Improvement of the qmosRT-PCR Assay and Its Application for the Detection and Quantitation of the Three Serotypes of the Novel Oral Polio Vaccine in Stool Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Viruses and Clinical Samples
2.2. Primers and TaqMan Oligoprobes Used for OPV/nOPV Serotype Identification and Quantification
2.3. Extraction of Viral RNA
2.4. qmosRT-PCR Amplification
2.5. Sensitivity of the qmosRT-PCR Assay
2.6. Spike of nOPV2 and Trivalent nOPV in Stool Supernatant
3. Results
3.1. Evaluation of the Specificity and Sensitivity of the Simultaneous Detection and Quantification of Each nOPV Strain in the Presence of High Concentrations of the Two Other Serotypes
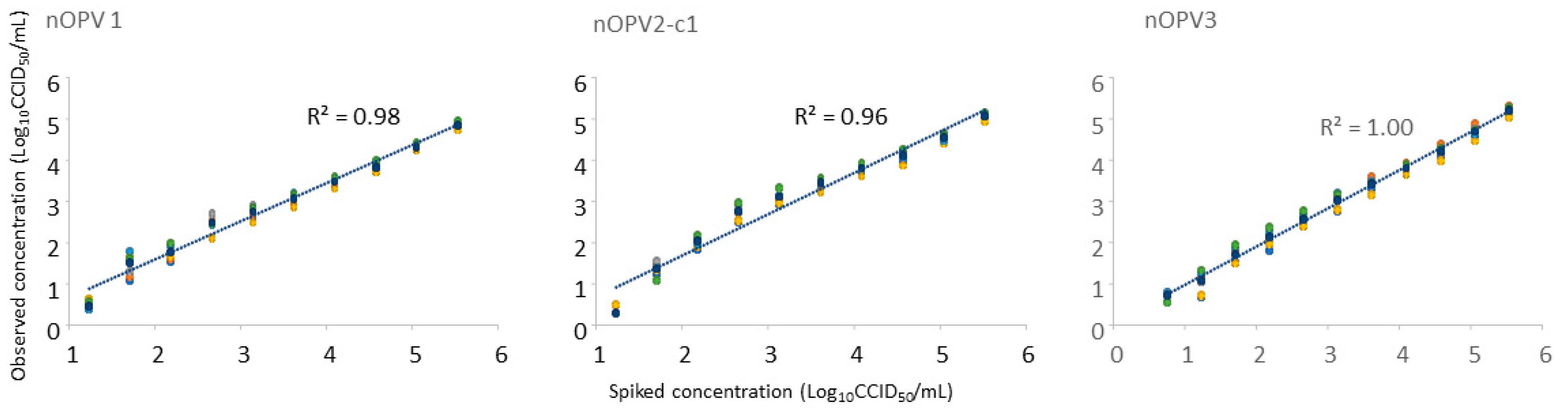
3.2. Analysis of Monovalent nOPV2-c1 and Trivalent nOPV Spiked in Stool Supernatant
3.3. Analysis of Stool Samples from nOPV2 Clinical Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutter, R.W.; Kew, O.M.; Cochi, S.L.; Aylward, R.B. Poliovirus vaccine—Live. In Vaccines, 6th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 598–645. [Google Scholar]
- WHO. Global eradication of wild poliovirus type 2 declared. Declaration further milestone for globally-coordinated vaccine switch in 2016 [cited 20 September 2015]. 2016. Available online: https://polioeradication.org/news-post/global-eradication-of-wild-poliovirus-type-2-declared/ (accessed on 1 September 2023).
- WHO. Two out of three wild poliovirus strains eradicated. Global eradication of wild poliovirus type 3 declared on World Polio Day2019. 2019. Available online: https://www.who.int/news-room/feature-stories/detail/two-out-of-three-wild-poliovirus-strains-eradicated (accessed on 1 September 2023).
- John, J.; Giri, S.; Karthikeyan, A.S.; Iturriza-Gomara, M.; Muliyil, J.; Abraham, A.; Grassly, N.C.; Kang, G. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: An open-label, randomised controlled trial. Lancet 2014, 384, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.; Ottosen, A.; Ghazieh, A.; Fournier-Caruana, J.; Ntow, A.K.; Gonzalez, A.R. Managing the Planned Cessation of a Global Supply Market: Lessons Learned From the Global Cessation of the Trivalent Oral Poliovirus Vaccine Market. J. Infect. Dis. 2017, 216 (Suppl. 1), S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.L.; Jaikaran, E.S.; Davies, J.R.; Tomlinson, A.J.; Mason, P.J.; Barnes, J.M.; Beale, A.J. A study of poliovaccination in infancy: Excretion following challenge with live virus by children given killed or living poliovaccine. J. Hyg. 1966, 64, 105–120. [Google Scholar] [CrossRef]
- Hird, T.R.; Grassly, N.C. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012, 8, e1002599. [Google Scholar] [CrossRef] [PubMed]
- Brickley, E.B.; Strauch, C.B.; Wieland-Alter, W.F.; Connor, R.I.; Lin, S.; Weiner, J.A.; Ackerman, M.E.; Arita, M.; Oberste, M.S.; Weldon, W.C.; et al. Intestinal Immune Responses to Type 2 Oral Polio Vaccine (OPV) Challenge in Infants Previously Immunized with Bivalent OPV and Either High-Dose or Standard Inactivated Polio Vaccine. J. Infect. Dis. 2018, 217, 371–380. [Google Scholar] [CrossRef]
- Thompson, K.M.; Duintjer Tebbens, R.J. Lessons from the Polio Endgame: Overcoming the Failure to Vaccinate and the Role of Subpopulations in Maintaining Transmission. J. Infect. Dis. 2017, 216 (Suppl. 1), S176–S182. [Google Scholar] [CrossRef]
- Wright, P.F.; Connor, R.I.; Wieland-Alter, W.F.; Hoen, A.G.; Boesch, A.W.; Ackerman, M.E.; Oberste, M.S.; Gast, C.; Brickley, E.B.; Asturias, E.J.; et al. Vaccine-induced mucosal immunity to poliovirus: Analysis of cohorts from an open-label, randomised controlled trial in Latin American infants. Lancet Infect. Dis. 2016, 16, 1377–1384. [Google Scholar] [CrossRef]
- Wang, H. Why Have cVDPV2 Outbreaks Increased Globally after the Polio Immunization Strategy Switch: Challenges for the Polio Eradication Endgame. China CDC Wkly. 2020, 2, 176–179. [Google Scholar] [CrossRef]
- Blake, I.M.; Pons-Salort, M.; Molodecky, N.A.; Diop, O.M.; Chenoweth, P.; Bandyopadhyay, A.S.; Zaffran, M.; Sutter, R.W.; Grassly, N.C. Type 2 Poliovirus Detection after Global Withdrawal of Trivalent Oral Vaccine. N. Engl. J. Med. 2018, 379, 834–845. [Google Scholar] [CrossRef]
- Jorba, J.; Diop, O.M.; Iber, J.; Henderson, E.; Zhao, K.; Quddus, A.; Sutter, R.; Vertefeuille, J.F.; Wenger, J.; Wassilak, S.G.F.; et al. Update on Vaccine-Derived Poliovirus Outbreaks—Worldwide, January 2018–June 2019. MMWR Morb. Mortal Wkly. Rep. 2019, 68, 1024–1028. [Google Scholar] [CrossRef]
- Cann, A.J.; Stanway, G.; Hughes, P.J.; Minor, P.D.; Evans, D.M.; Schild, G.C.; Almond, J.W. Reversion to neurovirulence of the live-attenuated Sabin type 3 oral poliovirus vaccine. Nucleic Acids Res. 1984, 12, 7787–7792. [Google Scholar] [CrossRef] [PubMed]
- Macadam, A.J.; Ferguson, G.; Burlison, J.; Stone, D.; Skuce, R.; Almond, J.W.; Minor, P.D. Correlation of RNA secondary structure and attenuation of Sabin vaccine strains of poliovirus in tissue culture. Virology 1992, 189, 415–422. [Google Scholar] [CrossRef]
- Macadam, A.J.; Pollard, S.R.; Ferguson, G.; Skuce, R.; Wood, D.; Almond, J.W.; Minor, P.D. Genetic basis of attenuation of the Sabin type 2 vaccine strain of poliovirus in primates. Virology 1993, 192, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Colbere-Garapin, F.; Macadam, A.; Taffs, L.F.; Marsden, S.; Minor, P.; Horaud, F. Mapping of mutations associated with neurovirulence in monkeys infected with Sabin 1 poliovirus revertants selected at high temperature. J. Virol. 1990, 64, 4922–4929. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.; Begg, N.T.; Cammack, N.; Minor, P.D. Virus excretion and mutation by infants following primary vaccination with live oral poliovaccine from two sources. J. Med. Virol. 1990, 32, 92–95. [Google Scholar]
- Laassri, M.; Lottenbach, K.; Belshe, R.; Rennels, M.; Plotkin, S.; Chumakov, K. Analysis of reversions in the 5′-untranslated region of attenuated poliovirus after sequential administration of inactivated and oral poliovirus vaccines. J. Infect. Dis. 2006, 193, 1344–1349. [Google Scholar] [CrossRef]
- Konopka-Anstadt, J.L.; Campagnoli, R.; Vincent, A.; Shaw, J.; Wei, L.; Wynn, N.T.; Smithee, S.E.; Bujaki, E.; Te Yeh, M.; Laassri, M.; et al. Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization. NPJ Vaccines 2020, 5, 26. [Google Scholar] [CrossRef]
- Macadam, A.J.; Ferguson, G.; Stone, D.M.; Meredith, J.; Knowlson, S.; Auda, G.; Almond, J.W.; Minor, P.D. Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: Relevance to poliomyelitis eradication. J. Virol. 2006, 80, 8653–8663. [Google Scholar] [CrossRef]
- Yeh, M.T.; Smith, M.; Carlyle, S.; Konopka-Anstadt, J.L.; Burns, C.C.; Konz, J.; Andino, R.; Macadam, A. Genetic stabilization of attenuated oral vaccines against poliovirus types 1 and 3. Nature 2023, 619, 135–142. [Google Scholar] [CrossRef]
- Saez-Llorens, X.; Bandyopadhyay, A.S.; Gast, C.; Leon, T.; DeAntonio, R.; Jimeno, J.; Caballero, M.I.; Aguirre, G.; Oberste, M.S.; Weldon, W.C.; et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: Two clinical trials. Lancet 2021, 397, 27–38. [Google Scholar] [CrossRef]
- Manukyan, H.; Zagorodnyaya, T.; Ruttimann, R.; Manor, Y.; Bandyopadhyay, A.; Shulman, L.; Chumakov, K.; Laassri, M. Quantitative multiplex one-step RT-PCR assay for identification and quantitation of Sabin strains of poliovirus in clinical and environmental specimens. J. Virol. Methods 2018, 259, 74–80. [Google Scholar] [CrossRef] [PubMed]
- WHO. Manual of Laboratory Methods for Testing the Potency of Final Vaccines Used in the WHO Expanded Programme on Immunization; Technical Report; WHO: Geneva, Switzerland, 1990. [Google Scholar]
- WHO. Recommendations for the Production and Control of Poliomyelitis Vaccine (Oral); Technical Report; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Khan, A.S.; Blumel, J.; Deforce, D.; Gruber, M.F.; Jungback, C.; Knezevic, I.; Mallet, L.; Mackay, D.; Matthijnssens, J.; O’Leary, M.; et al. Report of the second international conference on next generation sequencing for adventitious virus detection in biologics for humans and animals. Biologicals 2020, 67, 94–111. [Google Scholar] [CrossRef] [PubMed]
- GPEI. Novel Oral Polio Vaccine Type 2 (nOPV2) Granted EUL Recommendation; GPEI: Geneva, Switzerland, 2020. [Google Scholar]
- GPEI. GPEI Statement on cVDPV2 Detections in Burundi and Democratic Republic of the Congo; Global Polio Eradication Initiative: Geneva, Switzerland, 2023. [Google Scholar]
- Kilpatrick, D.R.; Yang, C.F.; Ching, K.; Vincent, A.; Iber, J.; Campagnoli, R.; Mandelbaum, M.; De, L.; Yang, S.J.; Nix, A.; et al. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J. Clin. Microbiol. 2009, 47, 1939–1941. [Google Scholar] [CrossRef] [PubMed]
| nOPV/OPV Serotype | Oligo Name | Location in nOPV Genome | Sequence 5′-->3′ | Size (nt) | Tm (°C, Basic) | Amplicon Size (bp) |
|---|---|---|---|---|---|---|
| 1 | 2771Sab1F | 2831-2849 | CAGCTTCCACCAAGAATAA | 19 | 43 | 92 |
| Sab1-2962R | 2922-2904 | GAAGAACTCCAATTTCCTC | 19 | 47 | ||
| Sab1-FAM | 2863-2877 | FAM-ACAGTGTGGAAGATC-NFQ | 15 | |||
| 2 | 2682TqS2F | 2748-2763 | CCAGAGACGAACGCGA | 16 | 49 | 122 |
| 2802TqS2R | 2869-2852 | AAACCGAAAACAATCTGC | 18 | 44 | ||
| Sab2-VIC | 2772-2787 | VIC-CACGGTTGAGTCATTC-NFQ | 16 | |||
| 3 | 1411TqS3F | 1479-1497 | GGGAAAATTTTACTCCCAA | 19 | 45 | 69 |
| Sab3-1487R | 1547-1527 | ACTGGGCAGAACTCTCTTTTT | 21 | 51 | ||
| Sab3-NED | 1510-1524 | NED-AACGCAGTAACATCC-NFQ | 15 |
| nOPV Serotypes | Expected Titers * | qmosRT-PCR Result γ | Expected Titers * | qmosRT-PCR Result γ | ||
|---|---|---|---|---|---|---|
| Ct | log10 CCID50/mL | Ct | log10 CCID50/mL | |||
| 1 | 3 | 34.96 | 1.35 ± 0.33 | 3 | UD | |
| 2 | 3 | 26.15 | 3.51 ± 0.08 | 7 | 15.95 | 6.52 ± 0.07 |
| 3 | 7 | 14.89 | 6.79 ± 0.08 | 7 | 17.06 | 6.22 ± 0.05 |
| 1 | 3 | 25.55 | 3.81 ± 0.03 | 4 | 32.34 | 2.04 ± 0.09 |
| 2 | 7 | 14.42 | 6.97 ± 0.06 | 7 | 15.83 | 6.56 ± 0.03 |
| 3 | 3 | 28.51 | 3.21 ± 0.18 | 7 | 17.04 | 6.23 ± 0.01 |
| 1 | 7 | 12.05 | 7.34 ± 0.04 | 2 | UD | |
| 2 | 3 | 25.78 | 3.62 ± 0.03 | 6 | 18.93 | 5.64 ± 0.05 |
| 3 | 3 | 28.62 | 3.18 ± 0.09 | 6 | 19.95 | 5.46 ± 0.05 |
| 1 | 7 | 12.04 | 7.35 ± 0.02 | 3 | 29.62 | 2.75 ± 0.01 |
| 2 | 7 | 14.00 | 7.1 ± 0.03 | 3 | 28.20 | 2.9 ± 0.01 |
| 3 | 3 | 36.28 | 1.16 ± 0.59 | 6 | 20.07 | 5.43 ± 0.05 |
| 1 | 7 | 12.08 | 7.33 ± 0.05 | 4 | 23.29 | 4.4 ± 0.11 |
| 2 | 3 | 26.89 | 3.29 ± 0.06 | 4 | 24.59 | 3.97 ± 0.07 |
| 3 | 7 | 15.12 | 6.73 ± 0.08 | 6 | 20.28 | 5.37 ± 0.09 |
| 1 | 3 | UD | 2 | UD | ||
| 2 | 7 | 14.19 | 7.04 ± 0.02 | 2 | 33.06 | 1.46 ± 0.08 |
| 3 | 7 | 15.15 | 6.72 ± 0.13 | 6 | 20.19 | 5.4 ± 0.04 |
| 1 | 2 | UD | ||||
| 2 | 2 | 31.17 | 2.02 ± 0.07 | |||
| 3 | 7 | 15.06 | 6.75 ± 0.04 | |||
| 1 | 2 | 30.83 | 2.43 ± 0.02 | |||
| 2 | 7 | 14.36 | 6.99 ± 0.02 | |||
| 3 | 2 | 33.26 | 1.96 ± 0.07 | |||
| 1 | 7 | 12.08 | 7.33 ± 0.02 | |||
| 2 | 2 | 28.37 | 2.85 ± 0.09 | |||
| 3 | 2 | 40.54 | 0.04 ± 0.47 | |||
| nOPV Serotypes | Expected Titer * | qmosRT-PCR Result γ | |
|---|---|---|---|
| Ct | log10 CCID50/mL | ||
| 1 | 7 | 12.87 | 7.00 ± 0.02 |
| 2 | 7 | 16.58 | 6.90 ± 0.03 |
| 3 | NT | UD | |
| 1 | 7 | 12.75 | 7.03 ± 0.02 |
| 2 | NT | UD | |
| 3 | 7 | 14.79 | 6.91 ± 0.02 |
| 1 | NT | UD | |
| 2 | 7 | 17.28 | 6.70 ± 0.01 |
| 3 | 7 | 14.91 | 6.87 ± 0.02 |
| 1 | 7 | 12.84 | 7.01 ± 0.01 |
| 2 | NT | UD | |
| 3 | NT | UD | |
| 1 | NT | UD | |
| 2 | 7 | 17.89 | 6.52 ± 0.02 |
| 3 | NT | UD | |
| 1 | NT | UD | |
| 2 | NT | UD | |
| 3 | 7 | 15.04 | 6.84 ± 0.03 |
| 1 | 7 | 12.89 | 6.99 ± 0.03 |
| 2 | 7 | 16.55 | 6.91 ± 0.05 |
| 3 | 7 | 14.95 | 6.86 ± 0.03 |
| nOPV Serotypes | Expected Titer * | qmosRT-PCR Result | |
|---|---|---|---|
| Ct | log10 CCID50/mL γ | ||
| 1 | 7 | 12.98 | 6.97 ± 0.01 |
| 2 | 7 | 16.63 | 6.89 ± 0.01 |
| 3 | 1 | 37.04 | 0.55 ± 0.38 |
| 1 | 7 | 12.55 | 7.09 ± 0.01 |
| 2 | 1 | 41.79 | −0.25 ± 0.40 |
| 3 | 7 | 14.67 | 6.94 ± 0.05 |
| 1 | 1 | 33.43 | 0.83 ± 0.07 |
| 2 | 7 | 17.27 | 6.70 ± 0.00 |
| 3 | 7 | 14.98 | 6.85 ± 0.01 |
| 1 | 6 | 16.44 | 5.93 ± 0.02 |
| 2 | 6 | 20.48 | 5.78 ± 0.03 |
| 3 | 1 | 34.04 | 1.40 ± 0.33 |
| 1 | 6 | 16.24 | 5.99 ± 0.00 |
| 2 | 1 | 39.16 | 0.51 ± 0.39 |
| 3 | 6 | 18.14 | 5.94 ± 010 |
| 1 | 1 | 32.77 | 1.04 ± 0.12 |
| 2 | 6 | 21.07 | 5.61 ± 0.01 |
| 3 | 6 | 18.41 | 5.87 ± 0.13 |
| 1 | 6 | 16.36 | 5.95 ± 0.02 |
| 2 | 3 | 30.49 | 2.90 ± 0.03 |
| 3 | 1 | 35.09 | 1.01 ± 0.11 |
| 1 | 1 | 32.44 | 1.13 ± 0.03 |
| 2 | 6 | 22.47 | 5.21 ± 0.03 |
| 3 | 3 | 28.54 | 2.92 ± 012 |
| 1 | 3 | 25.82 | 3.11 ± 0.04 |
| 2 | 1 | 37.46 | 0.91 ± 0.17 |
| 3 | 6 | 18.56 | 5.82 ± 0.09 |
| Trivalent nOPV | Monovalent nOPV2-C1 | ||||
|---|---|---|---|---|---|
| Expected Titers * | nOPV1 Titers γ (Average Ct) | nOPV2-C1 Titers γ (Average Ct) | nOPV3 Titers γ (Average Ct) | Expected Titers * | nOPV2-C1 Titers γ (Average Ct) |
| 5.52 | 4.86 ± 0.06 (22) | 5.08 ± 0.07(22) | 5.21 ± 0.10 (22) | 5.55 | 4.11 ± 0.09 (26) |
| 5.05 | 4.32 ± 0.08 (24) | 4.55 ± 0.09 (24) | 4.70 ± 0.14 (24) | 5.08 | 3.60 ± 0.10 (27) |
| 4.57 | 3.83 ± 0.10 (26) | 4.11 ± 0.14 (25) | 4.21 ± 0.13 (26) | 4.60 | 3.17 ± 0.05 (28) |
| 4.09 | 3.48 ± 0.11 (27) | 3.81 ± 0.12 (26) | 3.81 ± 0.12 (27) | 4.12 | 2.83 ± 0.04 (29) |
| 3.61 | 3.06 ± 0.15 (29) | 3.45 ± 0.12 (27) | 3.43 ± 0.15 (28) | 3.64 | 2.54 ± 0.11 (31) |
| 3.14 | 2.75 ± 0.16 (30) | 3.11 ± 0.15(29) | 3.03 ± 0.18 (30) | 3.17 | 2.30 ± 0.12 (32) |
| 2.66 | 2.49 ± 0.19 (30) | 2.77 ± 0.19 (30) | 2.57 ± 0.15 (31) | 2.69 | 1.89 ± 0.13 (34) |
| 2.18 | 1.77 ± 0.18 (33) | 2.03 ± 0.14 (32) | 2.12 ± 0.20 (33) | 2.21 | 1.54 ± 0.24 (34) |
| 1.71 | 1.51 ± 0.29 (34) | 1.36 ± 0.16(35) | 1.72 ± 0.20 (34) | 1.74 | 1.05 ± 0.31 (35) |
| 1.23 | 0.45 ± 0.12 (37) | 0.29 ± 0.38 (38) | 1.08 ± 0.28 (36) | 1.26 | 1.09 ± 0.71 (38) |
| 0.75 | 0.72 ± 0.13 (37) | 0.78 | |||
| 0.27 | 0.30 | ||||
| Sample Codes | Detection Results | Titers (log10 CCID50/mL) | |||||
|---|---|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | All Types | Type 1 | Type 2 | Type 3 | |
| 2 | Neg | Pos | Neg | 2 | 2.74 | ||
| 3 | Neg | Pos | Neg | 2 | 3.43 | ||
| 4 | Neg | Pos | Pos | 2, 3 | 3.64 | 4.55 | |
| 5 | Neg | Pos | Neg | 2 | 2.76 | ||
| 6 | Neg | Pos | Neg | 2 | 2.15 | ||
| 7 | Neg | Pos | Neg | 2 | 2.15 | ||
| 8 | Neg | Pos | Neg | 2 | 1.3 | ||
| 9 | Neg | Pos | Neg | 2 | 2.28 | ||
| 10 | Neg | Pos | Neg | 2 | 2.71 | ||
| 11 | Neg | Pos | Neg | 2 | 1.99 | ||
| 12 | Pos | Pos | Pos | 1, 2, 3 | −0.27 | 5.26 | 0.04 |
| 13 | Pos | Pos | Pos | 1, 2, 3 | −0.34 | 4.09 | 0.61 |
| 14 | Neg | Pos | Neg | 2 | 2.46 | ||
| 15 | Neg | Pos | Neg | 2 | 2.54 | ||
| 16 | Neg | Pos | Neg | 2 | 5.11 | ||
| 17 | Neg | Pos | Neg | 2 | 2.83 | ||
| 18 | Neg | Pos | Neg | 2 | 2.69 | ||
| 19 | Neg | Pos | Neg | 2 | 0.55 | ||
| 20 | Pos | Pos | Pos | 1, 2, 3 | 1.85 | 2.47 | 0.11 |
| 21 | Neg | Pos | Neg | 2 | 2.95 | ||
| 22 | Neg | Pos | Neg | 2 | 2.25 | ||
| 23 | Neg | Pos | Neg | 2 | 5.45 | ||
| 24 | Neg | Pos | Neg | 2 | 3.35 | ||
| 25 | Neg | Pos | Neg | 2 | 3.43 | ||
| 26 | Neg | Pos | Neg | 2 | 4.43 | ||
| 27 | Neg | Pos | Neg | 2 | 4.77 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manukyan, H.; Tritama, E.; Wahid, R.; Anstadt, J.; Konz, J.; Chumakov, K.; Laassri, M. Improvement of the qmosRT-PCR Assay and Its Application for the Detection and Quantitation of the Three Serotypes of the Novel Oral Polio Vaccine in Stool Samples. Vaccines 2023, 11, 1729. https://doi.org/10.3390/vaccines11111729
Manukyan H, Tritama E, Wahid R, Anstadt J, Konz J, Chumakov K, Laassri M. Improvement of the qmosRT-PCR Assay and Its Application for the Detection and Quantitation of the Three Serotypes of the Novel Oral Polio Vaccine in Stool Samples. Vaccines. 2023; 11(11):1729. https://doi.org/10.3390/vaccines11111729
Chicago/Turabian StyleManukyan, Hasmik, Erman Tritama, Rahnuma Wahid, Jennifer Anstadt, John Konz, Konstantin Chumakov, and Majid Laassri. 2023. "Improvement of the qmosRT-PCR Assay and Its Application for the Detection and Quantitation of the Three Serotypes of the Novel Oral Polio Vaccine in Stool Samples" Vaccines 11, no. 11: 1729. https://doi.org/10.3390/vaccines11111729





