Comparative Immune Response after Vaccination with SOBERANA® 02 and SOBERANA® plus Heterologous Scheme and Natural Infection in Young Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Ethics
2.2. Blood Collection and Isolation of Peripheral Blood Mononuclear Cells
2.3. Antibody Determinations
2.4. Staining of Cells and Multicolor Flow Cytometry
2.5. IFN-γ Secretion Assay for an Evaluation of the T Cell Specific Response
2.6. Determination of Cytokine Release
2.7. Statistics
3. Results
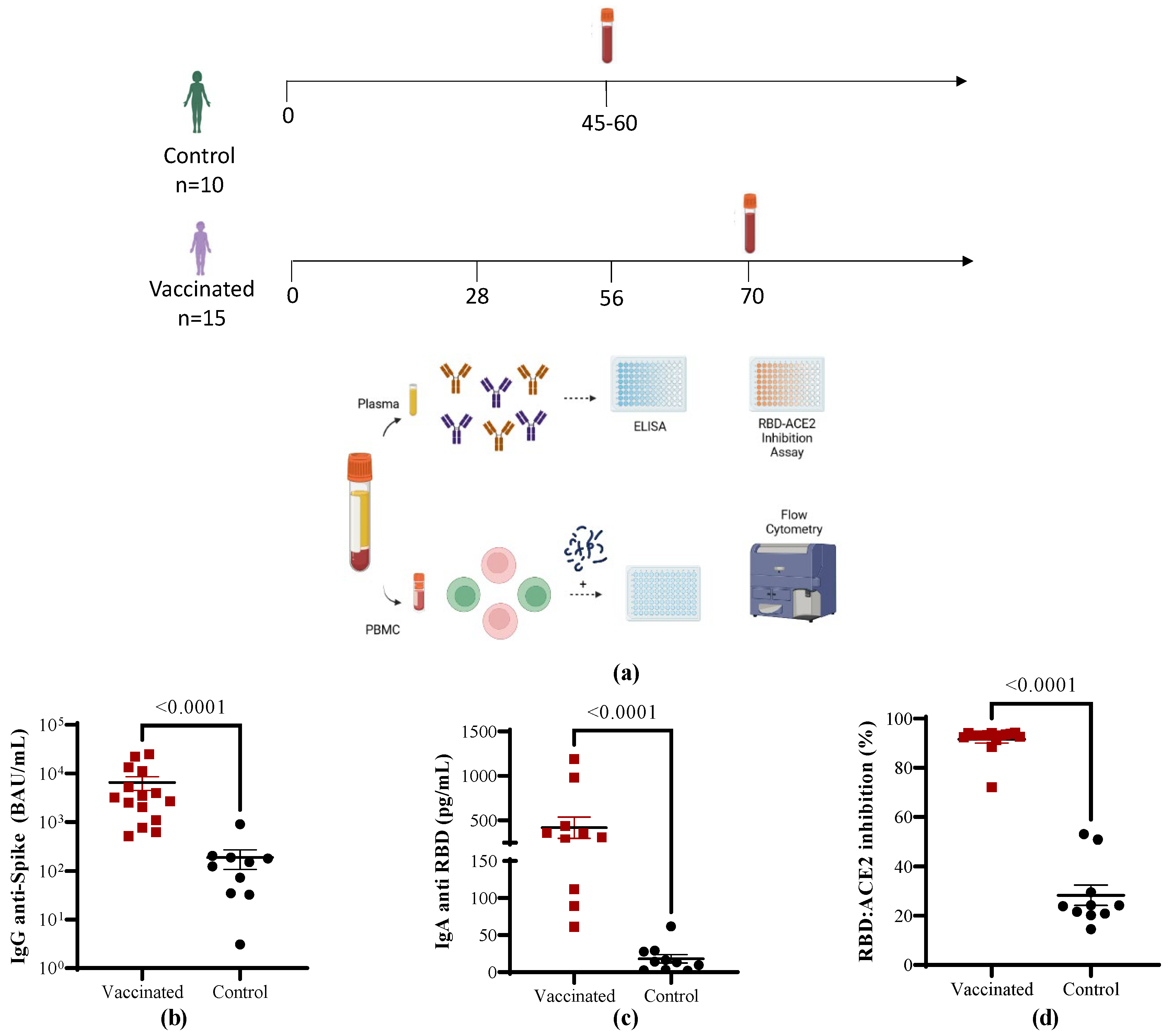
3.1. Characterization of the Study Cohort
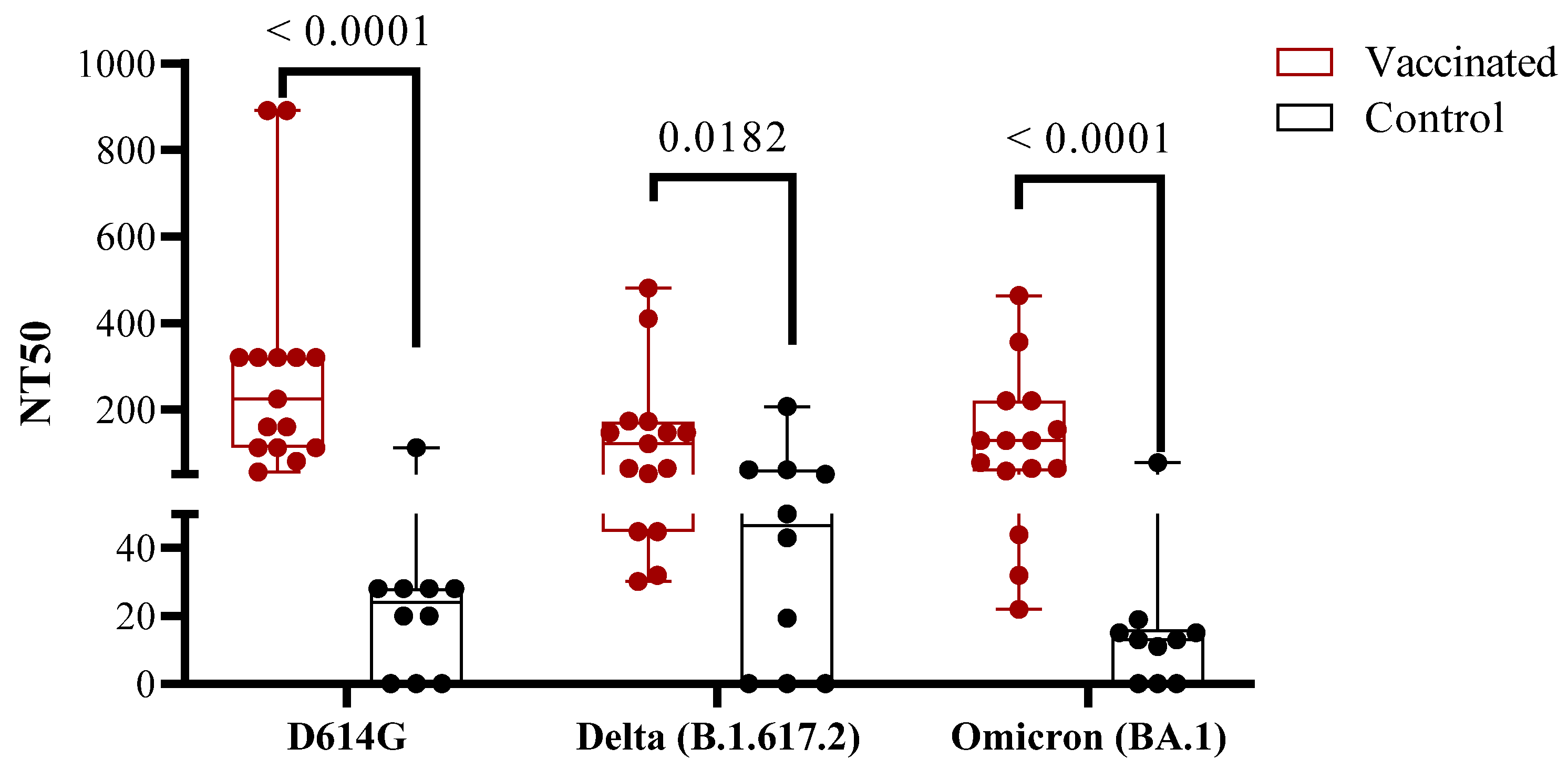
3.2. Antibody Immune Responses in Vaccinated Children Compared to COVID-19 Recovered Children
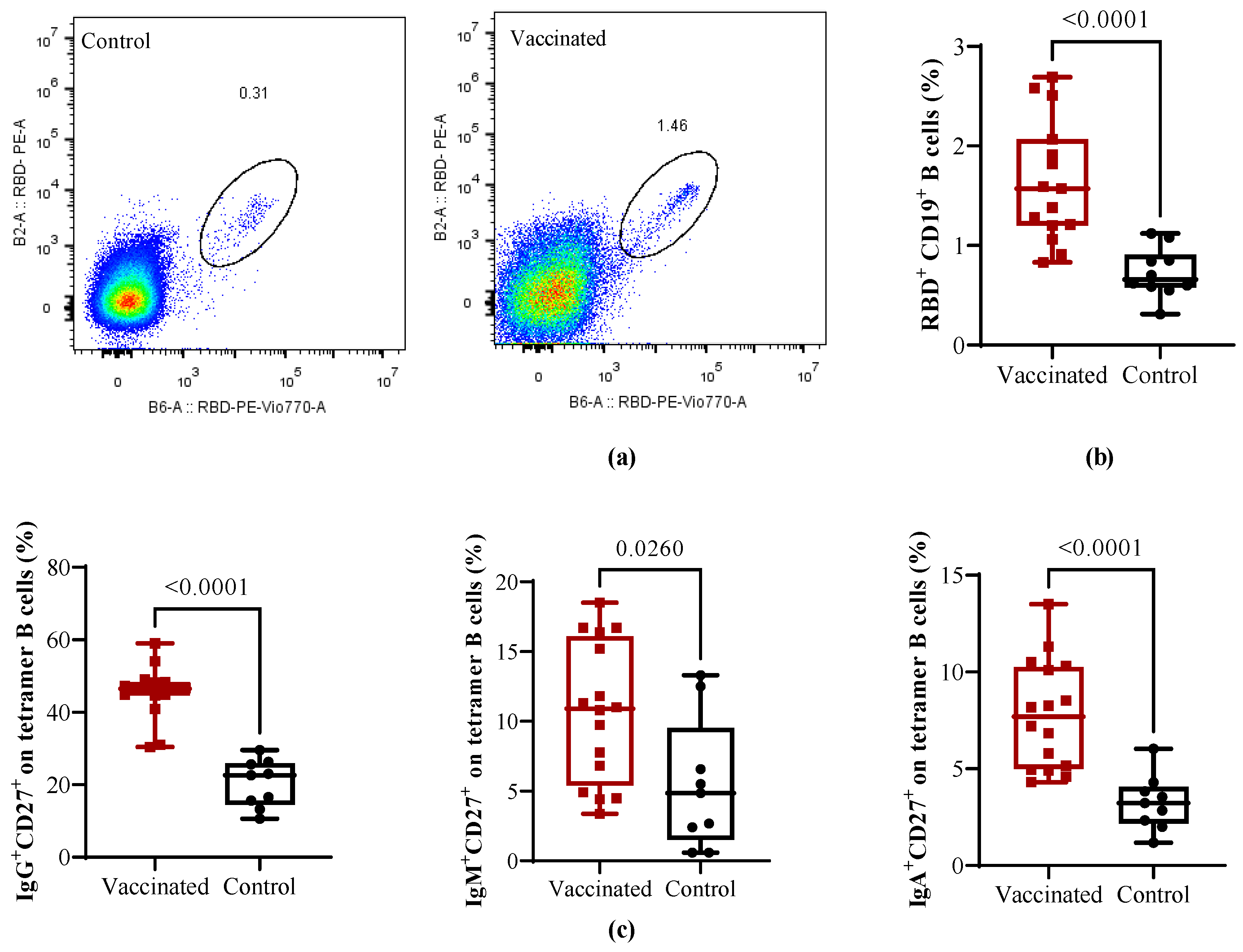
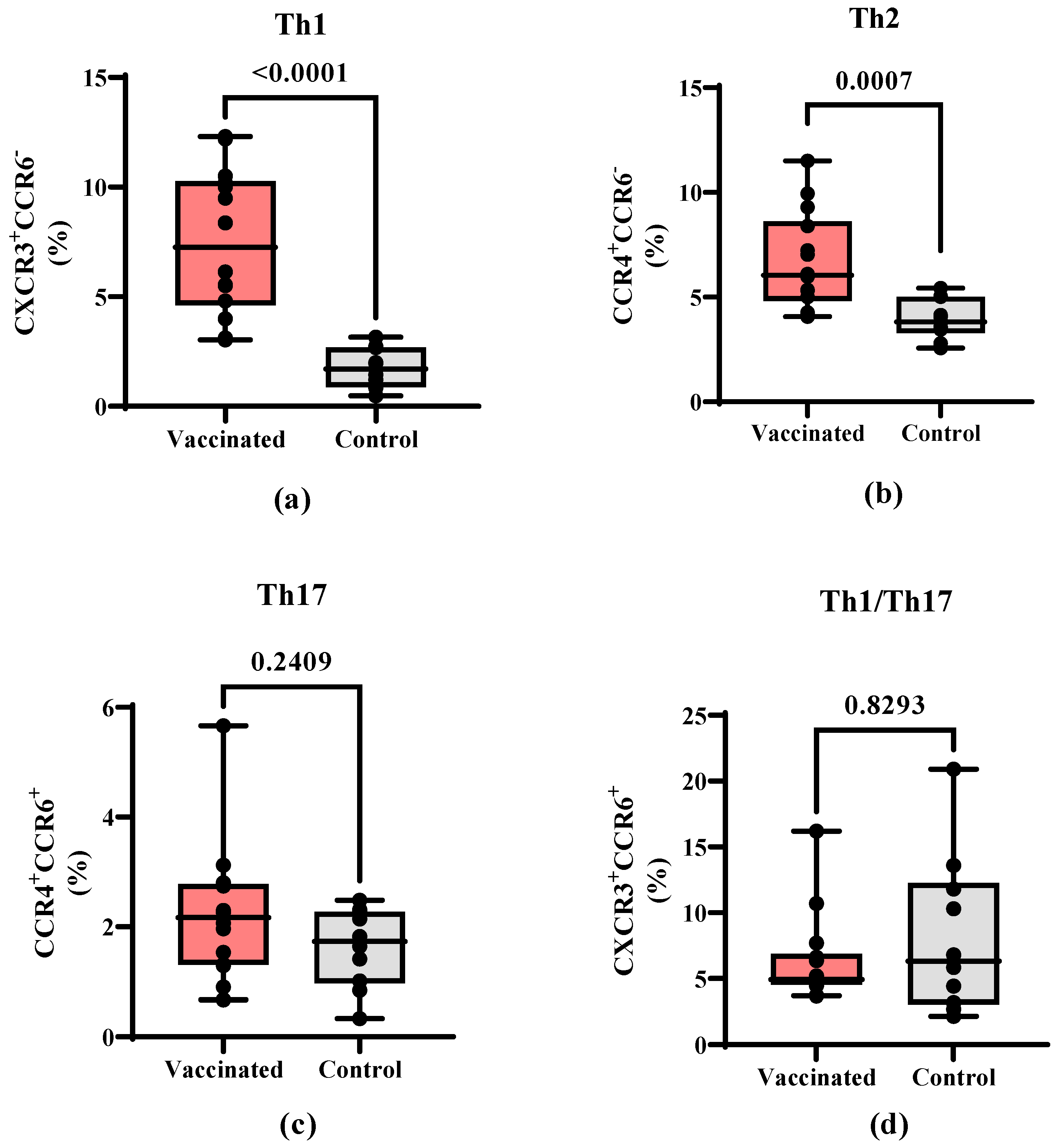
3.3. Total and RBD-Specific B Cells and T Helper Populations in Vaccinated and COVID-19 Recovered Children
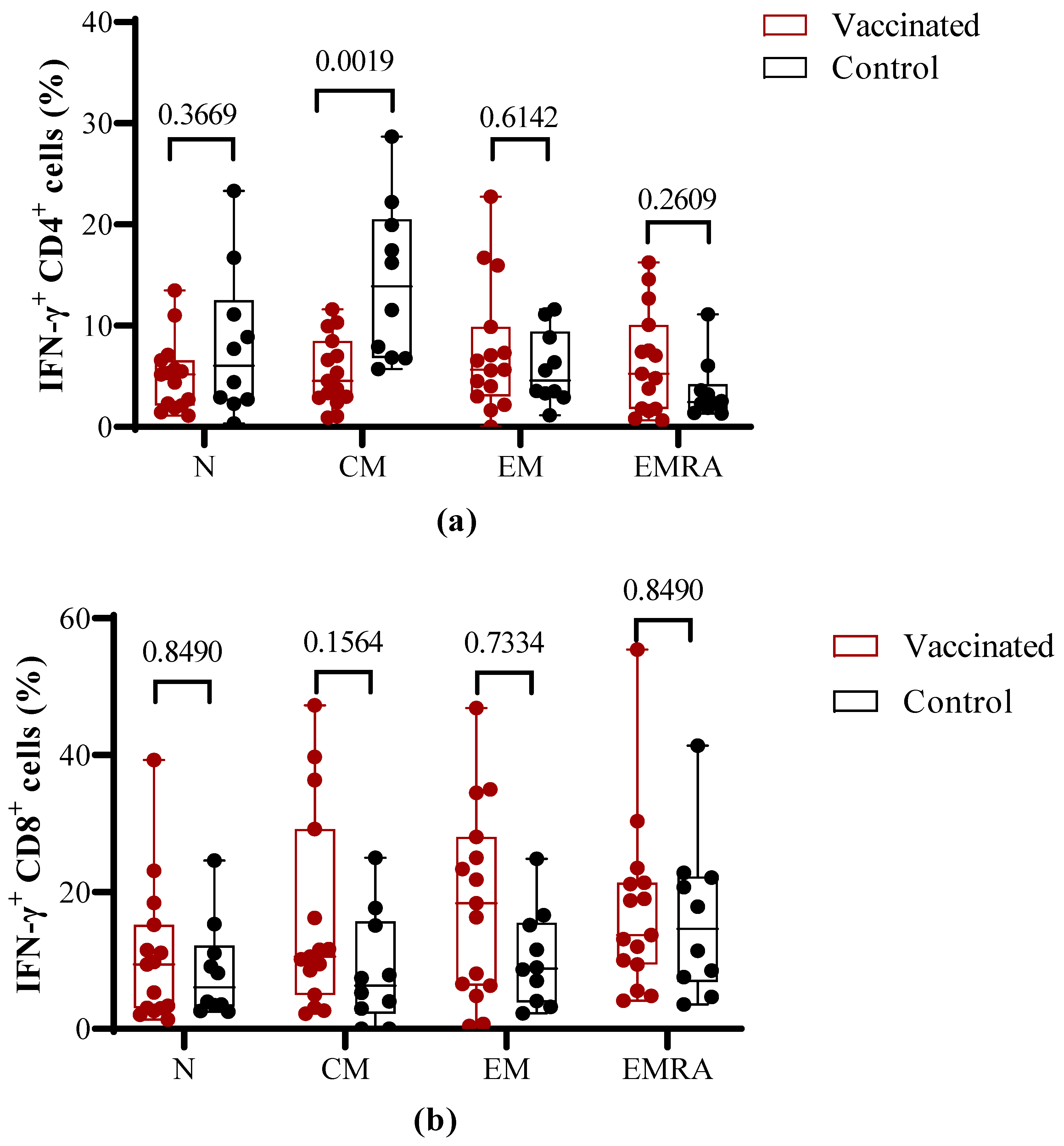
3.4. Functional Properties of T Cells after Antigen-Specific In Vitro Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McVernon, J.; Liberman, J. WHO keeps COVID-19 a public health emergency of international concern. BMJ 2023, 380, p504. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; DeWitt, P.E.; Russell, S.; Anand, A.; Bradwell, K.R.; Bremer, C.; Gabriel, D.; Girvin, A.T.; Hajagos, J.G.; McMurry, J.A.; et al. Characteristics, Outcomes, and Severity Risk Factors Associated with SARS-CoV-2 Infection among Children in the US National COVID Cohort Collaborative. JAMA Netw. Open 2022, 5, e2143151. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, J.; Kuno, T.; Takagi, H.; Sumitomo, N. Clinical characteristics of COVID-19 in children: A systematic review. Pediatr. Pulmonol. 2020, 55, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. COVID vaccines and kids: Five questions as trials begin. Nature 2021, 592, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Chua, G.T.; Wong, J.S.C.; Lam, I.; Ho, P.P.K.; Chan, W.H.; Yau, F.Y.S.; Rosa Duque, J.S.; Ho, A.C.C.; Siu, K.K.; Cheung, T.W.Y.; et al. Clinical Characteristics and Transmission of COVID-19 in Children and Youths during 3 Waves of Outbreaks in Hong Kong. JAMA Netw. Open 2021, 4, e218824. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus (COVID-19) Vaccinations. Available online: http://ourworldindata.org/covid-vaccinations (accessed on 30 August 2023).
- Piechotta, V.; Siemens, W.; Thielemann, I.; Toews, M.; Koch, J.; Vygen-Bonnet, S.; Kothari, K.; Grummich, K.; Braun, C.; Kapp, P.; et al. Safety and effectiveness of vaccines against COVID-19 in children aged 5–11 years: A systematic review and meta-analysis. Lancet Child Adolesc. Health 2023, 7, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, L.; Shi, Y. Safety, Efficacy, and Immunogenicity of Varying Types of COVID-19 Vaccines in Children Younger Than 18 Years: An Update of Systematic Review and Meta-Analysis. Vaccines 2023, 11, 87. [Google Scholar] [CrossRef]
- Watanabe, A.; Kani, R.; Iwagami, M.; Takagi, H.; Yasuhara, J.; Kuno, T. Assessment of Efficacy and Safety of mRNA COVID-19 Vaccines in Children Aged 5 to 11 Years: A Systematic Review and Meta-analysis. JAMA Pediatr. 2023, 177, 384–394. [Google Scholar] [CrossRef]
- Frenkel, L.D. The global burden of vaccine-preventable infectious diseases in children less than 5 years of age: Implications for COVID-19 vaccination. How can we do better? In Allergy and Asthma Proceedings; OceanSide Publications, Inc.: East Providence, RI, USA, 2021; pp. 378–385. [Google Scholar]
- Valdes-Balbin, Y.; Santana-Mederos, D.; Quintero, L.; Fernández, S.; Rodriguez, L.; Sanchez Ramirez, B.; Perez-Nicado, R.; Acosta, C.; Méndez, Y.; Ricardo, M.G.; et al. SARS-CoV-2 RBD-Tetanus Toxoid Conjugate Vaccine Induces a Strong Neutralizing Immunity in Preclinical Studies. ACS Chem. Biol. 2021, 16, 1223–1233. [Google Scholar] [CrossRef]
- Ochoa-Azze, R.; Chang-Monteagudo, A.; Climent-Ruiz, Y.; Macías-Abraham, C.; Valenzuela-Silva, C.; de los Ángeles García-García, M.; Jerez-Barceló, Y.; Triana-Marrero, Y.; Ruiz-Villegas, L.; Dairon Rodríguez-Prieto, L.; et al. Safety and immunogenicity of the FINLAY-FR-1A vaccine in COVID-19 convalescent participants: An open-label phase 2a and double-blind, randomised, placebo-controlled, phase 2b, seamless, clinical trial. Lancet Respir. Med. 2022, 10, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Romaní, M.E.; García-Carmenate, M.; Valenzuela-Silva, C.; Baldoquín-Rodríguez, W.; Martínez-Pérez, M.; Rodríguez-González, M.; Paredes-Moreno, B.; Mendoza-Hernández, I.; González-Mujica Romero, R.; Samón-Tabio, O.; et al. Safety and efficacy of the two doses conjugated protein-based SOBERANA-02 COVID-19 vaccine and of a heterologous three-dose combination with SOBERANA-Plus: A double-blind, randomised, placebo-controlled phase 3 clinical trial. Lancet Reg. Health—Am. 2023, 18, 100423. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Romani, M.E.; García-Carmenate, M.; Verdecia-Sánchez, L.; Pérez-Rodríguez, S.; Rodriguez-González, M.; Valenzuela-Silva, C.; Paredes-Moreno, B.; Sanchez-Ramirez, B.; González-Mugica, R.; Hernández-Garcia, T.; et al. Safety and immunogenicity of anti-SARS-CoV-2 heterologous scheme with SOBERANA 02 and SOBERANA Plus vaccines: Phase IIb clinical trial in adults. Med 2022, 3, 760–773.e765. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Romaní, M.E.; Verdecia-Sánchez, L.; Rodríguez-González, M.; Rodríguez-Noda, L.; Valenzuela-Silva, C.; Paredes-Moreno, B.; Sánchez-Ramírez, B.; Pérez-Nicado, R.; González-Mugica, R.; Hernández-García, T.; et al. Safety and immunogenicity of anti-SARS CoV-2 vaccine SOBERANA 02 in homologous or heterologous scheme: Open label phase I and phase IIa clinical trials. Vaccine 2022, 40, 4220–4230. [Google Scholar] [CrossRef] [PubMed]
- Puga-Gómez, R.; Ricardo-Delgado, Y.; Rojas-Iriarte, C.; Céspedes-Henriquez, L.; Piedra-Bello, M.; Vega-Mendoza, D.; Pérez, N.P.; Paredes-Moreno, B.; Rodríguez-González, M.; Valenzuela-Silva, C.; et al. Open-label phase I/II clinical trial of SARS-CoV-2 receptor binding domain-tetanus toxoid conjugate vaccine (FINLAY-FR-2) in combination with receptor binding domain-protein vaccine (FINLAY-FR-1A) in children. Int. J. Infect. Dis. 2023, 126, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Eugenia-Toledo-Romani, M.; Valenzuela-Silva, C.; Montero-Diaz, M.; Iniguez-Rojas, L.; Rodriguez-González, M.; Martinez-Cabrera, M.; Puga-Gómez, R.; German-Almeida, A.; Fernandez-Castillo, S.; Climent-Ruiz, Y.; et al. Real-World Effectiveness of the OBERANA02 and SOBERANA-Plus Vaccine Combination in Children 2 to 11 Years of Age during the SARS-CoV-2 Omicron Wave in Cuba: A Regression Discontinuity Study; SSRN: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Paul, K.; Sibbertsen, F.; Weiskopf, D.; Lütgehetmann, M.; Barroso, M.; Danecka, M.K.; Glau, L.; Hecher, L.; Hermann, K.; Kohl, A.; et al. Specific CD4+ T Cell Responses to Ancestral SARS-CoV-2 in Children Increase with Age and Show Cross-Reactivity to Beta Variant. Front. Immunol. 2022, 13, 867577. [Google Scholar] [CrossRef] [PubMed]
- Osterholm, M.T.; Rabadán-Diehl, C.; Anzinger, J.; Bottazzi, M.E.; Christie-Samuels, C.; Erondu, N.; Marrazzo, J.; Milan, S.; Quashie, P.K.; Schwaab, T.; et al. EXECUTIVE SUMMARY Insights from Cuba’s COVID-19 Vaccine Enterprise: Report from a High Level Fact-Finding Delegation to Cuba. MEDICC Rev. 2022, 24, 109–128. [Google Scholar] [CrossRef]
- Lagousi, T.; Routsias, J.; Mavrouli, M.; Papadatou, I.; Geropeppa, M.; Spoulou, V. Comparative Characterization of Human Antibody Response Induced by BNT162b2 Vaccination vs. SARS-CoV-2 Wild-Type Infection. Vaccines 2022, 10, 1210. [Google Scholar] [CrossRef]
- Soto, J.A.; Melo-González, F.; Gutierrez-Vera, C.; Schultz, B.M.; Berríos-Rojas, R.V.; Rivera-Pérez, D.; Piña-Iturbe, A.; Hoppe-Elsholz, G.; Duarte, L.F.; Vázquez, Y.; et al. Inactivated Vaccine-Induced SARS-CoV-2 Variant-Specific Immunity in Children. mBio 2022, 13, e01311–e01322. [Google Scholar] [CrossRef]
- Dowell, A.C.; Powell, A.A.; Davis, C.; Scott, S.; Logan, N.; Willett, B.J.; Bruton, R.; Ayodele, M.; Jinks, E.; Gunn, J.; et al. mRNA or ChAd0x1 COVID-19 Vaccination of Adolescents Induces Robust Antibody and Cellular Responses with Continued Recognition of Omicron Following mRNA-1273. Front. Immunol. 2022, 13, 882515. [Google Scholar] [CrossRef]
- Cinicola, B.L.; Piano Mortari, E.; Zicari, A.M.; Agrati, C.; Bordoni, V.; Albano, C.; Fedele, G.; Schiavoni, I.; Leone, P.; Fiore, S.; et al. The BNT162b2 vaccine induces humoral and cellular immune memory to SARS-CoV-2 Wuhan strain and the Omicron variant in children 5 to 11 years of age. Front. Immunol. 2022, 13, 1094727. [Google Scholar] [CrossRef] [PubMed]
- Tsang, H.W.; Chua, G.T.; To, K.K.W.; Wong, J.S.C.; Tu, W.; Kwok, J.S.Y.; Wong, W.H.S.; Wang, X.; Zhang, Y.; Rosa Duque, J.S.; et al. Assessment of SARS-CoV-2 Immunity in Convalescent Children and Adolescents. Front. Immunol. 2021, 12, 797919. [Google Scholar] [CrossRef] [PubMed]
- Sieber, J.; Mayer, M.; Schmidthaler, K.; Kopanja, S.; Camp, J.V.; Popovitsch, A.; Dwivedi, V.; Hoz, J.; Schoof, A.; Weseslindtner, L.; et al. Long-Lived Immunity in SARS-CoV-2-Recovered Children and Its Neutralizing Capacity against Omicron. Front. Immunol. 2022, 13, 882456. [Google Scholar] [CrossRef] [PubMed]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Kared, H.; Wolf, A.-S.; Alirezaylavasani, A.; Ravussin, A.; Solum, G.; Tran, T.T.; Lund-Johansen, F.; Vaage, J.T.; Nissen-Meyer, L.S.; Nygaard, U.C.; et al. Immune responses in Omicron SARS-CoV-2 breakthrough infection in vaccinated adults. Nat. Commun. 2022, 13, 4165. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Todeschini, M.; Casiraghi, F.; Mister, M.; Pezzotta, A.; Peracchi, T.; Tomasoni, S.; Trionfini, P.; Benigni, A.; Remuzzi, G. Long-term adaptive response in COVID-19 vaccine recipients and the effect of a booster dose. Front. Immunol. 2023, 14, 1123158. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- José-Cascón, M.S.; de la Varga-Martínez, R.; Campos-Caro, A.; Rodríguez, C. Dynamics of B-Cell Responses after SARS-CoV-2 Vaccination in Spain. Vaccines 2022, 10, 1615. [Google Scholar] [CrossRef]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.-C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]
- Piano Mortari, E.; Russo, C.; Vinci, M.R.; Terreri, S.; Fernandez Salinas, A.; Piccioni, L.; Alteri, C.; Colagrossi, L.; Coltella, L.; Ranno, S.; et al. Highly Specific Memory B Cells Generation after the 2nd Dose of BNT162b2 Vaccine Compensate for the Decline of Serum Antibodies and Absence of Mucosal IgA. Cells 2021, 10, 2541. [Google Scholar] [CrossRef]
- Kaku, C.I.; Bergeron, A.J.; Ahlm, C.; Normark, J.; Sakharkar, M.; Forsell, M.N.E.; Walker, L.M. Recall of preexisting cross-reactive B cell memory after Omicron BA.1 breakthrough infection. Sci. Immunol. 2022, 7, eabq3511. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; van den Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2–specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef] [PubMed]
- Fazolo, T.; Lima, K.; Fontoura, J.C.; de Souza, P.O.; Hilario, G.; Zorzetto, R.; Júnior, L.R.; Pscheidt, V.M.; de Castilhos Ferreira Neto, J.; Haubert, A.F.; et al. Pediatric COVID-19 patients in South Brazil show abundant viral mRNA and strong specific anti-viral responses. Nat. Commun. 2021, 12, 6844. [Google Scholar] [CrossRef] [PubMed]
- Cotugno, N.; Ruggiero, A.; Bonfante, F.; Petrara, M.R.; Zicari, S.; Pascucci, G.R.; Zangari, P.; De Ioris, M.A.; Santilli, V.; Manno, E.C.; et al. Virological and immunological features of SARS-CoV-2-infected children who develop neutralizing antibodies. Cell Rep. 2021, 34, 108852. [Google Scholar] [CrossRef] [PubMed]
- Gil-Etayo, F.J.; Suàrez-Fernández, P.; Cabrera-Marante, O.; Arroyo, D.; Garcinuño, S.; Naranjo, L.; Pleguezuelo, D.E.; Allende, L.M.; Mancebo, E.; Lalueza, A.; et al. T-Helper Cell Subset Response Is a Determining Factor in COVID-19 Progression. Front. Cell. Infect. Microbiol. 2021, 11, 624483. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Cohen, C.A.; Li, A.P.Y.; Hachim, A.; Hui, D.S.C.; Kwan, M.Y.W.; Tsang, O.T.Y.; Chiu, S.S.; Chan, W.H.; Yau, Y.S.; Kavian, N.; et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat. Commun. 2021, 12, 4678. [Google Scholar] [CrossRef]
- Conway, S.R.; Lazarski, C.A.; Field, N.E.; Jensen-Wachspress, M.; Lang, H.; Kankate, V.; Durkee-Shock, J.; Kinoshita, H.; Suslovic, W.; Webber, K.; et al. SARS-CoV-2-Specific T Cell Responses Are Stronger in Children With Multisystem Inflammatory Syndrome Compared to Children With Uncomplicated SARS-CoV-2 Infection. Front. Immunol. 2022, 12, 793197. [Google Scholar] [CrossRef]
- International Register Clinical Trials. Identifier RPCEC00000374. Phase I–II Study, Sequential during Phase I, Open-Label, Adaptive and Multicenter to Evaluate the Safety, Reactogenicity and Immunogenicity of a Heterologous Two-Dose Schedule of the Prophylactic Anti-SARS-CoV-2 Vaccine Candidate, FINLAY-FR-2 and a Dose of FINLAY-FR-1A. 2021. Available online: https://rpcec.sld.cu/trials/RPCEC00000374-en (accessed on 9 August 2023).

| Vaccinated Children | Children Recovered from COVID-19 | |
|---|---|---|
| N | 15 | 10 |
| Sex | ||
| Female | 6 (40.0%) | 4 (40.0%) |
| Male | 9 (60.0%) | 6 (60.0%) |
| Skin color | ||
| White | 12 (80.0%) | 3 (30.0%) |
| Black | 1 (6.6%) | 1 (10.0%) |
| Multiracial | 2 (12.3%) | 6 (60.0%) |
| Age (years) | ||
| Mean (SD) | 5.3 (2.1) | 7.9 (2.9) |
| Median (IQR) | 9.0 (6.0) | 7.5 (5.0) |
| Range | (5; 11) | (4; 11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Nicado, R.; Massa, C.; Rodríguez-Noda, L.M.; Müller, A.; Puga-Gómez, R.; Ricardo-Delgado, Y.; Paredes-Moreno, B.; Rodríguez-González, M.; García-Ferrer, M.; Palmero-Álvarez, I.; et al. Comparative Immune Response after Vaccination with SOBERANA® 02 and SOBERANA® plus Heterologous Scheme and Natural Infection in Young Children. Vaccines 2023, 11, 1636. https://doi.org/10.3390/vaccines11111636
Pérez-Nicado R, Massa C, Rodríguez-Noda LM, Müller A, Puga-Gómez R, Ricardo-Delgado Y, Paredes-Moreno B, Rodríguez-González M, García-Ferrer M, Palmero-Álvarez I, et al. Comparative Immune Response after Vaccination with SOBERANA® 02 and SOBERANA® plus Heterologous Scheme and Natural Infection in Young Children. Vaccines. 2023; 11(11):1636. https://doi.org/10.3390/vaccines11111636
Chicago/Turabian StylePérez-Nicado, Rocmira, Chiara Massa, Laura Marta Rodríguez-Noda, Anja Müller, Rinaldo Puga-Gómez, Yariset Ricardo-Delgado, Beatriz Paredes-Moreno, Meiby Rodríguez-González, Marylé García-Ferrer, Ilianet Palmero-Álvarez, and et al. 2023. "Comparative Immune Response after Vaccination with SOBERANA® 02 and SOBERANA® plus Heterologous Scheme and Natural Infection in Young Children" Vaccines 11, no. 11: 1636. https://doi.org/10.3390/vaccines11111636
APA StylePérez-Nicado, R., Massa, C., Rodríguez-Noda, L. M., Müller, A., Puga-Gómez, R., Ricardo-Delgado, Y., Paredes-Moreno, B., Rodríguez-González, M., García-Ferrer, M., Palmero-Álvarez, I., Garcés-Hechavarría, A., Rivera, D. G., Valdés-Balbín, Y., Vérez-Bencomo, V., García-Rivera, D., & Seliger, B. (2023). Comparative Immune Response after Vaccination with SOBERANA® 02 and SOBERANA® plus Heterologous Scheme and Natural Infection in Young Children. Vaccines, 11(11), 1636. https://doi.org/10.3390/vaccines11111636






