, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Crough, T.; Khanna, R. Immunobiology of Human Cytomegalovirus: From Bench to Bedside. Clin. Microbiol. Rev. 2009, 22, 76–98. [Google Scholar] [CrossRef] [PubMed]
- Chee, S.-P.; Bacsal, K.; Jap, A.; Se-Thoe, S.-Y.; Cheng, C.L.; Tan, B.H. Clinical Features of Cytomegalovirus Anterior Uveitis in Immunocompetent Patients. Am. J. Ophthalmol. 2008, 145, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Miserocchi, E.; Fogliato, G.; Bianchi, I.; Bandello, F.; Modorati, G. Clinical Features of Ocular Herpetic Infection in an Italian Referral Center. Cornea 2014, 33, 565–570. [Google Scholar] [CrossRef]
- Pleyer, U.; Chee, S.-P. Current Aspects on the Management of Viral Uveitis in Immunocompetent Individuals. Clin. Ophthalmol. 2015, 9, 1017–1028. [Google Scholar] [CrossRef]
- De Groot-Mijnes, J.D.F.; de Visser, L.; Rothova, A.; Schuller, M.; van Loon, A.M.; Weersink, A.J.L. Rubella Virus Is Associated with Fuchs Heterochromic Iridocyclitis. Am. J. Ophthalmol. 2006, 141, 212–214.e1. [Google Scholar] [CrossRef]
- Kamoi, K.; Mochizuki, M. HTLV-1 Uveitis. Front. Microbiol. 2012, 3, 270. [Google Scholar] [CrossRef]
- Kong, K.; Ding, X.; Ni, Y. Resolution of Harada Disease-like Uveitis after Quadrivalent Human Papillomavirus Vaccination: A Case Report. Hum. Vaccines Immunother. 2022, 18, 1–4. [Google Scholar] [CrossRef]
- Ye, H.; Feng, H.; Zhao, P.; Fei, P. Case Report: Posterior Uveitis after Divalent Human Papillomavirus Vaccination in an Asian Female. Optom. Vis. Sci. 2020, 97, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Dansingani, K.K.; Suzuki, M.; Naysan, J.; Samson, C.M.; Spaide, R.F.; Fisher, Y.L. Panuveitis with Exudative Retinal Detachments After Vaccination Against Human Papilloma Virus. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, L.D.; Kaiser, G.M.; Steinmetz, R.L.; Srivastava, S.K. Acute Retinal Necrosis after Herpes Zoster Vaccination. Arch. Ophthalmol. 2011, 129, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.A.; Levison, A.L.; Stewart, J.M.; Acharya, N.R.; Margolis, T.P. Retinal Necrosis Following Varicella-Zoster Vaccination. Arch. Ophthalmol. 2012, 130, 1355–1356. [Google Scholar] [CrossRef] [PubMed]
- Heath, G.; Depledge, D.P.; Brown, J.R.; Hale, A.D.; Tutil, H.; Williams, R.; Breuer, J. Acute Retinal Necrosis Caused by the Zoster Vaccine Virus. Clin. Infect. Dis. 2017, 65, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.J.; Wingelaar, M.J.; Armbrust, K.R.; Kopplin, L.J. Uveitis Reactivation Following Recombinant Zoster Vaccination. Am. J. Ophthalmol. Case Rep. 2021, 23, 101115. [Google Scholar] [CrossRef]
- Fried, M.; Conen, D.; Conzelmann, M.; Steinemann, E. Uveitis after hepatitis B vaccination. Lancet 1987, 330, 631–632. [Google Scholar] [CrossRef]
- Brézin, A.P.; Massin-Korobelnik, P.; Boudin, M.; Gaudric, A.; LeHoang, P. Acute Posterior Multifocal Placoid Pigment Epitheliopathy after Hepatitis B Vaccine. Arch. Ophthalmol. 1995, 113, 297–300. [Google Scholar] [CrossRef]
- Baglivo, E.; Safran, A.B.; Borruat, F.X. Multiple Evanescent White Dot Syndrome after Hepatitis B Vaccine. Am. J. Ophthalmol. 1996, 122, 431–432. [Google Scholar] [CrossRef]
- Sood, A.B.; O’Keefe, G.; Bui, D.; Jain, N. Vogt-Koyanagi-Harada Disease Associated with Hepatitis B Vaccination. Ocul. Immunol. Inflamm. 2019, 27, 524–527. [Google Scholar] [CrossRef]
- Khalifa, Y.M.; Monahan, P.M.; Acharya, N.R. Ampiginous Choroiditis Following Quadrivalent Human Papilloma Virus Vaccine. Br. J. Ophthalmol. 2010, 94, 137–139. [Google Scholar] [CrossRef]
- Ogino, K.; Kishi, S.; Yoshimura, N. Multiple Evanescent White Dot Syndrome after Human Papillomavirus Vaccination. Case Rep. Ophthalmol. 2014, 5, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chang, Y.-H.; Lee, Y.-C. Panuveitis Following Administration of Quadrivalent Human Papillomavirus Vaccine. Tzu Chi Med. J. 2014, 26, 44–46. [Google Scholar] [CrossRef]
- Sawai, T.; Shimizu, M.; Sakai, T.; Yachie, A. Tubulointerstitial Nephritis and Uveitis Syndrome Associated with Human Papillomavirus Vaccine. J. Pediatr. Ophthalmol. Strabismus 2016, 53, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Hector, R.E. Acute Posterior Multifocal Placoid Pigment Epitheliopathy. Am. J. Ophthalmol. 1978, 86, 424–425. [Google Scholar] [CrossRef]
- Blumberg, S.; Bienfang, D.; Kantrowitz, F.G. A Possible Association Between Influenza Vaccination and Small-Vessel Vasculitis. Arch. Intern. Med. 1980, 140, 847–848. [Google Scholar] [CrossRef]
- Blanche, P.; Decrette, C.; Sicard, D. Development of Uveitis Following Vaccination for Influenza. Clin. Infect. Dis. 1994, 19, 979. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Yilmaz, T.; Foster, C.S. Vogt-Koyanagi-Harada Syndrome Associated with Bilateral Serous Macular Detachments Responsive to Immunomodulatory Therapy. Ophthalmic Surg. Lasers Imaging Retin. 2009, 40, 345–347. [Google Scholar] [CrossRef]
- Wells, M.B.; Garg, S. Bilateral panuveitis after influenza vaccination. Retin. Cases Brief Rep. 2009, 3, 386. [Google Scholar] [CrossRef]
- Mendrinos, E.; Baglivo, E. Acute Posterior Multifocal Placoid Pigment Epitheliopathy Following Influenza Vaccination. Eye 2010, 24, 180–181. [Google Scholar] [CrossRef]
- Tao, Y.; Chang, L.-B.; Zhao, M.; Li, X.-X. Two Cases of Exudative Retina Detachment and Uveitis Following H1N1 Influenza Vaccination. Chin. Med. J. 2011, 124, 3838–3840. [Google Scholar] [PubMed]
- Rothova, A.; de Groot, J.D.F.; Mudrikova, T. Reactivation of Acute Retinal Necrosis after Flu H1N1 Vaccination. Br. J. Ophthalmol. 2011, 95, 291. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Nazarian, S.M.; Thayi, D.R.; Hammond, F.; Petrovic, V. Multiple Evanescent White Dot Syndrome Following Recent Influenza Vaccination. Can. J. Ophthalmol. 2013, 48, e115–e116. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.S.; Evans, S.; Yeo, D.; Al-bermani, A. Retinal Artery Vasculitis Secondary to Administration of Influenza Vaccine. BMJ Case Rep. 2015, 2015, bcr2015211971. [Google Scholar] [CrossRef]
- Gonome, T.; Suzuki, Y.; Metoki, T.; Takahashi, S.; Nakazawa, M. Acute Posterior Multifocal Placoid Pigment Epitheliopathy and Granulomatous Uveitis Following Influenza Vaccination. Am. J. Ophthalmol. Case Rep. 2016, 4, 60–63. [Google Scholar] [CrossRef]
- Branisteanu, D.; Bilha, A. Acute posterior multifocal placoid pigment epitheliopathy following influenza vaccination. Rom. J. Ophthalmol. 2015, 59, 52–58. [Google Scholar]
- Manusow, J.S.; Rai, A.; Yeh, S.; Mandelcorn, E.D. Two Cases of Panuveitis with Orbital Inflammatory Syndrome after Influenza Vaccination. Can. J. Ophthalmol. 2015, 50, e71–e74. [Google Scholar] [CrossRef]
- Kim, M. Vogt-Koyanagi-Harada Syndrome Following Influenza Vaccination. Indian J. Ophthalmol. 2016, 64, 98. [Google Scholar] [CrossRef]
- Abou-Samra, A.; Tarabishy, A.B. Multiple Evanescent White Dot Syndrome Following Intradermal Influenza Vaccination. Ocul. Immunol. Inflamm. 2019, 27, 528–530. [Google Scholar] [CrossRef]
- Murtaza, F.; Pereira, A.; Mandelcorn, M.S.; Kaplan, A.J. Vogt-Koyanagi-Harada Disease Following Influenza Vaccination. Am. J. Ophthalmol. Case Rep. 2022, 26, 101516. [Google Scholar] [CrossRef]
- Islam, S.M.M.; El-Sheikh, H.F.; Tabbara, K.F. Anterior Uveitis Following Combined Vaccination for Measles, Mumps and Rubella (MMR): A Report of Two Cases. Acta Ophthalmol. Scand. 2000, 78, 590–592. [Google Scholar] [CrossRef]
- Sedaghat, M.; Zarei-Ghanavati, S.; Shokoohi, S.; Ghasemi, A. Panuveitis and Dermal Vasculitis Following MMR Vaccination. East. Mediterr. Health J. 2007, 13, 470–474. [Google Scholar]
- Ferrini, W.; Aubert, V.; Balmer, A.; Munier, F.L.; Abouzeid, H. Anterior Uveitis and Cataract after Rubella Vaccination: A Case Report of a 12-Month-Old Girl. Pediatrics 2013, 132, e1035–e1038. [Google Scholar] [CrossRef]
- Esmaeli-Gutstein, B.; Winkelman, J.Z. Uveitis Associated with Varicella Virus Vaccine. Am. J. Ophthalmol. 1999, 127, 733–734. [Google Scholar] [CrossRef]
- Naseri, A.; Good, W.V.; Cunningham, E.T. Herpes Zoster Virus Sclerokeratitis and Anterior Uveitis in a Child Following Varicella Vaccination. Am. J. Ophthalmol. 2003, 135, 415–417. [Google Scholar] [CrossRef]
- Fine, H.F.; Kim, E.; Flynn, T.E.; Gomes, N.L.; Chang, S. Acute Posterior Multifocal Placoid Pigment Epitheliopathy Following Varicella Vaccination. Br. J. Ophthalmol. 2010, 94, 282–283. [Google Scholar] [CrossRef]
- Sham, C.W.; Levinson, R.D. Uveitis Exacerbation After Varicella-Zoster Vaccination in an Adult. Arch. Ophthalmol. 2012, 130, 793–794. [Google Scholar] [CrossRef]
- Weinlander, E.J.; Wang, A.L.; Jaru-Ampornpan, P.; Altaweel, M.M.; Nork, T.M. Two Cases of Acute Retinal Necrosis Due to Varicella Zoster Despite Prior Shingles Vaccination. Retin. Cases Brief Rep. 2019, 13, 241. [Google Scholar] [CrossRef]
- Heydari-Kamjani, M.; Vante, I.; Uppal, P.; Demory Beckler, M.; Kesselman, M.M. Uveitis Sarcoidosis Presumably Initiated After Administration of Shingrix Vaccine. Cureus 2019, 11, e4920. [Google Scholar] [CrossRef]
- Chen, R.I.; Deaner, J.D.; Srivastava, S.K.; Lowder, C.Y. Acute Retinal Necrosis Following Recombinant Subunit Varicella-Zoster Virus Vaccine. Am. J. Ophthalmol. Case Rep. 2020, 20, 100962. [Google Scholar] [CrossRef]
- Menghini, M.; Raja, V.; Raiter, J.; Balaratnasingam, C.; Constable, I.J. Acute retinal necrosis associated with Herpes Zoster vaccination. Retin. Cases Brief Rep. 2021, 15, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, A.L.; Moraes, H.V. de Anterior and Intermediate Uveitis Following Yellow Fever Vaccination with Fractional Dose: Case Reports. Ocul. Immunol. Inflamm. 2019, 27, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Volkov, L.; Grard, G.; Bollaert, P.-E.; Durand, G.A.; Cravoisy, A.; Conrad, M.; Nace, L.; Courte, G.; Marnai, R.; Leparc-Goffart, I.; et al. Viscerotropic Disease and Acute Uveitis Following Yellow Fever Vaccination: A Case Report. BMC Infect. Dis. 2020, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Campos, W.R.; Cenachi, S.P.F.; Soares, M.S.; Gonçalves, P.F.; Vasconcelos-Santos, D.V. Vogt-Koyanagi-Harada-like Disease Following Yellow Fever Vaccination. Ocul. Immunol. Inflamm. 2021, 29, 124–127. [Google Scholar] [CrossRef]
- Pereima, R.R.; Bonatti, R.; Crotti, F.; Furtado, J.M.; Lopes, M.H.; Yamamoto, J.H.; Kreuz, A.C. Ocular Adverse Events Following Yellow Fever Vaccination: A Case Series. Ocul. Immunol. Inflamm. 2022, 30, 1425–1429. [Google Scholar] [CrossRef]
- Fine, L.; Fine, A.; Cunningham, E.T. Multiple Evanescent White Dot Syndrome Following Hepatitis a Vaccination. Arch. Ophthalmol. 2001, 119, 1856–1858. [Google Scholar] [CrossRef]
- Yang, J.; Chen, C.; Hu, Y.; Zeng, R. Multiple Evanescent White Dot Syndrome Following Rabies Vaccination: A Case Report. BMC Ophthalmol. 2018, 18, 312. [Google Scholar] [CrossRef]
- Stangos, A.; Zaninetti, M.; Petropoulos, I.; Baglivo, E.; Pournaras, C. Multiple Evanescent White Dot Syndrome Following Simultaneous Hepatitis-A and Yellow Fever Vaccination. Ocul. Immunol. Inflamm. 2006, 14, 301–304. [Google Scholar] [CrossRef]
- Cohen, S.M. Multiple Evanescent White Dot Syndrome After Vaccination for Human Papilloma Virus and Meningococcus. J. Pediatr. Ophthalmol. Strabismus 2010, 47, 1–3. [Google Scholar] [CrossRef]
- Escott, S.; Tarabishy, A.B.; Davidorf, F.H. Multifocal Choroiditis Following Simultaneous Hepatitis A, Typhoid, and Yellow Fever Vaccination. Clin. Ophthalmol. 2013, 7, 363–365. [Google Scholar] [CrossRef]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human Papillomavirus and Cervical Cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic Human Papillomavirus Infection. Nat. Rev. Dis. Prim. 2016, 2, 16086. [Google Scholar] [CrossRef] [PubMed]
- WHO. Human Papillomavirus Vaccines: WHO Position Paper, December 2022. Available online: https://www.who.int/publications-detail-redirect/who-wer9750-645-672 (accessed on 28 July 2023).
- WHO. Immunization Coverage. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed on 18 October 2023).
- WHO. List of Prequalified Vaccines. Available online: https://extranet.who.int/pqweb/vaccines/list-prequalified-vaccines (accessed on 28 July 2023).
- Slade, B.A.; Leidel, L.; Vellozzi, C.; Woo, E.J.; Hua, W.; Sutherland, A.; Izurieta, H.S.; Ball, R.; Miller, N.; Braun, M.M.; et al. Postlicensure Safety Surveillance for Quadrivalent Human Papillomavirus Recombinant Vaccine. JAMA 2009, 302, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Lv, W.; Zhang, L.; Chen, J.; Chen, H. Association of HLA-DR4/HLA-DRB1*04 with Vogt-Koyanagi-Harada Disease: A Systematic Review and Meta-Analysis. Sci. Rep. 2014, 4, 6887. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Khochtali, S.; Kahloun, R.; Ammous, D.; Jelliti, B.; Ben Yahia, S.; Zaouali, S.; Khairallah, M. Clinical and Multimodal Imaging Characteristics of Acute Vogt–Koyanagi–Harada Disease Unassociated with Clinically Evident Exudative Retinal Detachment. Int. Ophthalmol. 2016, 36, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Mackensen, F.; Billing, H. Tubulointerstitial Nephritis and Uveitis Syndrome. Curr. Opin. Ophthalmol. 2009, 20, 525. [Google Scholar] [CrossRef]
- Edwards, I.R.; Biriell, C. Harmonisation in Pharmacovigilance. Drug Saf. 1994, 10, 93–102. [Google Scholar] [CrossRef]
- Natale, C.; Giannini, T.; Lucchese, A.; Kanduc, D. Computer-Assisted Analysis of Molecular Mimicry between Human Papillomavirus 16 E7 Oncoprotein and Human Protein Sequences. Immunol. Cell Biol. 2000, 78, 580–585. [Google Scholar] [CrossRef]
- Sonneveld, M.J.; Zoutendijk, R.; Janssen, H.L.A. Hepatitis B Surface Antigen Monitoring and Management of Chronic Hepatitis B. J. Viral Hepat. 2011, 18, 449–457. [Google Scholar] [CrossRef]
- Hadziyannis, S.J.; Papatheodoridis, G.V. Hepatitis B e Antigen-Negative Chronic Hepatitis B: Natural History and Treatment. Semin. Liver Dis. 2006, 26, 130–141. [Google Scholar] [CrossRef]
- Ocama, P.; Opio, C.K.; Lee, W.M. Hepatitis B Virus Infection: Current Status. Am. J. Med. 2005, 118, 1413. [Google Scholar] [CrossRef] [PubMed]
- Shepard, C.W.; Simard, E.P.; Finelli, L.; Fiore, A.E.; Bell, B.P. Hepatitis B Virus Infection: Epidemiology and Vaccination. Epidemiol. Rev. 2006, 28, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Benage, M.; Fraunfelder, F.W. Vaccine-Associated Uveitis. Mo. Med. 2016, 113, 48–52. [Google Scholar] [PubMed]
- Grob, P.J.; Martenet, A.C.; Witmer, R. Nonspecific Immune Parameters and Hepatitis B Antigens in Patients with Uveitis. Mod. Probl. Ophthalmol. 1976, 16, 254–258. [Google Scholar] [PubMed]
- Murray, P.I.; Prasad, J.; Rahi, A.H. Status of Hepatitis B Virus in the Aetiology of Uveitis in Great Britain. Br. J. Ophthalmol. 1983, 67, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.; Carmichael, L.; Bistner, S. Corneal Endothelium in Viral Induced Anterior Uveitis. Ultrastructural Changes Following Canine Adenovirus Type 1 Infection. Arch. Ophthalmol. 1975, 93, 219–224. [Google Scholar] [CrossRef]
- London, W.T. Hepatitis B Virus and Antigen-Antibody Complex Diseases. N. Engl. J. Med. 1977, 296, 1528–1529. [Google Scholar] [CrossRef]
- Farthing, C.F.; Howard, R.S.; Thin, R.N. Papillitis and Hepatitis B. Br. Med. J. Clin. Res. Ed. 1986, 292, 1712. [Google Scholar] [CrossRef]
- Yokochi, T.; Fujii, Y.; Nakashima, I.; Asai, J.; Kiuchi, M.; Kojima, K.; Kato, N. A Murine Model of Experimental Autoimmune Lens-Induced Uveitis Using Klebsiella O3 Lipopolysaccharide as a Potent Immunological Adjuvant. Int. J. Exp. Pathol. 1993, 74, 573–582. [Google Scholar]
- Geier, D.A.; Geier, M.R. A Case-Control Study of Serious Autoimmune Adverse Events Following Hepatitis B Immunization. Autoimmunity 2005, 38, 295–301. [Google Scholar] [CrossRef]
- Verstraeten, T.; Descamps, D.; David, M.-P.; Zahaf, T.; Hardt, K.; Izurieta, P.; Dubin, G.; Breuer, T. Analysis of Adverse Events of Potential Autoimmune Aetiology in a Large Integrated Safety Database of AS04 Adjuvanted Vaccines. Vaccine 2008, 26, 6630–6638. [Google Scholar] [CrossRef]
- Altman, A.; Szyper-Kravitz, M.; Shoenfeld, Y. HBV Vaccine and Dermatomyositis: Is There an Association? Rheumatol. Int. 2008, 28, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Mikaeloff, Y.; Caridade, G.; Rossier, M.; Suissa, S.; Tardieu, M. Hepatitis B Vaccination and the Risk of Childhood-Onset Multiple Sclerosis. Arch. Pediatr. Adolesc. Med. 2007, 161, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Mikaeloff, Y.; Caridade, G.; Suissa, S.; Tardieu, M. Hepatitis B Vaccine and the Risk of CNS Inflammatory Demyelination in Childhood. Neurology 2009, 72, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Influenza Vaccine: Recommendation of the Public Health Service Advisory Committee on Immunization Practices. Ann. Intern. Med. 1978, 89, 657–659. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 31 July 2023).
- World Health Organization. Influenza Vaccination Coverage and Effectiveness. Available online: https://www.who.int/europe/news-room/fact-sheets/item/influenza-vaccination-coverage-and-effectiveness (accessed on 18 October 2023).
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–2022 Influenza Season. MMWR Recomm. Rep. 2021, 70, 1–28. [Google Scholar] [CrossRef]
- Thurairajan, G.; Hope-Ross, M.W.; Situnayake, R.D.; Murray, P.I. Polyarthropathy, Orbital Myositis and Posterior Scleritis: An Unusual Adverse Reaction to Influenza Vaccine. Br. J. Rheumatol. 1997, 36, 120–123. [Google Scholar] [CrossRef]
- Solomon, A.; Siganos, C.S.; Frucht-Pery, J. Adverse Ocular Effects Following Influenza Vaccination. Eye 1999, 13, 381–382. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Strauss, B.; De Serres, G.; MacDonald, D.; Marion, S.A.; Naus, M.; Patrick, D.M.; Kendall, P. Oculo-Respiratory Syndrome: A New Influenza Vaccine-Associated Adverse Event? Clin. Infect. Dis. 2003, 36, 705–713. [Google Scholar] [CrossRef]
- Walter, R.; Hartmann, K.; Fleisch, F.; Reinhart, W.H.; Kuhn, M. Reactivation of Herpesvirus Infections after Vaccinations? Lancet 1999, 353, 810. [Google Scholar] [CrossRef]
- Watad, A.; Quaresma, M.; Brown, S.; Cohen Tervaert, J.W.; Rodríguez-Pint, I.; Cervera, R.; Perricone, C.; Shoenfeld, Y. Autoimmune/Inflammatory Syndrome Induced by Adjuvants (Shoenfeld’s Syndrome)—An Update. Lupus 2017, 26, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E.T.; Moorthy, R.S. Vaccine-Associated Posterior Uveitis. Retina 2020, 40, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Rec, W.E. Rubella Vaccines: WHO Position Paper. Wkly Epidemiol. Rec. 2000, 86, 301–316. [Google Scholar]
- Birnbaum, A.D.; Tessler, H.H.; Schultz, K.L.; Farber, M.D.; Gao, W.; Lin, P.; Oh, F.; Goldstein, D.A. Epidemiologic Relationship between Fuchs Heterochromic Iridocyclitis and the United States Rubella Vaccination Program. Am. J. Ophthalmol. 2007, 144, 424–428. [Google Scholar] [CrossRef]
- De Groot-Mijnes, J.D.F.; de Visser, L.; Zuurveen, S.; Martinus, R.A.; Völker, R.; ten Dam-van Loon, N.H.; de Boer, J.H.; Postma, G.; de Groot, R.J.; van Loon, A.M.; et al. Identification of New Pathogens in the Intraocular Fluid of Patients with Uveitis. Am. J. Ophthalmol. 2010, 150, 628–636. [Google Scholar] [CrossRef]
- Quentin, C.D.; Reiber, H. Fuchs Heterochromic Cyclitis: Rubella Virus Antibodies and Genome in Aqueous Humor. Am. J. Ophthalmol. 2004, 138, 46–54. [Google Scholar] [CrossRef]
- Groen-Hakan, F.; van de Laar, S.; van der Eijk-Baltissen, A.A.; van Loon, N.T.D.; de Boer, J.; Rothova, A. Clinical Manifestations, Prognosis, and Vaccination Status of Patients with Rubella Virus–Associated Uveitis. Am. J. Ophthalmol. 2019, 202, 37–46. [Google Scholar] [CrossRef]
- Johnson, R.W.; Wasner, G.; Saddier, P.; Baron, R. Herpes Zoster and Postherpetic Neuralgia: Optimizing Management in the Elderly Patient. Drugs Aging 2008, 25, 991–1006. [Google Scholar] [CrossRef]
- Johnson, R.W.; Rice, A.S.C. Pain Following Herpes Zoster: The Influence of Changing Population Characteristics and Medical Developments. Pain 2007, 128, 3–5. [Google Scholar] [CrossRef]
- Sanford, M.; Keating, G.M. Zoster Vaccine (Zostavax): A Review of Its Use in Preventing Herpes Zoster and Postherpetic Neuralgia in Older Adults. Drugs Aging 2010, 27, 159–176. [Google Scholar] [CrossRef]
- Hesse, E.M. Postlicensure Safety Surveillance of Recombinant Zoster Vaccine (Shingrix)—United States, October 2017–June 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 91–94. [Google Scholar] [CrossRef]
- Bharucha, T.; Ming, D.; Breuer, J. A Critical Appraisal of ‘Shingrix’, a Novel Herpes Zoster Subunit Vaccine (HZ/Su or GSK1437173A) for Varicella Zoster Virus. Hum. Vaccines Immunother. 2017, 13, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Schöberl, F.; Csanadi, E.; Eren, O.; Dieterich, M.; Kümpfel, T. NMOSD Triggered by Yellow Fever Vaccination—An Unusual Clinical Presentation with Segmental Painful Erythema. Mult. Scler. Relat. Disord. 2017, 11, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Hedges, T.R.; Sinclair, S.H.; Gragoudas, E.S. Evidence for Vasculitis in Acute Posterior Multifocal Placoid Pigment Epitheliopathy. Ann. Ophthalmol. 1979, 11, 539–542. [Google Scholar] [PubMed]
- Wong, M.; Campos-Baniak, M.G.; Colleaux, K. Acute Idiopathic Blind Spot Enlargement Syndrome Following Measles, Mumps and Rubella Vaccination. Can. J. Ophthalmol. 2019, 54, e199–e203. [Google Scholar] [CrossRef] [PubMed]
- Abu-Yaghi, N.E.; Hartono, S.P.; Hodge, D.O.; Pulido, J.S.; Bakri, S.J. White Dot Syndromes: A 20-Year Study of Incidence, Clinical Features and Outcomes. Ocul. Immunol. Inflamm. 2011, 19, 426–430. [Google Scholar] [CrossRef]
- Dhaliwal, R.S.; Maguire, A.M.; Flower, R.W.; Arribas, N.P. Acute Posterior Multifocal Placoid Pigment Epitheliopathy. An Indocyanine Green Angiographic Study. Retina 1993, 13, 317–325. [Google Scholar] [CrossRef]
- Jones, N.P. Acute Posterior Multifocal Placoid Pigment Epitheliopathy. Br. J. Ophthalmol. 1995, 79, 384–389. [Google Scholar] [CrossRef]
- Du, L.; Kijlstra, A.; Yang, P. Vogt-Koyanagi-Harada Disease: Novel Insights into Pathophysiology, Diagnosis and Treatment. Prog. Retin. Eye Res. 2016, 52, 84–111. [Google Scholar] [CrossRef]
- Imai, Y.; Sugita, M.; Nakamura, S.; Toriyama, S.; Ohno, S. Cytokine Production and Helper T Cell Subsets in Vogt-Koyanagi-Harada’s Disease. Curr. Eye Res. 2001, 22, 312–318. [Google Scholar] [CrossRef]
- Holland, G.N. Standard Diagnostic Criteria for the Acute Retinal Necrosis Syndrome. Executive Committee of the American Uveitis Society. Am. J. Ophthalmol. 1994, 117, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Hillenkamp, J.; Nölle, B.; Bruns, C.; Rautenberg, P.; Fickenscher, H.; Roider, J. Acute Retinal Necrosis: Clinical Features, Early Vitrectomy, and Outcomes. Ophthalmology 2009, 116, 1971–1975.e2. [Google Scholar] [CrossRef] [PubMed]
- Young, N.J.; Bird, A.C. Bilateral Acute Retinal Necrosis. Br. J. Ophthalmol. 1978, 62, 581–590. [Google Scholar] [CrossRef]
- Palay, D.A.; Sternberg, P.; Davis, J.; Lewis, H.; Holland, G.N.; Mieler, W.F.; Jabs, D.A.; Drews, C. Decrease in the Risk of Bilateral Acute Retinal Necrosis by Acyclovir Therapy. Am. J. Ophthalmol. 1991, 112, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.; Margo, C.E. Ocular Adverse Events Following Vaccination: Overview and Update. Surv. Ophthalmol. 2022, 67, 293–306. [Google Scholar] [CrossRef]
- Scalabrin, S.; Becco, A.; Vitale, A.; Nuzzi, R. Ocular Effects Caused by Viral Infections and Corresponding Vaccines: An Overview of Varicella Zoster Virus, Measles Virus, Influenza Viruses, Hepatitis B Virus, and SARS-CoV-2. Front. Med. 2022, 9, 999251. [Google Scholar] [CrossRef] [PubMed]
- Ness, T.; Hengel, H. Impfschäden am Auge. Ophthalmologe 2016, 113, 615–622. [Google Scholar] [CrossRef]
- Cunningham, E.T.; Moorthy, R.S.; Fraunfelder, F.W.; Zierhut, M. Vaccine-Associated Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 517–520. [Google Scholar] [CrossRef]
- Cordero-Coma, M.; Salazar-Méndez, R.; Garzo-García, I.; Yilmaz, T. Drug-Induced Uveitis. Expert Opin. Drug Saf. 2015, 14, 111–126. [Google Scholar] [CrossRef]
- Moorthy, R.S.; Moorthy, M.S.; Cunningham, E.T.J. Drug-Induced Uveitis. Curr. Opin. Ophthalmol. 2018, 29, 588. [Google Scholar] [CrossRef]
- Cunningham, E.T.; London, N.J.S.; Moorthy, R.; Garg, S.J.; Zierhut, M. Drugs, Inflammation, and the Eye. Ocul. Immunol. Inflamm. 2016, 24, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Fraunfelder, F.W.; Suhler, E.B.; Fraunfelder, F.T. Hepatitis B Vaccine and Uveitis: An Emerging Hypothesis Suggested by Review of 32 Case Reports. Cutan. Ocul. Toxicol. 2010, 29, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Holt, H.D.; Hinkle, D.M.; Falk, N.S.; Fraunfelder, F.T.; Fraunfelder, F.W. Human Papilloma Virus Vaccine Associated Uveitis. Curr. Drug Saf. 2014, 9, 65–68. [Google Scholar] [CrossRef]
- Fiore, T.; Iaccheri, B.; Androudi, S.; Papadaki, T.G.; Anzaar, F.; Brazitikos, P.; D’Amico, D.J.; Foster, C.S. Acute Posterior Multifocal Placoid Pigment Epitheliopathy: Outcome and Visual Prognosis. Retina 2009, 29, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Lavezzo, M.M.; Sakata, V.M.; Morita, C.; Rodriguez, E.E.C.; Abdallah, S.F.; da Silva, F.T.G.; Hirata, C.E.; Yamamoto, J.H. Vogt-Koyanagi-Harada Disease: Review of a Rare Autoimmune Disease Targeting Antigens of Melanocytes. Orphanet J. Rare Dis. 2016, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, T.F.; Silvestri, G.; McDowell, C.; Foot, B.; McAvoy, C.E. Acute Retinal Necrosis in the United Kingdom: Results of a Prospective Surveillance Study. Eye 2012, 26, 370–377; quiz 378. [Google Scholar] [CrossRef]
- Gherardi, R.K.; Crépeaux, G.; Authier, F.-J. Myalgia and Chronic Fatigue Syndrome Following Immunization: Macrophagic Myofasciitis and Animal Studies Support Linkage to Aluminum Adjuvant Persistency and Diffusion in the Immune System. Autoimmun. Rev. 2019, 18, 691–705. [Google Scholar] [CrossRef]
- Read, R.W. Uveitis: Advances in Understanding of Pathogenesis and Treatment. Curr. Rheumatol. Rep. 2006, 8, 260–266. [Google Scholar] [CrossRef]
- Waisbren, B.A. Acquired Autoimmunity after Viral Vaccination Is Caused by Molecular Mimicry and Antigen Complimentarity in the Presence of an Immunologic Adjuvant and Specific HLA Patterns. Med. Hypotheses 2008, 70, 346–348. [Google Scholar] [CrossRef]
- Broekhuyse, R.M.; Kuhlmann, E.D.; Winkens, H.J. Experimental Autoimmune Anterior Uveitis (EAAU). III. Induction by Immunization with Purified Uveal and Skin Melanins. Exp. Eye Res. 1993, 56, 575–583. [Google Scholar] [CrossRef]


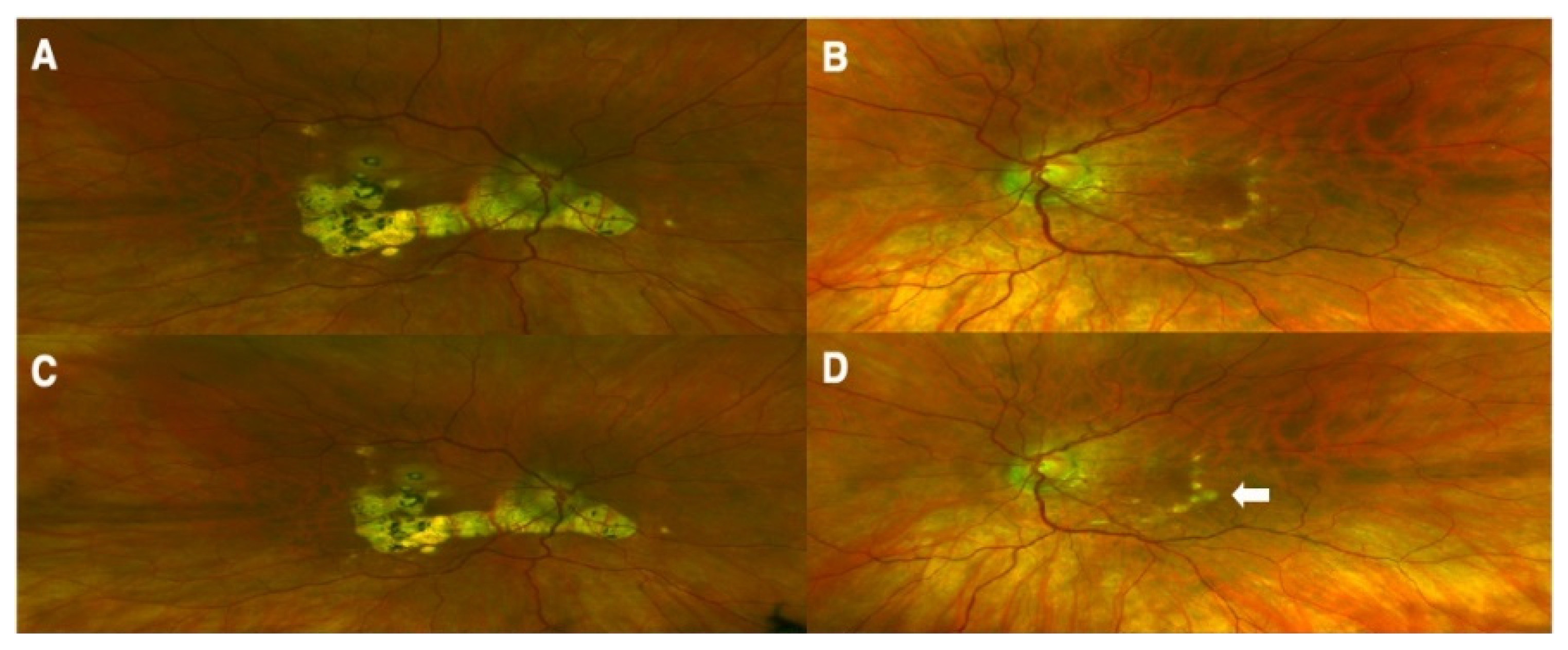
| Reference | Diagnosis | Vaccine Type | Age */Gender | Symptoms/Signs/Lab Tests/Medical History | Interval Post-Vaccination ^ | Treatment | Outcome/Follow-Up |
|---|---|---|---|---|---|---|---|
| Hepatitis B virus (HBV) vaccine | |||||||
| Fried 1987 [28] | Bilateral posterior uveitis | Recombinant | 20/F | Headache | 1st: 3 days after the second dose 2nd: 4 days after the third dose | NR | Recurrence due to re-exposure/not reported |
| Brézin 1995 [29] | Bilateral APMPPE | Recombinant | 31/M | None | 3 days after the fourth dose | NR | A residual paracentral scotoma/9 m |
| Bilateral APMPPE | Recombinant | 30/M | None | 14 days after the third dose | NR | A residual paracentral scotoma OS 4 m | |
| Baglivo 1996 [30] | Bilateral MEWDS | Recombinant | 23/F | None | 24 h after the third dose | NR | None/3 m |
| Sood 2019 [31] | Bilateral VKH (pan-uveitis) | Recombinant | 43/M | Hearing loss, tinnitus, integumentary changes. | 3 days after the first dose | po/io steroids | VKH with long-term steroids treatment/5 m |
| Human papillomavirus (HPV) vaccine | |||||||
| Khalifa 2010 [32] | Bilateral ampiginous choroiditis | Quadrivalent/recombinant | 17/F | None | 3 weeks after the first dose | po steroids | Extensive macular scarring remained/3 m |
| Ogino 2014 [33] | MEWDS (left eye) | Bivalent/recombinant | 16/F | Throat pain, headache | 2 weeks after the second dose | iv steroids and anti-histamine | FA revealed recurrent leakage/2 y |
| Chen, Y.-H. 2014 [34] | Bilateral pan-uveitis | Quadrivalent/recombinant | 27/F | Bilateral knee pain, erythematous papules vertigo, and hearing impairment | 4 days after the third dose | io/po steroids | None/2 y |
| Dansingani 2015 [23] | Bilateral pan-uveitis and ERD resembling VKH | Quadrivalent/recombinant | 20/F | HLA-DRB1*0405(+) | 3 weeks after the second dose | po steroids | None/5 m |
| Sawai 2016 [35] | Bilateral anterior uveitis/TINU | Recombinant | 14/F | Fever, general malaise, low back pain | 4 days after the first dose | io/gtt steroid | long-term steroids treatment for uveitis/3 y |
| Bilateral anterior uveitis/TINU | Recombinant | 14/F | NR | 10 weeks after the third dose | io/gtt steroid | None/NR | |
| Ye, H. 2020 [22] | Bilateral posterior uveitis resembling Harada disease | Divalent/recombinant | 29/F | HLA-DR04 and 07(+) | 7 days after the third dose | io/po steroids | None/4 m |
| Kong 2022 [21] | Bilateral Harada disease-like uveitis(posterior) | Quadrivalent/recombinant | 37/F | None | 10 days after the third dose | gtt steroids | None/3 m |
| Influenza virus vaccine | |||||||
| Hector 1978 [36] | Bilateral APMPPE | Live attenuated | 21/M | Fever, chills, headache | 48 h after the first dose | None | Residual RPE changes/6 m |
| Blumberg 1980 [37] | Iritis (OD) and optic neuritis (OS) | Inactivated | 27/M | Fever, arthralgias, and myalgias | 14 days after vaccination | Systematic/gtt steroids | C3: 91 mg/dL; ESR: 40 mm/h/1 m |
| Blanche 1994 [38] | Bilateral anterior and posterior uveitis | Inactivated | 68/F | Fever | 48 h after the first dose | gtt steroids | None/3 m |
| Gallagher 2009 [39] | Bilateral VKH (pan-uveitis) | NR | 44/F | Tinnitus | 1 month after vaccination | iv/po steroids with steroids-sparing and long-term immunomodulation | None/NR |
| Wells 2009 [40] | Bilateral pan-uveitis | NR | 70/M | NR | 1 days after the first dose | io/gtt steroids | None/3 m |
| Mendrinos 2010 [41] | Bilateral APMPPE | NR | 27/M | Flu-like symptoms | 14 days after the vaccination | po steroids | Residual RPE changes/3 m |
| Tao 2011 [42] | Posterior uveitis and ERD (right) | Live attenuated (H1N1) | 10/M | None | 10 days after vaccination | iv/po steroids | None/1 m |
| Bilateral posterior uveitis and ERD | Live attenuated (H1N1) | 47/F | High fever, bilateral headache | 2 days after vaccination | iv/po steroids | Visual acuity: 0.01/NR | |
| Rothova 2011 [43] | Bilateral VZV-associated pan-uveitis | Live attenuated (H1N1) | 60/M | NR | 4 days after vaccination | systematic/io steroids | The intraocular inflammation slowly subsided/NR |
| Goyal 2013 [46] | MEWDS (right eye) | NR | 53/M | NR | 10 days after vaccination | None | A paracentral scotoma/1 m |
| Williams 2015 [45] | Retinal artery vasculitis (right eye; anterior uveitis) | Live attenuated | 78/F | Right-sided headache | 8 weeks after vaccination | gtt steroids | None/9 m |
| Branisteanu 2015 [47] | Bilateral APMPEE | NR | 18/F | Intermittent headaches | 14 days after vaccination | po steroids | Residual RPE changes/5 y |
| Manusow 2015 [48] | Bilateral pan-uveitis with OIS | Live attenuated | 49/F | Polyarthritis, fever, tender cervical lymphadenopathy | 4 days after vaccination | iv/po steroids | No light perception OS/1 y |
| Pan-uveitis with OIS (right) | Live attenuated | 57/M | Mild jaundice, confusion and disorientation to place and time/ESR-84(+) | 3 days after vaccination | iv/po steroids | LP, 20/50/3 m | |
| Gonome 2016 [46] | Bilateral AMPEE and granulomatous uveitis (pan-uveitis) | NR | 30/F | Fever, cough, and nausea | 17 days after vaccination | Initiate iv/gtt NSAIDs; After the granulomatous uveitis appearance; gtt steroids | None/1 m |
| Kim 2016 [49] | Bilateral VKH (pan-uveitis) | Live attenuated | 52/F | Tinnitus | 1 month after vaccination | iv/po steroid | None/NR |
| Abou-Samra 2019 [50] | MEWDS (right eye) | NR | 27/F | Fever, rash, oral ulcers, arthralgias, headache, or vertigo. | 14 days after vaccination | None | Residual RPE changes/8 w |
| Murtaza 2022 [51] | Bilateral VKH (pan-uveitis) | Inactivated | 30/M | Headache, tinnitus, and HLA-DR4(+) | 2 days after vaccination | io/po steroids | Sunset glow fundus from choroidal depigmentation/6 m |
| Measles–Mumps–Rubella (MMR) vaccine | |||||||
| Islam 2000 [52] | Bilateral anterior uveitis | Live attenuated | 12/F | NR | 6 weeks after vaccination | gtt steroids | None/1 y |
| Bilateral anterior uveitis | Live attenuated | 14/M | NR | 4 weeks after vaccination | po/gtt steroids | None/NR | |
| Sedaghat 2007 [53] | Bilateral pan-uveitis and dermal vasculitis | Live attenuated | 17/F | Fever, chills, skin rash and knee arthritis | 5 days after vaccination | po/gtt steroids | None/6 m |
| Ferrini 2013 [54] | Anterior uveitis (left eye) with iris heterochromia and cataract | Live attenuated | 12 month/F | HLA-B51, rubella IgG (+) | 3 months after vaccination | Systematic/gtt/io steroids cataract extraction | None/3 m |
| Varicella zoster virus (VZV) vaccine | |||||||
| Esmaeli-Gutstein 1999 [55] | Anterior and intermediate uveitis (left eye) | Live attenuated | 16/F | Generalized vesicular rash | 1 week after vaccination | po acyclovir, gtt steroids | None/NR |
| Naseri 2003 [56] | Herpes zoster virus sclerokeratitis and anterior uveitis (left eye) | Live attenuated | 9/M | rash in left face, wild-type VZV DNA (+) | 3 years after vaccination | po acyclovir/gtt steroids | NR/NR |
| Fine 2010 [57] | Bilateral APMPPE | Live attenuated | 11/F | Severe headaches and tinnitus/VZV Ab (+) | 10 days after vaccination | po steroids | NR/1 y |
| Charkoudian 2011 [24] | ARN (left eye) | Live attenuated | 77/F | VZV DNA (+)/diabetes mellitus | 6 days after vaccination | po/iv antiviral drugs, vitrectomy | NR/NR |
| Bilateral ARN | Live attenuated | 80/M | rash and fever/VZV DNA (+), immunosuppressant use for renal transplantation | 2 months after vaccination | po/iv antiviral drugs, io foscarnet, bilateral vitrectomy | NR/NR | |
| Gonzales 2012 [25] | Bilateral ARN | Live attenuated | 20/M | Oka strain VZV DNA (+)/ immunosuppressant for an inflammatory gastroenteropathy | 1 month after vaccination | io foscarnet, antiviral drugs, pars plana vitrectomy | NR/NR |
| Sham 2012 [58] | Exacerbation of anterior uveitis (right eye) | Live attenuated | 86/M | Medical history of HZO with anterior uveitis | 3 weeks after vaccination | po Valacyclovir, gtt steroids | None/NR |
| Heath 2017 [26] | ARN (left eye) | Live attenuated | 78/F | Oka strain VZV DNA (+)/immunosuppressant for autoimmune diabetes | 6 weeks after vaccination | po valaciclovir, gtt steroids, pars plana vitrectomy | A pigmented scar/NR |
| Weinlander 2019 [59] | ARN (left eye) | Live attenuated | 64/M | Wild-type VZV DNA (+)/metabolic syndrome and impaired glucose tolerance | 16 months after vaccination | po Valacyclovir, po/gtt steroids | None/6 m |
| ARN (left eye) | Live attenuated | 62/M | Wild-type VZV DNA (+)/Cirrhosis and diabetes mellitus type 2 | 7 months after vaccination | po Valacyclovir, gtt steroids | Died from complications of his cirrhosis/6 m | |
| Heydari-Kamjani 2019 [60] | Bilateral uveitis sarcoidosis | Recombinant zoster vaccine | 53/F | Headaches | 4 days after vaccination | gtt steroids | None/NR |
| Chen R.I. 2020 [61] | ARN (left eye) | Recombinant zoster vaccine | 65/F | Immunomodulator for multiple myeloma/wild-type VZV DNA (+) | 6 weeks after vaccination | io foscarnet, iv/po antiviral drugs | None/19 w |
| Menghini 2021 [62] | ARN with obliterative angiopathy (left eye) | Live attenuated | 76/M | Insulin-dependent diabetes mellitus, chronic lymphocytic leukemia/wild-type VZV DNA (+) | 2 days after vaccination | io foscarnet, iv/po/iv antiviral drugs, iv/po steroids | Left eye visual acuity dropped to perception only/NR |
| Richards 2021 [27] | Recurrent bilateral multifocal choroiditis | Recombinant zoster vaccine | 57/F | arm swelling at the injection site, chills, malaise, subjective fever, and tinnitus/immunosuppressant for multifocal choroiditis | 24 h after the first dose | po steroids and continued methotrexate | Intravitreal bevacizumab for a secondary choroidal neovascular membrane/2 m |
| Recurrent bilateral anterior and mild intermediate uveitis | Recombinant zoster vaccine | 69/M | Headache/gtt steroids for uveitis | 1 month after the second dose | po valacyclovir/gtt steroids | None/1 m | |
| Recurrent anterior uveitis (left eye) | Recombinant zoster vaccine | 70/F | gtt steroids and po valacyclovir for viral keratouveitis | 2 weeks after the first dose | po valacyclovir, po/gtt steroids | None/6 w | |
| Yellow Fever virus vaccine | |||||||
| Biancardi 2019 [63] | Anterior uveitis (right eye) | Live attenuated | 35/F | None | 10 days after vaccination | gtt steroids | None/NR |
| Intermediate uveitis (left eye) | Live attenuated | 21/F | low fever, body ache, and mild headache | 14 days after vaccination | po steroids | None/6 w | |
| Volkov 2020 [64] | Viscerotropic disease followed by bilateral acute anterior and intermediate uveitis | Live attenuated | 37/M | Fever, cough, dyspnea, malaise, sore throat, non-bloody diarrhea, and morbilliform skin rash of the chest/YFV RNA (+) | 2–3 weeks after vaccination | io/gtt steroids | Persistent fatigue for few month/NR |
| Campos 2021 [65] | Bilateral VKH (pan-uveitis) | Live attenuated | 34/M | tinnitus, headache | 12 days after a booster dose | Iv/po steroids | None/2 y |
| Pereima 2022 [66] | Bilateral Acute VKH (pan-uveitis) | Live attenuated | 45/M | tinnitus, headache | 2 weeks after vaccination | Iv/po steroids | Sunset glow fundus and dark dots/30 m |
| Hepatitis A virus (HAV) vaccine | |||||||
| Fine 2001 [67] | MEWDS (left eye) | Inactivated | 30/M | Not reported | 13 days after booster vaccination | None | None/6 w |
| Rabies vaccine | |||||||
| Yang 2018 [68] | MEWDS (left eye) | Inactivated | 33/F | None | 7 days after the third dose | io steroids | None/3 y |
| Co-administration | |||||||
| Stangos 2006 [69] | MEWDS (left eye) | HAV/yellow fever | 50/F | None | 1 week after vaccination | None | None/6 w |
| Cohen 2010 [70] | MEWDS (left eye) | HPV/Meningococcus | 17/F | HLA-B27(+) | 1 months after vaccination | None | None/2 m |
| Escott 2013 [71] | Acute multifocal choroiditis (right eye) | HAV/typhoid/yellow fever | 33/M | fever, rash, oral ulcers, arthralgias, headache, vertigo | 3 weeks after vaccination | None | RPE atrophy/8 w |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Kamoi, K.; Zong, Y.; Zhang, J.; Yang, M.; Ohno-Matsui, K. Ocular Inflammation Post-Vaccination. Vaccines 2023, 11, 1626. https://doi.org/10.3390/vaccines11101626
Zou Y, Kamoi K, Zong Y, Zhang J, Yang M, Ohno-Matsui K. Ocular Inflammation Post-Vaccination. Vaccines. 2023; 11(10):1626. https://doi.org/10.3390/vaccines11101626
Chicago/Turabian StyleZou, Yaru, Koju Kamoi, Yuan Zong, Jing Zhang, Mingming Yang, and Kyoko Ohno-Matsui. 2023. "Ocular Inflammation Post-Vaccination" Vaccines 11, no. 10: 1626. https://doi.org/10.3390/vaccines11101626
APA StyleZou, Y., Kamoi, K., Zong, Y., Zhang, J., Yang, M., & Ohno-Matsui, K. (2023). Ocular Inflammation Post-Vaccination. Vaccines, 11(10), 1626. https://doi.org/10.3390/vaccines11101626







