Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations
Abstract
1. Introduction
2. Adjuvants and Their Importance
2.1. Traditional Adjuvants
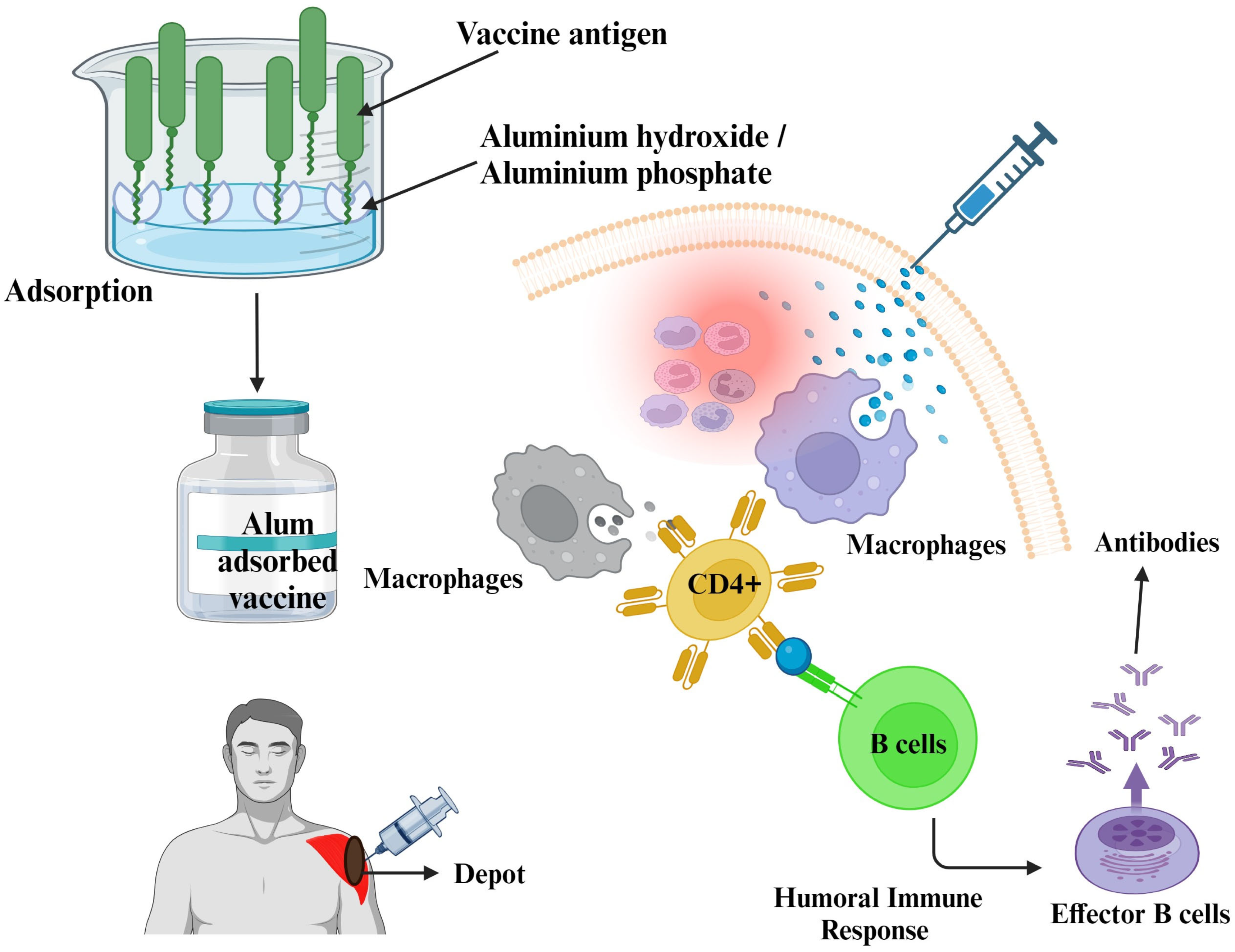
2.1.1. Mineral Salts
- a.
- Aluminum Compounds
| Aluminum Adjuvant | Characteristics | Examples of Vaccines | References |
|---|---|---|---|
| Aluminum hydroxide | White, gel-like substance; insoluble in water | Hepatitis A, Hepatitis B, Human Papillomavirus | [31,33,34] |
| Aluminum phosphate | White, crystalline powder | Anthrax, Diphtheria-Tetanus-Pertussis, Haemophilus influenzae type b | [31,33,34] |
| Amorphous aluminum hydroxy phosphate sulfate (AAHS) | Nanoparticulate adjuvant, composed of very small particles | Human Papillomavirus, Hepatitis B | [31,33,34] |
- b.
- Calcium phosphate
2.1.2. Oil Emulsion Adjuvants
- a.
- Freund’s Adjuvants
- Complete Freund’s Adjuvant (CFA)
- Incomplete Freund’s adjuvant (IFA)
- b.
- Montanide
- c.
- Adjuvant 65-4
- d.
- MF 59
2.1.3. Immune-Stimulating Complexes (ISCOMs)
- a.
- Quil A
- b.
- QS-21
- c.
- AS01
- d.
- AS02
- e.
- AS03
- f.
- AS04
2.2. Modern Adjuvant Platforms
2.2.1. Bacterial Derivatives
2.2.2. Virus-like Particles
2.2.3. Bacteriophages as Vectors
2.2.4. Liposomes
2.2.5. Nanosomes
2.2.6. Nanoparticles
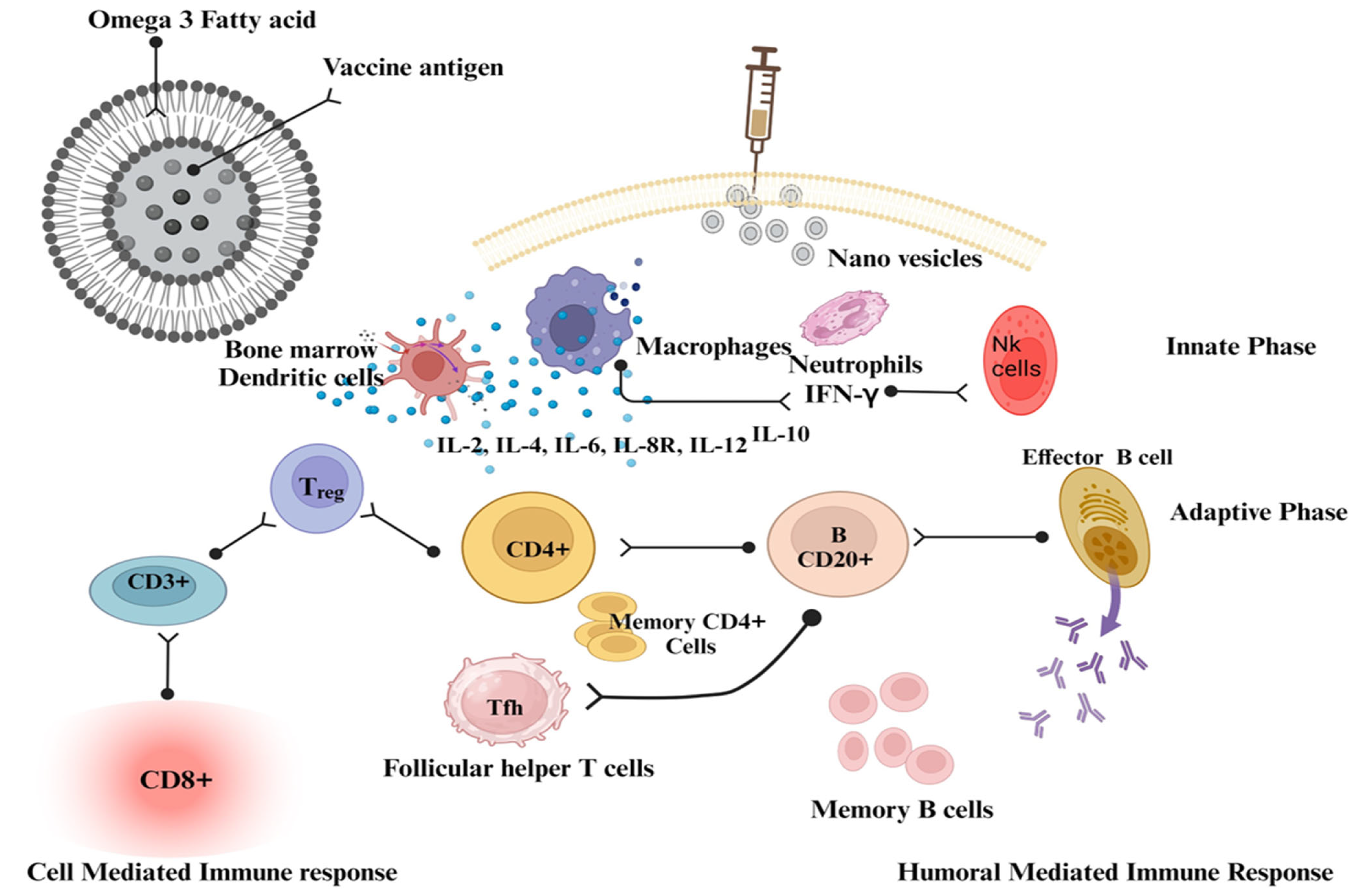
2.2.7. Nanovesicles
3. Future Trends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephan, R. Edward Jenner and the history of smallpox and vaccination. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon, UK, 2005; Volume 18, pp. 21–25. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Amanna, I.J.; Slifka, M.K. Successful Vaccines. In Current Topics in Microbiology and Immunology; Springer Science and Business Media LLC: Berlin, Germany, 2020; Volume 428, pp. 1–30. [Google Scholar] [CrossRef]
- Sivakumar, S.M.; Safhi, M.M.; Kannadasan, M.; Sukumaran, N. Vaccine adjuvants—Current status and prospects on controlled release adjuvancity. Saudi Pharm. J. 2011, 19, 197–206. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Pashine, A.; Valiante, N.M.; Ulmer, J.B. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 2005, 11 (Suppl. S4), S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef]
- Hoebe, K.; Janssen, E.; Beutler, B. The interface between innate and adaptive immunity. Nat. Immunol. 2004, 5, 971–974. [Google Scholar] [CrossRef]
- Teixeira, T.; Kweder, S.L.; Saint-Raymond, A. Are the European Medicines Agency, US Food and Drug Administration, and Other International Regulators Talking to Each Other? Clin. Pharmacol. Ther. 2020, 107, 507–513. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Ramon, G. Sur l’augmentation anormale de l’antitoxine chez les chevaux producteurs de se’rum antidiphte’rique. Bull. Soc. Centr. Med. Vet. 1925, 101, 227. [Google Scholar]
- Ramon, G. Procedes pour accroitre la production des antitoxins. Ann. Inst. Pasteur. 1926, 40, 1–10. [Google Scholar]
- Vogel, F.R. Adjuvants in perspective. Dev. Biol. Stand. 1998, 92, 241–248. [Google Scholar]
- Olafsdottir, T.; Lindqvist, M.; Harandi, A.M. Molecular signatures of vaccine adjuvants. Vaccine 2015, 33, 5302–5307. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Shaw, C.A.; Tomljenovic, L. Aluminum in the central nervous system (CNS): Toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol. Res. 2013, 56, 304–316. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Xu, J. Better Adjuvants for Better Vaccines: Progress in Adjuvant Delivery Systems, Modifications, and Adjuvant–Antigen Codelivery. Vaccines 2020, 8, 128. [Google Scholar] [CrossRef]
- Garçon, N.; Segal, L.; Tavares, F.; Van Mechelen, M. The safety evaluation of adjuvants during vaccine development: The AS04 experience. Vaccine 2011, 29, 4453–4459. [Google Scholar] [CrossRef]
- O’Hagan, D.T. New-Generation Vaccine Adjuvants. In eLS; Wiley: Hoboken, NJ, USA, 2015; pp. 1–7. [Google Scholar] [CrossRef]
- McAleer, W.J.; Buynak, E.B.; Maigetter, R.Z.; Wampler, D.E.; Miller, W.J.; Hilleman, M.R. Human hepatitis B vaccine from recombinant yeast. Nature 1984, 307, 178–180. [Google Scholar] [CrossRef]
- Aguilar, J.C.; Rodríguez, E.G. Vaccine adjuvants revisited. Vaccine 2007, 25, 3752–3762. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Rappuoli, R. Vaccine adjuvants: The dream becomes real. Hum. Vaccines 2008, 4, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, E.B. Mineral adjuvants. In Immunopotentiators in Modern Vaccines; Academic Press: Cambridge, MA, USA, 2006; pp. 217–233. [Google Scholar] [CrossRef]
- Maschmann, E.; Küster, E.; Fischer, W. Über die Fähigkeit des Tonerde-Präparates B, Diphtherie-Toxin zu adsorbieren. Ber. Dtsch. Chem. Ges. 1931, 64, 2174–2178. [Google Scholar] [CrossRef]
- Ericsson, H. Purification and adsorption of diphtheria toxoid. Nature 1946, 158, 350–351. [Google Scholar] [CrossRef]
- Holt, L.B. Purified precipitated diphtheria toxoid of constant composition. Lancet 1947, 1, 282–285. [Google Scholar] [CrossRef]
- Gupta, R.K.; Roast, B.E.; Relyveld, E.; Siber, G.R. Adjuvant properties of aluminum and calcium compounds. In Vaccine Design: The Subunit and Adjuvant Approach; Powell, M.F., Newman, M.F., Eds.; Plenum Publishing Corporation: New York, NY, USA, 1995; pp. 229–248. [Google Scholar] [CrossRef]
- Majgaard Jensen, O.; Koch, C. On the effect of Al(OH)3 as an immunological adjuvant. APMIS 1988, 96, 257–264. [Google Scholar] [CrossRef]
- Exley, C.; Siesjö, P.; Eriksson, H. The immunobiology of aluminium adjuvants: How do they really work? Trends Immunol. 2010, 31, 103–109. [Google Scholar] [CrossRef]
- Marrack, P.; McKee, A.; Munks, M. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009, 9, 287–293. [Google Scholar] [CrossRef]
- Flarend, R.E.; Hem, S.L.; White, J.L.; Elmore, D.; Suckow, M.A.; Rudy, A.C.; Dandashli, E.A. In vivo absorption of aluminium-containing vaccine adjuvants using 26Al. Vaccine 1997, 15, 1314–1318. [Google Scholar] [CrossRef]
- Lindblad, E.B.; Spräck, J.V. Basic Concepts in the Application of Immunological Adjuvants. Scand. J. Lab. Anim. Sci. 1987, 14. [Google Scholar] [CrossRef]
- Baylor, N.W.; Egan, W.; Richman, P. Aluminum salts in vaccines—US perspective. Vaccine 2002, 20, S18–S23. [Google Scholar] [CrossRef] [PubMed]
- Volk, V.K.; Bunney, W.E. Diphtheria Immunization with Fluid Toxoid and Alum Precipitated Toxoid—Preliminary Report. Am. J. Public Health Nations Health 1939, 29, 197–204. [Google Scholar] [CrossRef]
- Volk, V.K.; Bunney, W.E. Diphtheria Immunization with Fluid Toxoid and Alum-Precipitated Toxoid. Am. J. Public Health Nations Health 1942, 32, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.R.; Voyce, M.A.; Burland, W.L.; Marjorie, L.H. Advantages of Aluminium Hydroxide Adsorbed Combined Diphtheria, Tetanus, And Pertussis Vaccines For The Immunization of Infants. Br. Med. J. 1969, 1, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Straw, B.E.; MacLachlan, N.J.; Corbett, W.T.; Carter, P.B.; Schey, H.M. Comparison of tissue reactions produced by Haemophilus pleuropneumoniae vaccines made with six different adjuvants in swine. Can. J. Comp. Med. 1985, 49, 149–151. [Google Scholar] [PubMed]
- Men, Y.; Thomasin, C.; Merkle, H.P.; Gander, B.; Corradin, G. A single administration of tetanus toxoid in biodegradable microspheres elicits T cell and antibody responses similar or superior to those obtained with aluminum hydroxide. Vaccine 1995, 13, 683–709. [Google Scholar] [CrossRef]
- Walls, R.S. Eosinophil response to alum adjuvants: Involvement of T cells in non-antigen-dependent mechanisms. Proc. Soc. Exp. Biol. Med. 1977, 156, 431–435. [Google Scholar] [CrossRef]
- Nagel, J.; Svec, D.; Waters, T.; Fireman, P. IgE synthesis in man. I. Development of specific IgE antibodies after immunization with tetanus–diphtheria (Td) toxoids. J. Immunol. 1977, 118, 334–341. [Google Scholar] [CrossRef]
- Djurisic, S.; Jakobsen, J.C.; Petersen, S.B.; Kenfelt, M.; Klingenberg, S.L.; Gluud, C. Aluminium adjuvants used in vaccines. Cochrane Database Syst. Rev. 2018, 2018, CD013086. [Google Scholar] [CrossRef]
- Goto, N.; Kato, H.; Maeyama, J.-I.; Eto, K.; Yoshihara, S. Studies on the toxicities of aluminium hydroxide and calcium phosphate as immunological adjuvants for vaccines. Vaccine 1993, 11, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; Aguilar, J.C. Vaccine adjuvants: Current state and future trends. Immuology Cell Biol. 2004, 82, 488–496. [Google Scholar] [CrossRef] [PubMed]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. NPJ Vaccines 2018, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Davenport, F.M. Seventeen years’ experience with mineral oil adjuvant influenza virus vaccines. Ann. Allergy 1968, 26, 288–292. [Google Scholar]
- Ulmer, J.B.; DeWitt, C.M.; Chastain, M.; Friedman, A.; Donnelly, J.J.; McClements, W.L.; Caulfield, M.J.; Bohannon, K.E.; Volkin, D.B.; Evans, R.K. Enhancement of DNA vaccine potency using conventional aluminum adjuvants. Vaccine 1999, 18, 18–28. [Google Scholar] [CrossRef]
- Kwissa, M.; Lindblad, E.B.; Schirmbeck, R.; Reimann, J. Codelivery of a DNA vaccine and a protein vaccine with aluminum phosphate stimulates a potent and multivalent immune response. J. Mol. Med. 2003, 81, 502–510. [Google Scholar] [CrossRef]
- Alving, C.R.; Peachman, K.K.; Rao, M.; Reed, S.G. Adjuvants for human vaccines. Curr. Opin. Immunol. 2012, 24, 310–315. [Google Scholar] [CrossRef]
- Warren, H.S.; Chedid, L.A. Future prospects for vaccine adjuvants. Crit. Rev. Immunol. 1988, 8, 83–101. [Google Scholar]
- Morefield, G.L.; Sokolovska, A.; Jiang, D.; HogenEsch, H.; Robinson, J.P.; Hem, S.L. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 2005, 23, 1588–1595. [Google Scholar] [CrossRef]
- Brewer, J.M.; Conacher, M.; Hunter, C.A.; Mohrs, M.; Brombacher, F.; Alexander, J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J. Immunol. 1999, 163, 6448–6454. [Google Scholar] [CrossRef]
- Mody, K.T.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale 2013, 5, 5167–5179. [Google Scholar] [CrossRef]
- Relyveld, E.H. Preparation and use of calcium phosphate adsorbed vaccines. Dev. Biol. Stand. 1986, 65, 131–136. [Google Scholar]
- Relyveld, E.H. Calcium Phosphate Gel for Adsorbing Vaccines. U.S. Patent US4016252A, 5 April 1977. Available online: https://patents.google.com/patent/US4016252A/en (accessed on 5 November 2023).
- Meeusen, E.N.; Walker, J.; Peters, A.; Pastoret, P.P.; Jungersen, G. Current status of veterinary vaccines. Clin. Microbiol. Rev. 2007, 20, 489–510. [Google Scholar] [CrossRef]
- Relyveld, E.H.; Bizzini, B.; Gupta, R.K. Rational approaches to reduce adverse reactions in man to vaccines containing tetanus and diphtheria toxoids. Vaccine 1998, 16, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, T.L. Aluminium phosphate but not calcium phosphate stimulates the specific IgE response in guinea pigs to tetanus toxoid. Allergy 1978, 33, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ickovic, M.-R.; Relyveld, E.H.; Hénocq, E.; David, B.; Marie, F.N. Calcium-phosphate-adjuvanted allergens: Total and specific IgE levels before and after immunotherapy with house dust and Dermatophagoides pteronyssinus extracts. Ann. Immunol. 1983, 134, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Relyveld, E.H.; Ickovic, M.R.; Hénocq, E.; Garcelon, M. Calcium phosphate adjuvanted allergens. Ann. Allergy 1985, 54, 521–529. [Google Scholar] [PubMed]
- Coursaget, P.; Yvonnet, B.; Relyveld, E.H.; Barres, J.L.; Diop-Mar, I.; Chiron, J.P. Simultaneous administration of diphtheria/tetanus/pertussis/polio vaccine and hepatitis B vaccine in a simplified immunization programme. Dev. Biol. Stand. 1986, 65, 169–175. [Google Scholar]
- Jiang, D.; Premachandra, G.S.; Johnston, C.; Hem, S.L. Structure and adsorption properties of commercial calcium phosphate adjuvant. Vaccine 2004, 23, 693–698. [Google Scholar] [CrossRef]
- Matheis, W.; Zott, A.; Schwanig, M. The role of the adsorption process for production and control combined adsorbed vaccines. Vaccine 2001, 20, 67–73. [Google Scholar] [CrossRef]
- Olmedo, H.; Herrera, M.; Rojas, L.; Villalta, M.; Vargas, M.; Leiguez, E.; Teixeira, C.; Estrada, R.; Gutiérrez, J.M.; León, G.; et al. Comparison of the adjuvant activity of aluminum hydroxide and calcium phosphate on the antibody response towards Bothrops asper snake venom. J. Immunotoxicol. 2014, 11, 44–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Relyveld, E.; Chermann, J.C. Humoral response in rabbits immunized with calcium phosphate adjuvanted HIV-1 gp160 antigen. Biomed. Pharmacother. 1994, 48, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.-D.; Thibaudon, M.; Bélec, L.; Crépeaux, G. Calcium phosphate: A substitute for aluminum adjuvants? Expert Rev. Vaccines 2017, 16, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Rivera Gil, P.; Hühn, D.; del Mercato, L.L.; Sasse, D.; Parak, W.J. Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacol. Res. 2010, 20, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Amini, Y.; Moradi, B.; Tafaghodi, M.; Meshkat, Z.; Ghazvini, K.; Fasihi-Ramandi, M. TB trifusion antigen adsorbed on calcium phosphate nanoparticles stimulates strong cellular immunity in mice. Biotechnol. Bioproc. 2016, 21, 653–658. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A comprehensive review on emulsions and emulsion stability in chemical and energy industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef]
- Shah, R.R.; Brito, L.A.; O’Hagan, D.T.; Amiji, M.M. Emulsions as Vaccine Adjuvants. In Subunit Vaccine Delivery; Advances in Delivery Science and Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 59–76. [Google Scholar] [CrossRef]
- Le Moignic, P.; Pinoy, C.R. Les vaccins en emulsion dans les corps gras ou “lipo-vaccins. Comptes Rendus de la Societe de Biologie. Comptes Rendus Soc. Biol. 1916, 79, 201–203. [Google Scholar]
- Freund, J.; Casals, J.; Hosmer, E.P. Sensitization and Antibody Formation after Injection of Tubercle Bacilli and Paraffin Oil. Proc. Soc. Exp. Biol. Med. 1937, 37, 509–513. [Google Scholar] [CrossRef]
- Freund, J.; Thomson, K.J.; Hough, H.B.; Sommer, H.E.; Pisani, T.M. Antibody formation and sensitization with the aid of adjuvants. J. Immunol. 1948, 60, 383–398. [Google Scholar] [CrossRef]
- Freund, J. The mode of action of immunologic adjuvants. Bibl. Tuberc. 1956, 10, 130–148. [Google Scholar]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Saul, A.; Mahanty, S. Revisiting Freund’s incomplete adjuvant for vaccines in the developing world. Trends Parasitol. 2005, 21, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.P.; McClellan, H.A.; Rausch, K.M.; Zhu, D.; Whitmore, M.D.; Singh, S.; Laura, B.M.; Wu, Y.; Giersing, B.K.; Anthony, W.S.; et al. Montanide ISA 720 vaccines: Quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccines 2005, 23, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, E.; Liu, H.; Huckriede, A.; Hak, E. Safety and tolerability evaluation of the use of Montanide ISA™51 as vaccine adjuvant: A systematic review. Hum. Vaccines Immunother. 2016, 12, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Aucouturier, J.; Dupuis, L.; Deville, S.; Ascarateil, S.; Ganne, V. Montanide ISA 720 and 51: A new generation of water in oil emulsions as adjuvants for human vaccines. Expert. Rev. Vaccines 2002, 1, 111–118. [Google Scholar] [CrossRef]
- Smith, J.W.G.; Fletcher, W.B.; Peters, M.; Westwood, M.; Perkins, F.J. Response to influenza vaccine in adjuvant 65-4. J. Hyg. 1975, 74, 251–259. [Google Scholar] [CrossRef]
- Weibel, R.E.; McLean, A.; Woodhour, A.F.; Friedman, A.; Hilleman, M.R. Ten-year follow-up study for safety of adjuvant 65 influenza vaccine in man. Proc. Soc. Exp. Biol. Med. 1973, 143, 1053–1056. [Google Scholar] [CrossRef]
- Ko, E.-J.; Lee, Y.-T.; Kim, K.-H.; Jung, Y.-J.; Lee, Y.; Denning, T.L.; Kang, S.-M. Effects of MF59 Adjuvant on Induction of Isotype-Switched IgG Antibodies and Protection after Immunization with T-Dependent Influenza Virus Vaccine in the Absence of CD4+ T Cells. J. Virol. 2016, 90, 6976–6988. [Google Scholar] [CrossRef]
- Mastelic Gavillet, B.; Eberhardt, C.S.; Auderset, F.; Castellino, F.; Seubert, A.; Tregoning, J.S.; Lambert, P.H.; de Gregorio, E.; Del Giudice, G.; Siegrist, C.-A. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J. Immunol. 2015, 194, 4836–4845. [Google Scholar] [CrossRef]
- Singh, M.; Mildred, U.; Jina, K.; James, C.; Elawati, S.; Donatella, M.; Francesca, T.; Mario, C.; Gianfranco, V.; Giuseppe, D.G.; et al. A preliminary evaluation of alternative adjuvants to alum using a range of established and new generation vaccine antigens. Vaccine 2006, 24, 1680–1686. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Wilson, K.L.; Xiang, S.D.; Plebanski, M. Inflammatory/noninflammatory adjuvants and nanotechnology—The secret to vaccine design. In Micro and Nanotechnology in Vaccine Development; William Andrew: Norwich, NY, USA, 2017; pp. 99–125. [Google Scholar] [CrossRef]
- Ko, E.-J.; Kang, S.-M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccines Immunother. 2018, 14, 3041–3045. [Google Scholar] [CrossRef] [PubMed]
- Kommareddy, S.; Singh, M.; O’Hagan, D.T. MF59. In Immunopotentiators in Modern Vaccines; Academic Press: Cambridge, MA, USA, 2017; pp. 249–263. [Google Scholar] [CrossRef]
- Morein, B.; Sundquist, B.; Höglund, S.; Dalsgaard, K.; Osterhaus, A.I. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 1984, 308, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Kensil, C.R. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 1996, 13, 1–55. [Google Scholar] [PubMed]
- Filipić, B.; Pantelić, I.; Nikolić, I.; Majhen, D.; Stojić-Vukanić, Z.; Savić, S.; Krajišnik, D. Nanoparticle-Based Adjuvants and Delivery Systems for Modern Vaccines. Vaccines 2023, 11, 1172. [Google Scholar] [CrossRef]
- Singh, M.; O’Hagan, D.T. Recent advances in veterinary vaccine adjuvants. Int. J. Parasitol. 2003, 33, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Kensil, C.R.; Patel, U.; Lennick, M.; Marciani, D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 1991, 146, 431–437. [Google Scholar] [CrossRef]
- Skountzou, I.; Brock, N.; Lelutiu, N.; Compans, R.W. Adjuvants for Skin Vaccination. In Immunopotentiators in Modern Vaccines; Academic Press: Cambridge, MA, USA, 2017; pp. 399–419. [Google Scholar] [CrossRef]
- Kensil, C.R.; Kammer, R. QS-21: A water-soluble triterpene glycoside adjuvant. Expert Opin. Investig. Drugs 1998, 7, 1475–1482. [Google Scholar] [CrossRef]
- Sun, H.-X.; Xie, Y.; Ye, Y.-P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef]
- Song, X.; Hu, S. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine 2009, 27, 4883–4890. [Google Scholar] [CrossRef]
- Katayama, S.; Oda, K.; Ohgitani, T.; Hirahara, T.; Shimizu, Y. Influence of antigenic forms and adjuvants on the IgG subclass antibody response to Aujeszky’s disease virus in mice. Vaccine 1999, 17, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Marciani, D.J.; Kensil, C.R.; Beltz, G.A.; Hung, C.-H.; Cronier, J.; Aubert, A. Genetically-engineered subunit vaccine against feline leukaemia virus: Protective immune response in cats. Vaccine 1991, 9, 89–96. [Google Scholar] [CrossRef]
- Garçon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- The RTSS Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- Chlibek, R.; Pauksens, K.; Rombo, L.; van Rijckevorsel, G.; Richardus, J.H.; Plassmann, G.; Schwarz, T.F.; Catteau, G.; Lal, H.; Heineman, T.C. Long-term immunogenicity and safety of an investigational herpes zoster subunit vaccine in older adults. Vaccine 2016, 34, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, I.; Forgus, S.; De Boever, F.; Clement, F.; Demoitié, M.A.; Mettens, P.; Moris, P.; Ledent, E.; Leroux-Roels, G.; Ofori-Anyinam, O. M72 Study Group. Improved CD4+ T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: A randomized trial. Vaccine 2013, 31, 2196–2206. [Google Scholar] [CrossRef]
- Garçon, N.; Di Pasquale, A. From discovery to licensure, the Adjuvant System story. Hum. Vaccines Immunother. 2017, 13, 19–33. [Google Scholar] [CrossRef]
- Leleux, J.; Roy, K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: An immunological and materials perspective. Adv. Healthc. Mater. 2013, 2, 72–94. [Google Scholar] [CrossRef]
- Garçon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert. Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Camille, P.; Elouahabi, A.; Pol, H.; Harald, C.; et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef] [PubMed]
- Beran, J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients. Expert Opin. Biol. Ther. 2008, 8, 235–247. [Google Scholar] [CrossRef]
- Mbow, M.L.; De Gregorio, E.; Valiante, N.M.; Rappuoli, R. New adjuvants for human vaccines. Curr. Opin. Immunol. 2010, 22, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Collignon, C.; Bourguignon, P.; Wouters, S.; Fierens, K.; Fochesato, M.; Najoua, D.; Christelle, L.; Bernard, M.; Bart, N.L.; et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J. Immunol. 2014, 193, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; Romanowski, B. AS04-adjuvanted human papillomavirus (HPV) types 16 and 18 vaccine (Cervarix®): A review of its use in the prevention of premalignant cervical lesions and cervical cancer causally related to certain oncogenic HPV types. Drugs 2011, 71, 465–488. [Google Scholar]
- Garçon, N.; Morel, S.; Didierlaurent, A.; Descamps, D.; Wettendorff, M.; Van Mechelen, M. Development of an AS04-Adjuvanted HPV Vaccine with the Adjuvant System Approach. BioDrugs 2011, 25, 217–226. [Google Scholar] [CrossRef]
- Fabrizi, F.; Tarantino, A.; Castelnovo, C.; Martin, P.; Messa, P. Recombinant Hepatitis B Vaccine Adjuvanted With AS04 in Dialysis Patients: A Prospective Cohort Study. Kidney Blood Press. Res. 2015, 40, 584–592. [Google Scholar] [CrossRef]
- Garçon, N. Preclinical development of AS04. Methods Mol. Biol. 2010, 626, 15–27. [Google Scholar] [CrossRef]
- Ayub, M.A.; Bacci, M.R.; Fonseca, F.L.A.; Chehter, E.Z. Hemodialysis and hepatitis B vaccination: A challenge to physicians. Int. J. Gen. Med. 2014, 7, 109–114. [Google Scholar] [CrossRef]
- Cheng, E.; Cárdenas-Freytag, L.; Clements, J.D. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine 1999, 18, 38–49. [Google Scholar] [CrossRef]
- Walker, R.I. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine 1994, 12, 387–400. [Google Scholar] [CrossRef] [PubMed]
- McGhee, J.R.; Mestecky, J.; Dertzbaugh, M.T.; Eldridge, J.H.; Hirasawa, M.; Kiyono, H. The mucosal immune system: From fundamental concepts to vaccine development. Vaccine 1992, 10, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Cantó, E.; Moga, E.; Ricart, E.; Garcia-Bosch, O.; Garcia-Planella, E.; Juarez, C.; Vidal, S. MDP-Induced selective tolerance to TLR4 ligands: Impairment in NOD2 mutant Crohn’s disease patients. Inflamm. Bowel Dis. 2009, 15, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Audibert, F.M.; Lise, L.D. Adjuvants: Current status, clinical perspectives and future prospects. Immunol. Today 1993, 14, 281–284, Erratum in Immunol. Today 2008, 29, 149. [Google Scholar] [CrossRef]
- Zariri, A.; van der Ley, P. Biosynthetically engineered lipopolysaccharide as vaccine adjuvant. Expert Rev. Vaccines 2015, 14, 861–876. [Google Scholar] [CrossRef]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszyński, A.; et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Thompson, B.S.; Chilton, P.M.; Ward, J.R.; Evans, J.T.; Mitchell, T.C. The low-toxicity versions of LPS, MPL adjuvant and RC529, are efficient adjuvants for CD4+ T cells. J. Leukoc. Biol. 2005, 78, 1273–1280. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Yoshimatsu, K.; Koike, Y.; Hatsuse, R.; Yamanishi, K.; Tanishita, O.; Arikawa, J.; Azuma, I. Adjuvant activity of muramyl dipeptide derivatives to enhance immunogenicity of a hantavirus-inactivated vaccine. Vaccine 1998, 16, 216–224. [Google Scholar] [CrossRef]
- Johnson, D.A.; Keegan, D.S.; Sowell, C.G.; Livesay, M.T.; Johnson, C.L.; Taubner, L.M.; Harris, A.; Kent, R.M.; Jennifer, D.T.; Gary, L.G.; et al. 3-O-Desacyl monophosphoryl lipid A derivatives: Synthesis and immunostimulant activities. J. Med. Chem. 1999, 42, 4640–4649. [Google Scholar] [CrossRef]
- Hajam, I.A.; Dar, P.A.; Won, G.; John Hwa, L. Bacterial ghosts as adjuvants: Mechanisms and potential. Vet. Res. 2017, 48, 37. [Google Scholar] [CrossRef]
- Witte, A.; Wanner, G.; Bläsi, U.; Halfmann, G.; Szostak, M.; Lubitz, W. Endogenous transmembrane tunnel formation mediated by phi X174 lysis protein E. J. Bacteriol. 1990, 172, 4109–4114. [Google Scholar] [CrossRef]
- Huter, V.; Szostak, M.P.; Gampfer, J.; Prethaler, S.; Wanner, G.; Gabor, F.; Lubitz, W. Bacterial ghosts as drug carrier and targeting vehicles. J. Control. Release 1999, 61, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Harisa, G.I.; Sherif, A.Y.; Youssof, A.M.; Fars, K.A.; Salem-Bekhit, M.M. Bacteriosomes as a Promising Tool in Biomedical Applications: Immunotherapy and Drug Delivery. AAPS PharmSciTech 2020, 21, 168. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J. Control. Release 2020, 323, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Alaniz, R.C.; Deatherage, B.L.; Lara, J.C.; Cookson, B.T. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 2007, 179, 7692–7701. [Google Scholar] [CrossRef] [PubMed]
- Daleke-Schermerhorn, M.H.; Felix, T.; Soprova, Z.; Ten Hagen-Jongman, C.M.; Vikström, D.; Majlessi, L.; Joep, B.; Frank, F.; Karin de, P.; van der Wel, N.N.; et al. Decoration of outer membrane vesicles with multiple antigens by using an autotransporter approach. Appl. Environ. Microbiol. 2014, 80, 5854–5865. [Google Scholar] [CrossRef]
- Eko, F.O.; Lubitz, W.; McMillan, L.; Ramey, K.; Moore, T.T.; Ananaba, G.A.; Deborah, L.; Carolyn, M.B.; Joseph, U.I. Recombinant Vibrio cholerae ghosts as a delivery vehicle for vaccinating against Chlamydia trachomatis. Vaccine 2003, 21, 1694–1703. [Google Scholar] [CrossRef]
- Riedmann, E.M.; Kyd, J.M.; Smith, A.M.; Gomez-Gallego, S.; Jalava, K.; Cripps, A.W.; Lubitz, W. Construction of recombinant S-layer proteins (rSbsA) and their expression in bacterial ghosts—A delivery system for the nontypeableHaemophilus influenzae antigen Omp26. FEMS Immunol. Med. Microbiol. 2003, 37, 185–192. [Google Scholar] [CrossRef]
- Kim, C.S.; Hur, J.; Eo, S.K.; Park, S.Y.; Lee, J.H. Generation of Salmonella ghost cells expressing fimbrial antigens of enterotoxigenic Escherichia coli and evaluation of their antigenicity in a murine model. Can. J. Vet. Res. 2016, 80, 40–48. [Google Scholar]
- Gong, S.; Nan, N.; Sun, Y.; He, Z.; Li, J.; Chen, F.; Li, T.; Ning, N.; Wang, J.; Li, Z.; et al. Protective Immunity Elicited by VP1 Chimeric Antigens of Bacterial Ghosts against Hand-Foot-and-Mouth Disease Virus. Vaccines 2020, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Mizel, S.B.; Bates, J.T. Flagellin as an adjuvant: Cellular mechanisms and potential. J. Immunol. 2010, 185, 5677–5682. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, Y.; Yun, N.E.; Poussard, A.L.; Smith, J.N.; Smith, J.K.; Viktoriya, B.; Jenna, J.L.; Michele, A.Z.; Hong, L.; et al. Superior efficacy of a recombinant flagellin:H5N1 HA globular head vaccine is determined by the placement of the globular head within flagellin. Vaccine 2009, 27, 5875–5884. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.J.; Taylor, D.N.; Tussey, L.; Hay, C.; Nolan, C.; Fitzgerald, T.; Liu, G.; Uma, K.; Langzhou, S.; Irving, D.; et al. Safety and immunogenicity of a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125) in healthy young adults. Vaccine 2010, 28, 8268–8274. [Google Scholar] [CrossRef]
- Mani, R.; Gupta, M.; Malik, A.; Tandon, R.; Prasad, R.; Bhatnagar, R.; Banerjee, N. Adjuvant Potential of Poly-α-l-Glutamine from the Cell Wall of Mycobacterium tuberculosis. Infect. Immun. 2018, 86, e00537-18. [Google Scholar] [CrossRef]
- Rao, M.; Cadieux, N.; Fitzpatrick, M.; Reed, S.; Arsenian, S.; Valentini, D.; Parida, S.; Dodoo, E.; Zumla, A.; Maeurer, M. Mycobacterium tuberculosis proteins involved in cell wall lipid biosynthesis improve BCG vaccine efficacy in a murine TB model. Int. J. Infect. Dis. 2017, 56, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Vilander, A.C.; Dean, G.A. Adjuvant strategies for lactic acid bacterial mucosal vaccines. Vaccines 2019, 7, 150. [Google Scholar] [CrossRef]
- LeCureux, J.S.; Dean, G.A. Lactobacillus Mucosal Vaccine Vectors: Immune Responses against Bacterial and Viral Antigens. mSphere 2018, 3, e00061-18. [Google Scholar] [CrossRef]
- Back, Y.W.; Choi, S.; Choi, H.-G.; Shin, K.-W.; Son, Y.-J.; Paik, T.-H.; Hwa-Jung, K. Cell wall skeleton of Mycobacterium bovis BCG enhances the vaccine potential of antigen 85B against tuberculosis by inducing Th1 and Th17 responses. PLoS ONE 2019, 14, e0213536. [Google Scholar] [CrossRef]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert. Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Lentino, J.; Kopp, J.; Murray, L.; Ellison, W.; Rhee, M.; Gerald, S.; Lalith, A.; Kimberly, E.; William, L.H.; et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine 2018, 36, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.; Cooper, C. Review of hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine safety and efficacy. Expert Opin. Biol. Ther. 2007, 7, 1731–1737. [Google Scholar] [CrossRef]
- Kuo, T.Y.; Lin, M.Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.J.; Liu, L.T.C.; Cheng, J.; Wu, Y.C.; Wu, C.C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.M.; El-Shal, A.S.; Elbehidy, R.M.; Fouda, M.A.; Shalaby, S.M.; El Hawy, L.L.; Elsadek, A.F.; Neemat-Allah, M.A.A.; Ramadan, S.M.; Gohary, A.; et al. Hepatitis B Immunization Status in Children with Chronic Kidney Disease: Experience at a Single Center, Egypt. J. Clin. Med. 2023, 12, 1864. [Google Scholar] [CrossRef]
- Schiller, J.T.; Müller, M. Next generation prophylactic human papillomavirus vaccines. Lancet Oncol. 2015, 16, e217–e225. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, M.; Pan, H.; Lin, Z.; Wang, K.; Weng, Z.; Yibin, Z.; Lu, X.; Jun, Z.; Li, S.; et al. Robust manufacturing and comprehensive characterization of recombinant hepatitis E virus-like particles in Hecolin®. Vaccines 2014, 32, 4039–4050. [Google Scholar] [CrossRef]
- Herzog, C.; Hartmann, K.; Künzi, V.; Kürsteiner, O.; Mischler, R.; Lazar, H.; Glück, R. Eleven years of Inflexal V—A virosomal adjuvanted influenza vaccine. Vaccine 2009, 27, 4381–4387. [Google Scholar] [CrossRef]
- Ho, J.K.-T.; Jeevan-Raj, B.; Netter, H.-J. Hepatitis B Virus (HBV) Subviral Particles as Protective Vaccines and Vaccine Platforms. Viruses 2020, 12, 126. [Google Scholar] [CrossRef]
- Müller, H.; Fehling, S.K.; Dorna, J.; Urbanowicz, R.A.; Oestereich, L.; Krebs, Y.; Larissa, K.; Martin, S.; Verena, K.; N’Faly, M.; et al. Adjuvant formulated virus-like particles expressing native-like forms of the Lassa virus envelope surface glycoprotein are immunogenic and induce antibodies with broadly neutralizing activity. NPJ Vaccines 2020, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Jandus, C.; Maurer, P.; Hammann-Haenni, A.; Schwarz, K.; Bachmann, M.F.; Daniel, E.S.; Romero, P. Virus-like particles induce robust human T-helper cell responses. Eur. J. Immunol. 2012, 42, 330–340. [Google Scholar] [CrossRef]
- Upasani, V.; Rodenhuis-Zybert, I.; Cantaert, T. Antibody-independent functions of B cells during viral infections. PLoS Pathogens 2021, 17, e1009708. [Google Scholar] [CrossRef]
- Tak, W.M.; Mary, E.S. 23—Vaccines and Clinical Immunization. In The Immune Response; Tak, W., Mak, M., Saunders, E., Eds.; Academic Press: Cambridge, MA, USA, 2006; pp. 695–749. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. ‘World in motion’–emulsion adjuvants rising to meet the pandemic challenges. Npj Vaccines 2021, 6, 158. [Google Scholar] [CrossRef]
- Ojha, R.; Pandey, R.K.; Prajapati, V.K. Vaccine delivery systems against tuberculosis. In Nanotechnology Based Approaches for Tuberculosis Treatment; Elsevier Science: Amsterdam, The Netherlands, 2020; pp. 75–90. [Google Scholar] [CrossRef]
- Raska, M.; Turanek, J. DNA vaccines for the induction of immune responses in mucosal tissues. In Mucosal Immunology; Academic Press: Cambridge, MA, USA, 2015; pp. 1307–1335. [Google Scholar] [CrossRef]
- Kammer, A.R.; Amacker, M.; Rasi, S.; Westerfeld, N.; Gremion, C.; Neuhaus, D.; Zurbriggen, R. A new and versatile virosomal antigen delivery system to induce cellular and humoral immune responses. Vaccine 2007, 25, 7065–7074. [Google Scholar] [CrossRef]
- Cusi, M.G.; Zurbriggen, R.; Valassina, M.; Bianchi, S.; Durrer, P.; Valensin, P.E.; Donati, M.; Glück, R. Intranasal immunization with mumps virus DNA vaccine delivered by influenza virosomes elicits mucosal and systemic immunity. Virology 2000, 277, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Müller, M.; Kaeser, M.D.; Weydemann, U.; Amacker, M. Influenza virosomes as vaccine adjuvant and carrier system. Expert Rev. Vaccines 2013, 12, 779–791. [Google Scholar] [CrossRef]
- van der Velden, Y.U.; Grobben, M.; Caniels, T.G.; Burger, J.A.; Poniman, M.; Oomen, M.; Esther Siteur-van, R.; Khadija, T.; Denise, G.; Ronald, K.; et al. A SARS-CoV-2 Wuhan spike virosome vaccine induces superior neutralization breadth compared to one using the Beta spike. Sci. Rep. 2022, 12, 3884. [Google Scholar] [CrossRef]
- Weibel, R.E.; Woodhour, A.F.; Stokes, J., Jr.; Friedman, A.; McAleer, W.J.; Hilleman, M.R. New metabolizable immunologic adjuvant for human use. Am. J. Med. 1970, 48, 464–471. [Google Scholar] [CrossRef]
- Irving, M.B.; Pan, O.; Scott, J.K. Random-peptide libraries and antigen-fragment libraries for epitope mapping and the development of vaccines and diagnostics. Curr. Opin. Chem. Biol. 2001, 5, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Morris, C.; Brumlik, M.; Landry, S.J.; Finstad, K.; Nelson, A.; Virendra, J.; Christopher, H.; Xavier, A.; Andrew, L.; et al. Peptides identified through phage display direct immunogenic antigen to dendritic cells. J. Immunol. 2004, 172, 7425–7431. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Yu, M. Epitope identification and discovery using phage display libraries: Applications in vaccine development and diagnostics. Curr. Drug Targets 2004, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bastien, N.; Trudel, M.; Simard, C. Protective immune responses induced by the immunization of mice with a recombinant bacteriophage displaying an epitope of the human respiratory syncytial virus. Virology 1997, 234, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Tretiakova, D.S.; Vodovozova, E.L. Liposomes as adjuvants and vaccine delivery systems. Biochem. Suppl. Ser. A Membr. Cell Biol. 2022, 16, 1–20. [Google Scholar] [CrossRef]
- Agallou, M.; Margaroni, M.; Tsanaktsidou, E.; Badounas, F.; Kammona, O.; Kiparissides, C.; Karagouni, E. A liposomal vaccine promotes strong adaptive immune responses via dendritic cell activation in draining lymph nodes. J. Control. Release 2023, 356, 386–401. [Google Scholar] [CrossRef]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.-M. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine 2014, 10, 775–782. [Google Scholar] [CrossRef]
- Li, T.; Zehner, M.; He, J.; Próchnicki, T.; Horvath, G.; Latz, E.; Burgdorf, S.; Takeoka, S. NLRP3 inflammasome-activating arginine-based liposomes promote antigen presentations in dendritic cells. Int. J. Nanomed. 2019, 14, 3503–3516. [Google Scholar] [CrossRef]
- Li, T.; He, J.; Horvath, G.; Prochnicki, T.; Latz, E.; Takeoka, S. Lysine-containing cationic liposomes activate the NLRP3 inflammasome: Effect of a spacer between the head group and the hydrophobic moieties of the lipids. Nanomedicine 2017, 4, 279–288. [Google Scholar] [CrossRef]
- He, J.; Li, T.; Próchnicki, T.; Horvath, G.; Latz, E.; Takeoka, S. Membrane fusogenic lysine type lipid assemblies possess enhanced NLRP3 inflammasome activation potency. Biochem. Biophys. Rep. 2019, 18, 100623. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef] [PubMed]
- Shields, B.; Mills, J.; Ghildyal, R.; Gooley, P.; Meanger, J. Multiple heparin binding domains of respiratory syncytial virus G mediate binding to mammalian cells. Arch. Virol. 2003, 148, 1987–2003. [Google Scholar] [CrossRef] [PubMed]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.N.; Rito-Palomares, M.; Benavides, J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Gill, P. Nanocarriers, nanovaccines, and nanobacteria as nanobiotechnological concerns in modern vaccines. Scientia Iranica 2013, 20, 1003–1013. [Google Scholar]
- Marasini, N.; Ghaffar, K.A.; Skwarczynski, M.; Toth, I. Liposomes as a Vaccine Delivery System. In Micro and Nanotechnology in Vaccine Development; William Andrew: Norwich, NY, USA, 2017; pp. 221–239. [Google Scholar] [CrossRef]
- Karunakaran, B.; Gupta, R.; Patel, P.; Salave, S.; Sharma, A.; Desai, D.; Benival, D.; Kommineni, N. Emerging Trends in Lipid-Based Vaccine Delivery: A Special Focus on Developmental Strategies, Fabrication Methods, and Applications. Vaccines 2023, 11, 661. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Bramwell, V.W.; Christensen, D.; Agger, E.-M.; Andersen, P.; Perrie, Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J. Control. Release 2010, 142, 180–186. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Attia, M.A.; Essa, E.A.; Elebyary, T.T.; Faheem, A.M.; Elkordy, A.A. Brief on Recent Application of Liposomal Vaccines for Lower Respiratory Tract Viral Infections: From Influenza to COVID-19 Vaccines. Pharmaceuticals 2021, 14, 1173. [Google Scholar] [CrossRef]
- Foldvari, M. Biphasic vesicles: A novel topical drug delivery system. J. Biomed. Nanotechnol. 2010, 6, 543–557. [Google Scholar] [CrossRef]
- Hart, S.L. Lipid carriers for gene therapy. Curr. Drug Deliv. 2005, 2, 423–428. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jarrahian, C.; Zehrung, D.; Mitragotri, S.; Prausnitz, M.R. Delivery Systems for Intradermal Vaccination. In Intradermal Immunization; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–112. [Google Scholar] [CrossRef]
- Bose, R.J.; Kim, M.; Chang, J.H.; Ramasamy, P.; James, J.M.; Won-Gun, K.; Soo-Hong, L.; Hansoo, P. Biodegradable polymers for modern vaccine development. J. Ind. Eng. Chem. 2019, 77, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Lin, S.-J.; Yang, Y.-C.; Wang, D.-Y.; Cheng, H.-F.; Yeh, M.-K. Biodegradable polymeric microsphere-based vaccines and their applications in infectious diseases. Hum. Vaccines Immunother. 2015, 11, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Grego, E.A.; Siddoway, A.C.; Uz, M.; Liu, L.; Christiansen, J.C.; Ross, K.A.; Sean, M.K.; Surya, K.M.; Michael, J.W.; Balaji, N. Polymeric nanoparticle-based vaccine adjuvants and delivery vehicles. Curr. Top. Microbiol. Immunol. 2021, 433, 29–76. [Google Scholar] [CrossRef]
- Chesson, C.B.; Zloza, A. Nanoparticles: Augmenting tumor antigen presentation for vaccine and immunotherapy treatments of cancer. Nanomedicine 2017, 12, 2693–2706. [Google Scholar] [CrossRef]
- Mantegazza, A.R.; Magalhaes, J.G.; Amigorena, S.; Marks, M.S. Presentation of phagocytosed antigens by MHC class I and II. Traffic 2013, 14, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Picece, V.C.; Ou, B.S.; Luo, W.; Pulendran, B.; Appel, E.A. Designing spatial and temporal control of vaccine responses. Nat. Rev. Mater. 2022, 7, 174–195. [Google Scholar] [CrossRef]
- Sun, B.; Xia, T. Nanomaterial-based vaccine adjuvants. J. Mater. Chem. B 2016, 4, 5496–5509. [Google Scholar] [CrossRef]
- Curley, S.M.; Putnam, D. Biological nanoparticles in vaccine development. Front. Bioeng. Biotechnol. 2022, 10, 867119. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, K. Overview of vaccine adjuvants. Med. Drug Discov. 2021, 11, 100103. [Google Scholar] [CrossRef]
- Suresh, R.; Mosser, D.M. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 2013, 37, 284–291. [Google Scholar] [CrossRef]
- Bakkari, M.A.; Moni, S.S.; Alshammari, A.; Sultan, M.H.; Madkhali, O.A.; Almoshari, Y.; Alam, M.F.; Elmobark, M.E. Induction of Innate and Adaptive Immune Response against Recombinant HBsAg Protein Entrapped in Docosahexaenoic Acid Nanovesicles through Biomarkers. Vaccines 2023, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef] [PubMed]
- Irene, S.N.; Carol, F.; Cecilia, D.; Juan, A.T.P.; Simone, P.; Paula, M.; Francesca, G.; Ordovas-Montanes, J.; Samuel, W.K.; Svend, K.; et al. Novel in vitro booster vaccination to rapidly generate antigen-specific human monoclonal antibodies. J. Exp. Med. 2017, 214, 2471–2490. [Google Scholar] [CrossRef]
- Bakkari, M.A.; Moni, S.S.; Alshammari, A.; Salawi, A.; Sultan, M.H.; Madkhali, O.A.; Alqahtani, S.S.; Alam, M.F.; Shaheen, E.S.; Elmobark, M.E. Design, Characterization, and Immune Augmentation of Docosahexaenoic Acid Nanovesicles as a Potential Delivery System for Recombinant HBsAg Protein. Vaccines 2022, 10, 954. [Google Scholar] [CrossRef]
- Pattnaik, S.; Swain, K.; Singh, S.P.; Sirbaiya, A.K. Lipid vesicles: Potentials as drug delivery systems. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier Science: Amsterdam, The Netherlands, 2020; pp. 163–180. [Google Scholar] [CrossRef]
- L’homme, L.; Esser, N.; Riva, L.; Scheen, A.; Paquot, N.; Piette, J.; Legrand-Poels, S. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. J. Lipid Res. 2013, 54, 2998–3008. [Google Scholar] [CrossRef]
- Lin, C.; Chao, H.; Li, Z.; Xu, X.; Liu, Y.; Bao, Z.; Hou, L.; Liu, Y.; Wang, X.; You, Y.; et al. Omega-3 fatty acids regulate NLRP3 inflammasome activation and prevent behavior deficits after traumatic brain injury. Exp. Neurol. 2017, 290, 115–122. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef]
- Yin, Q.; Luo, W.; Mallajosyula, V.; Yang, B.; Jing, G.; Jinghang, X.; Meng, S.; Rohit, V.; Chunfeng, L.; Christian, M.C.; et al. A TLR7-nanoparticle adjuvant promotes a broad immune response against heterologous strains of influenza and SARS-CoV-2. Nat. Mater. 2023, 22, 380–390. [Google Scholar] [CrossRef]
- Bhagchandani, S.; Johnson, J.A.; Irvine, D.J. Evolution of Toll-like receptor 7/8 agonist therapeutics and their delivery approaches: From antiviral formulations to vaccine adjuvants. Adv. Drug Deliv. Rev. 2021, 175, 113803. [Google Scholar] [CrossRef]
- Dendritic Cell-Based Tumor Vaccine Adjuvant Immunotherapy of Human Glioblastoma Multiforme (WHO Grade IV Gliomas). 2016. Available online: https://clinicaltrials.gov/study/NCT02772094?cond=VACCINE%20ADJUVANTS&term=Clinical%20Trial&rank=1 (accessed on 29 June 2023).
- Dubois Cauwelaert, N.; Desbien, A.L.; Hudson, T.E.; Pine, S.O.; Reed, S.G.; Coler, R.N.; Orr, M.T. The TLR4 agonist vaccine adjuvant, GLA-SE, requires canonical and atypical mechanisms of action for TH1 induction. PLoS ONE 2016, 11, e0146372. [Google Scholar] [CrossRef]


| Calcium Adjuvant | Characteristics | Examples of Vaccines | References |
|---|---|---|---|
| Calcium phosphate | White, crystalline powder | Anthrax, Diphtheria–Tetanus–Pertussis, Haemophilus influenzae type b | [31,53] |
| Calcium carbonate | White, chalky powder | Human Papillomavirus | [53] |
| Calcium chloride | White, crystalline powder | Rabies | [31,53] |
| Calcium gluconate | White, crystalline powder | Influenza | [54,55] |
| Properties | References |
|---|---|
| [87] |
| [88] |
| [89] |
| [90] |
| Properties | References |
|---|---|
| [178] |
| [179] |
| [180] |
| [181,182,183,184] |
| [185,186,187,188,189] |
| Properties | References |
|---|---|
| [207,210,211] |
| [207,212] |
| [213] |
| [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moni, S.S.; Abdelwahab, S.I.; Jabeen, A.; Elmobark, M.E.; Aqaili, D.; Gohal, G.; Oraibi, B.; Farasani, A.M.; Jerah, A.A.; Alnajai, M.M.A.; et al. Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations. Vaccines 2023, 11, 1704. https://doi.org/10.3390/vaccines11111704
Moni SS, Abdelwahab SI, Jabeen A, Elmobark ME, Aqaili D, Gohal G, Oraibi B, Farasani AM, Jerah AA, Alnajai MMA, et al. Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations. Vaccines. 2023; 11(11):1704. https://doi.org/10.3390/vaccines11111704
Chicago/Turabian StyleMoni, Sivakumar S., Siddig Ibrahim Abdelwahab, Aamena Jabeen, Mohamed Eltaib Elmobark, Duaa Aqaili, Gassem Gohal, Bassem Oraibi, Abdulla Mohammed Farasani, Ahmed Ali Jerah, Mahdi Mohammed A. Alnajai, and et al. 2023. "Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations" Vaccines 11, no. 11: 1704. https://doi.org/10.3390/vaccines11111704
APA StyleMoni, S. S., Abdelwahab, S. I., Jabeen, A., Elmobark, M. E., Aqaili, D., Gohal, G., Oraibi, B., Farasani, A. M., Jerah, A. A., Alnajai, M. M. A., & Mohammad Alowayni, A. M. H. (2023). Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations. Vaccines, 11(11), 1704. https://doi.org/10.3390/vaccines11111704







