Increases in Cellular Immune Responses Due to Positive Effect of CVC1302-Induced Lysosomal Escape in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Piglets
2.2. Antigen, Adjuvant, and Immunizations
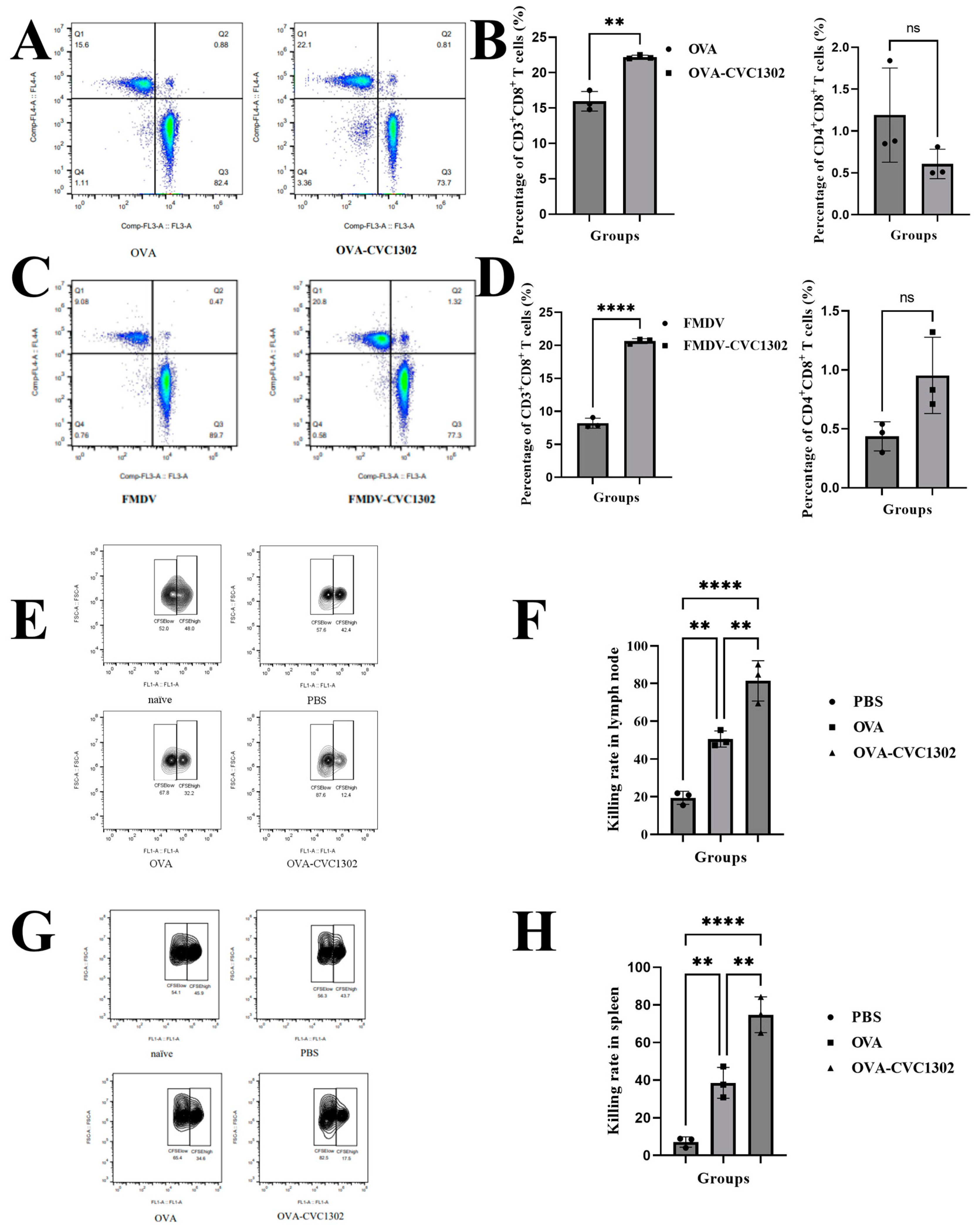
2.3. CD3+CD8+ T Cells Differentiation in Mice and Piglets
2.4. Cytotoxicity Assay
2.5. Preparation of Bone Marrow-Derived DCs (BMDCs)
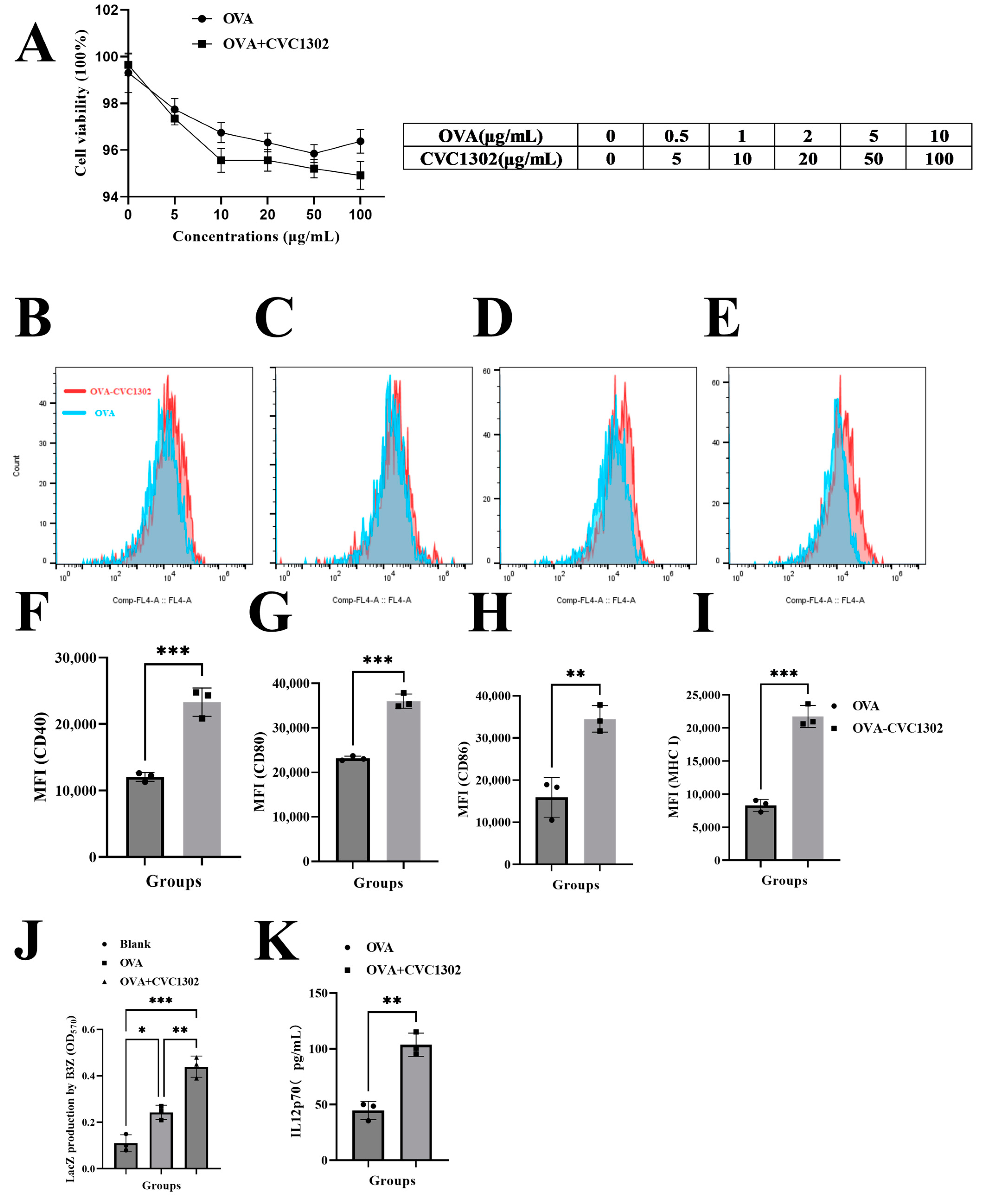
2.6. CCK-8 Assay
2.7. BMDCs Activation
2.8. Cytokine Detection from Cell Culture Supernatants
2.9. B3Z Cross-Presentation Assays
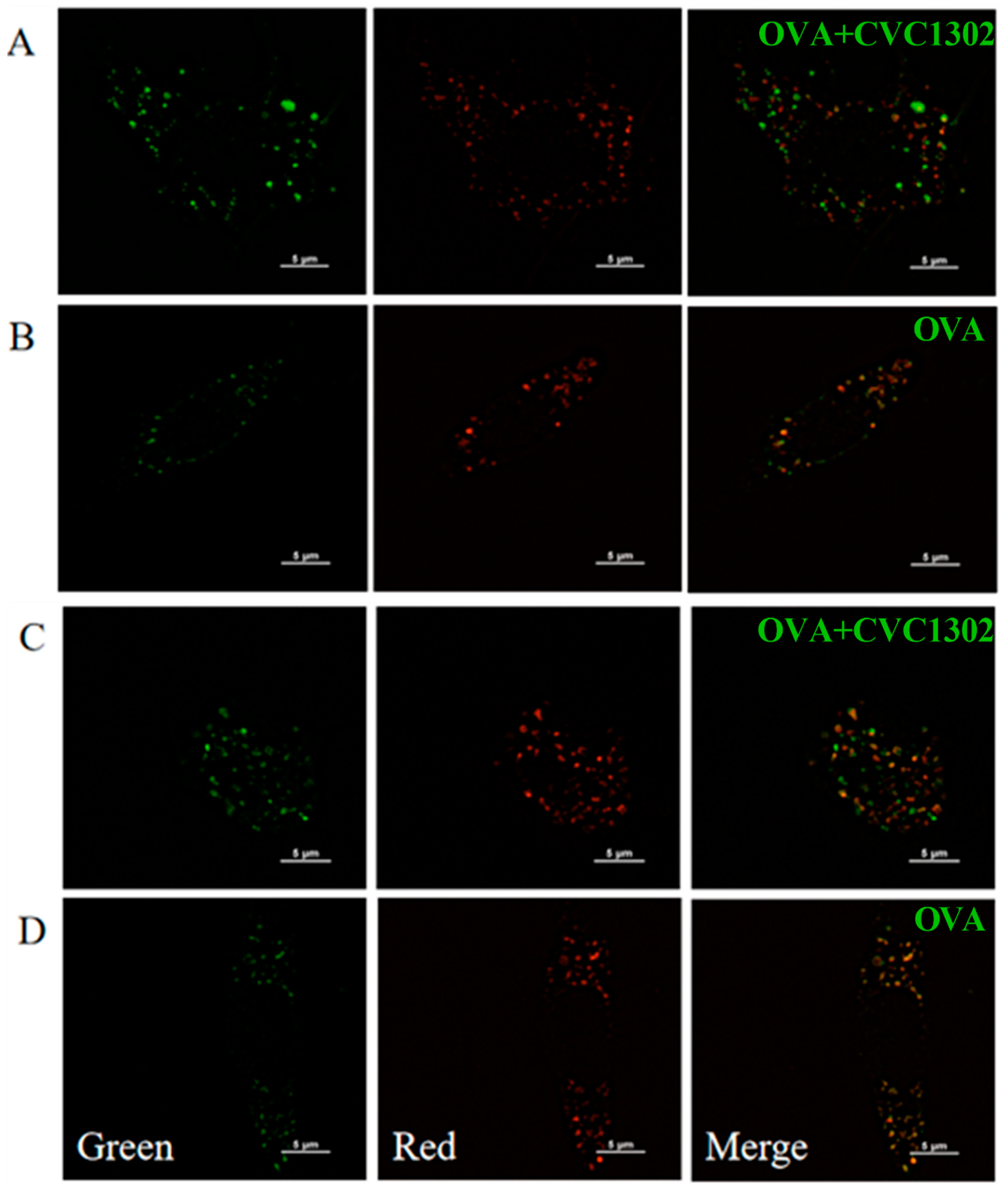
2.10. Confocal Microscopy
2.11. Real-Time PCR for Gene Expression
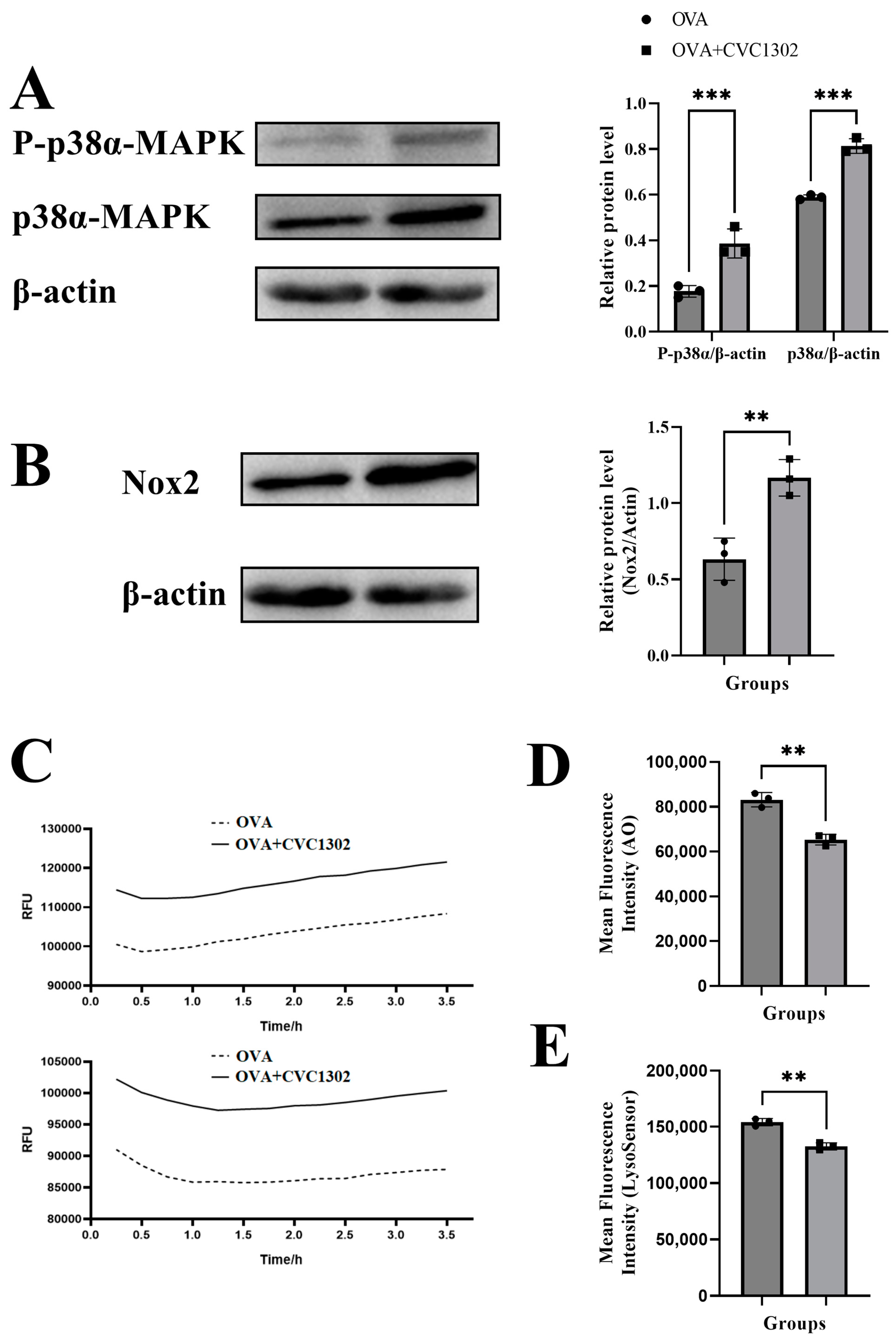
2.12. Immunoblot
2.13. ROS Formation—DCF Assay
2.14. AO Staining of BMDCs
2.15. LysoSensor Fluorescence Assay
2.16. Statistical Analysis
3. Results
3.1. CVC1302 Leads to an Enhanced CTL Response
3.2. CVC1302 Induces Improved Cross-Presentation by Flt3-L-Cultured BMDCs
3.3. CVC1302-Induced Lysosomal Escape of Antigen Is Indispensable for Cross-Presentation
3.4. CVC1302 Mediates Lysosomal Escape via p38α Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.; Suresh, M. Vaccine adjuvants to engage the cross-presentation pathway. Front. Immunol. 2022, 13, 940047. [Google Scholar] [CrossRef]
- Ho, N.I.; Huis In’t Veld, L.G.M.; Raaijmakers, T.K.; Adema, G.J. Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines? Front. Immunol. 2018, 9, 2874. [Google Scholar] [CrossRef] [PubMed]
- Den Brok, M.H.; Bull, C.; Wassink, M.; de Graaf, A.M.; Wagenaars, J.A.; Minderman, M.; Thakur, M.; Amigorena, S.; Rijke, E.O.; Schrier, C.C.; et al. Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation. Nat. Commun. 2016, 7, 13324. [Google Scholar] [CrossRef] [PubMed]
- Nutt, S.L.; Chopin, M. Transcriptional Networks Driving Dendritic Cell Differentiation and Function. Immunity 2020, 52, 942–956. [Google Scholar] [CrossRef]
- Greter, M.; Helft, J.; Chow, A.; Hashimoto, D.; Mortha, A.; Agudo-Cantero, J.; Bogunovic, M.; Gautier, E.L.; Miller, J.; Leboeuf, M.; et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity 2012, 36, 1031–1046. [Google Scholar] [CrossRef]
- Naik, S.H.; Proietto, A.I.; Wilson, N.S.; Dakic, A.; Schnorrer, P.; Fuchsberger, M.; Lahoud, M.H.; O’Keeffe, M.; Shao, Q.X.; Chen, W.F.; et al. Cutting edge: Generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 2005, 174, 6592–6597. [Google Scholar] [CrossRef]
- Elizondo, D.M.; Brandy, N.Z.D.; da Silva, R.L.L.; Haddock, N.L.; Kacsinta, A.D.; de Moura, T.R.; Lipscomb, M.W. Allograft Inflammatory Factor-1 Governs Hematopoietic Stem Cell Differentiation Into cDC1 and Monocyte-Derived Dendritic Cells Through IRF8 and RelB in vitro. Front. Immunol. 2019, 10, 173. [Google Scholar] [CrossRef]
- Sadiq, B.A.; Mantel, I.; Blander, J.M. A Comprehensive Experimental Guide to Studying Cross-Presentation in Dendritic Cells In Vitro. Curr. Protoc. Immunol. 2020, 131, e115. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef]
- Cruz, F.M.; Chan, A.; Rock, K.L. Pathways of MHC I cross-presentation of exogenous antigens. Semin. Immunol. 2023, 66, 101729. [Google Scholar] [CrossRef]
- Baljon, J.J.; Wilson, J.T. Bioinspired vaccines to enhance MHC class-I antigen cross-presentation. Curr. Opin. Immunol. 2022, 77, 102215. [Google Scholar] [CrossRef] [PubMed]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.C.; Gougerot-Pocidalo, M.A.; Dang, P.M. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Jancic, C.; Hugues, S.; Guermonprez, P.; Vargas, P.; Moura, I.C.; Lennon-Duménil, A.M.; Seabra, M.C.; Raposo, G.; Amigorena, S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 2006, 126, 205–218. [Google Scholar] [CrossRef]
- Mantegazza, A.R.; Savina, A.; Vermeulen, M.; Perez, L.; Geffner, J.; Hermine, O.; Rosenzweig, S.D.; Faure, F.; Amigorena, S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood 2008, 112, 4712–4722. [Google Scholar] [CrossRef] [PubMed]
- Lam, G.Y.; Huang, J.; Brumell, J.H. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin. Immunopathol. 2010, 32, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Yamamori, T.; Inanami, O.; Nagahata, H.; Cui, Y.; Kuwabara, M. Roles of p38 MAPK, PKC and PI3-K in the signaling pathways of NADPH oxidase activation and phagocytosis in bovine polymorphonuclear leukocytes. FEBS Lett. 2000, 467, 253–258. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, J.; Liu, C.; Guo, X.; Zhu, X.; Yao, Y.; Jiao, Y.; He, P.; Han, J.; Wu, L. p38alpha has an important role in antigen cross-presentation by dendritic cells. Cell. Mol. Immunol. 2018, 15, 246–259. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Seder, R.A.; Reddy, A.; Feldman, M.V. IL-12 in conjunction with dendritic cells enhances antiviral CD8+ CTL responses in vitro. J. Clin. Investig. 1996, 98, 715–722. [Google Scholar] [CrossRef]
- Watts, C.; West, M.A.; Zaru, R. TLR signalling regulated antigen presentation in dendritic cells. Curr. Opin. Immunol. 2010, 22, 124–130. [Google Scholar] [CrossRef]
- Du, L.; Hou, L.; Yu, X.; Cheng, H.; Chen, J.; Zheng, Q.; Hou, J. Pattern-Recognition Receptor Agonist-Containing Immunopotentiator CVC1302 Boosts High-Affinity Long-Lasting Humoral Immunity. Front. Immunol. 2021, 12, 697292. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Zheng, Q.; Hou, L.; Du, L.; Zhang, Y.; Qiao, X.; Hou, J.; Huang, K. The immunopotentiator CVC1302 enhances immune efficacy and protective ability of foot-and-mouth disease virus vaccine in pigs. Vaccine 2018, 36, 7929–7935. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, A.; Zhang, X.; Wu, J.; Freywald, A.; Xu, J.; Xiang, J. Novel exosome-targeted T-cell-based vaccine counteracts T-cell anergy and converts CTL exhaustion in chronic infection via CD40L signaling through the mTORC1 pathway. Cell. Mol. Immunol. 2017, 14, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sanchez, A.; Meza-Perez, S.; Flores-Langarica, A.; Donis-Maturano, L.; Estrada-Garcia, I.; Calderon-Amador, J.; Hernández-Pando, R.; Idoyaga, J.; Steinman, R.M.; Flores-Romo, L. ESAT-6 Targeting to DEC205+ Antigen Presenting Cells Induces Specific-T Cell Responses against ESAT-6 and Reduces Pulmonary Infection with Virulent Mycobacterium tuberculosis. PLoS ONE 2015, 10, e0124828. [Google Scholar] [CrossRef] [PubMed]
- Brasel, K.; De Smedt, T.; Smith, J.L.; Maliszewski, C.R. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 2000, 96, 3029–3039. [Google Scholar] [CrossRef]
- Su, X.; Song, H.; Niu, F.; Yang, K.; Kou, G.; Wang, X.; Chen, H.; Li, W.; Guo, S.; Li, J.; et al. Co-delivery of doxorubicin and PEGylated C16-ceramide by nanoliposomes for enhanced therapy against multidrug resistance. Nanomedicine 2015, 10, 2033–2050. [Google Scholar] [CrossRef]
- Nierkens, S.; den Brok, M.H.; Sutmuller, R.P.; Grauer, O.M.; Bennink, E.; Morgan, M.E.; Figdor, C.G.; Ruers, T.J.; Adema, G.J. In vivo colocalization of antigen and CpG [corrected] within dendritic cells is associated with the efficacy of cancer immunotherapy. Cancer Res. 2008, 68, 5390–5396. [Google Scholar] [CrossRef]
- Schlormann, W.; Horlebein, C.; Hubner, S.M.; Wittwer, E.; Glei, M. Potential Role of ROS in Butyrate- and Dietary Fiber-Mediated Growth Inhibition and Modulation of Cell Cycle-, Apoptosis- and Antioxidant-Relevant Proteins in LT97 Colon Adenoma and HT29 Colon Carcinoma Cells. Cancers 2023, 15, 440. [Google Scholar] [CrossRef]
- Yue, W.; Hamai, A.; Tonelli, G.; Bauvy, C.; Nicolas, V.; Tharinger, H.; Codogno, P.; Mehrpour, M. Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/progenitor cells interferes with their maintenance. Autophagy 2013, 9, 714–729. [Google Scholar] [CrossRef]
- Palmgren, M.G. Acridine orange as a probe for measuring pH gradients across membranes: Mechanism and limitations. Anal. Biochem. 1991, 192, 316–321. [Google Scholar] [CrossRef]
- Yang, K.; Lu, Y.; Xie, F.; Zou, H.; Fan, X.; Li, B.; Li, W.; Zhang, W.; Mei, L.; Feng, S.S.; et al. Cationic liposomes induce cell necrosis through lysosomal dysfunction and late-stage autophagic flux inhibition. Nanomedicine 2016, 11, 3117–3137. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Jin, S.; Tian, Y.; Zhang, X.; Zhao, Y.; Yu, L.; Liang, X.J. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 2011, 5, 8629–8639. [Google Scholar] [CrossRef] [PubMed]
- Pathni, A.; Ozcelikkale, A.; Rey-Suarez, I.; Li, L.; Davis, S.; Rogers, N.; Xiao, Z.; Upadhyaya, A. Cytotoxic T Lymphocyte Activation Signals Modulate Cytoskeletal Dynamics and Mechanical Force Generation. Front. Immunol. 2022, 13, 779888. [Google Scholar] [CrossRef] [PubMed]
- West, M.A.; Wallin, R.P.; Matthews, S.P.; Svensson, H.G.; Zaru, R.; Ljunggren, H.G.; Prescott, A.R.; Watts, C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science 2004, 305, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zhang, Y.; Hou, L.; Qiao, X.; Zhang, Y.; Cheng, H.; Lu, H.; Chen, J.; Du, L.; Zheng, Q.; et al. Increases in Cellular Immune Responses Due to Positive Effect of CVC1302-Induced Lysosomal Escape in Mice. Vaccines 2023, 11, 1718. https://doi.org/10.3390/vaccines11111718
Yu X, Zhang Y, Hou L, Qiao X, Zhang Y, Cheng H, Lu H, Chen J, Du L, Zheng Q, et al. Increases in Cellular Immune Responses Due to Positive Effect of CVC1302-Induced Lysosomal Escape in Mice. Vaccines. 2023; 11(11):1718. https://doi.org/10.3390/vaccines11111718
Chicago/Turabian StyleYu, Xiaoming, Yuanyuan Zhang, Liting Hou, Xuwen Qiao, Yuanpeng Zhang, Haiwei Cheng, Haiyan Lu, Jin Chen, Luping Du, Qisheng Zheng, and et al. 2023. "Increases in Cellular Immune Responses Due to Positive Effect of CVC1302-Induced Lysosomal Escape in Mice" Vaccines 11, no. 11: 1718. https://doi.org/10.3390/vaccines11111718
APA StyleYu, X., Zhang, Y., Hou, L., Qiao, X., Zhang, Y., Cheng, H., Lu, H., Chen, J., Du, L., Zheng, Q., Hou, J., & Tong, G. (2023). Increases in Cellular Immune Responses Due to Positive Effect of CVC1302-Induced Lysosomal Escape in Mice. Vaccines, 11(11), 1718. https://doi.org/10.3390/vaccines11111718




