mRNA and Adenoviral Vector Vaccine Platforms Utilized in COVID-19 Vaccines: Technologies, Ecosystem, and Future Directions
Abstract
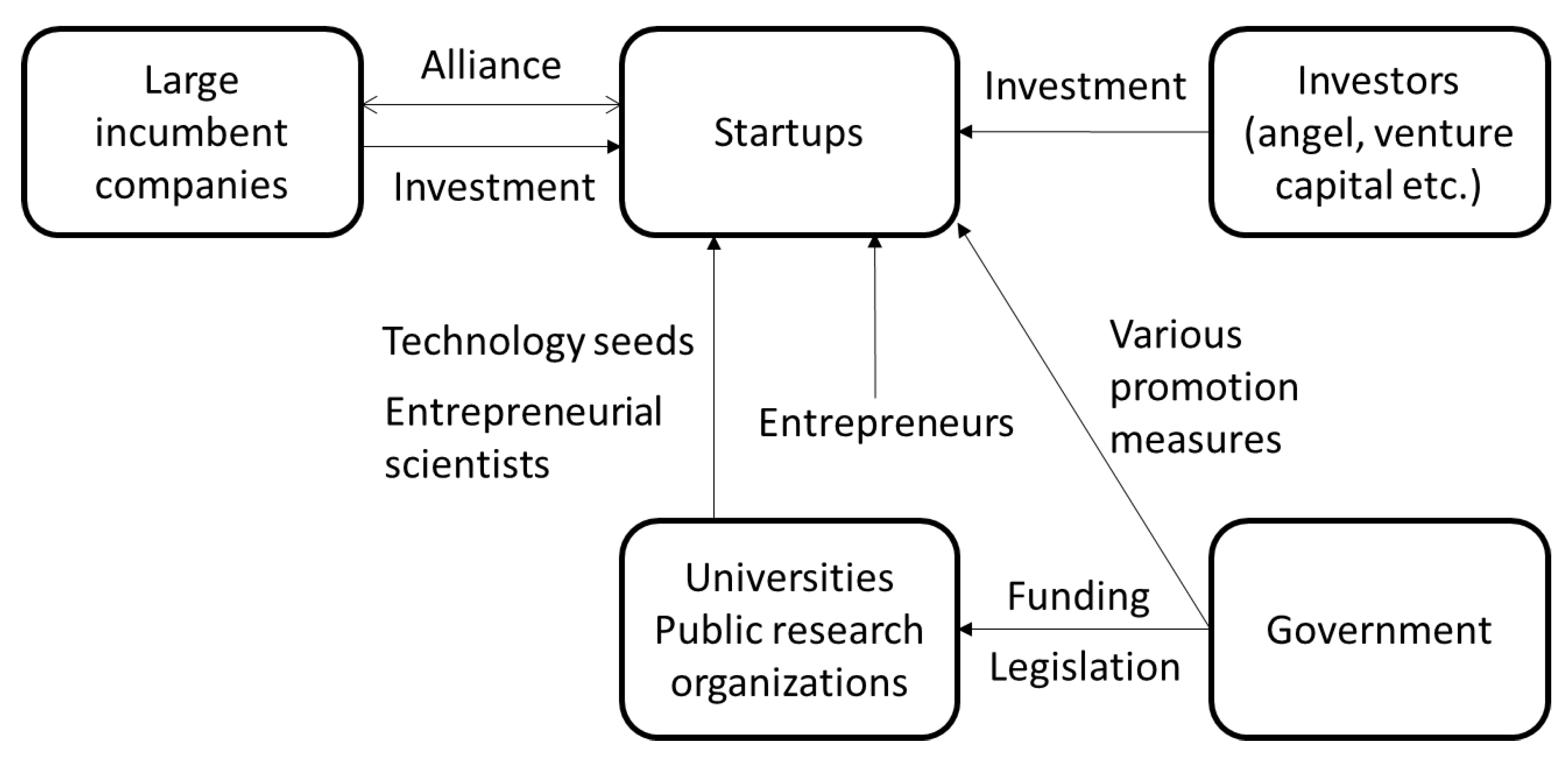
:1. Introduction
2. COVID-19 Vaccines Approved by the End of Year 2022
3. New Vaccine Technology Platforms Established through COVID-19 Vaccines
3.1. mRNA Vaccine
3.2. Viral Vector Vaccine
4. Research and Developmental History of mRNA and Adenoviral Vector Vaccines
4.1. Moderna
4.2. BioNTech
4.3. University of Oxford
4.4. The Case of Japan
5. Vaccine Development in the Post-COVID-19 Era
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Zhang, B. RNA therapeutics: Updates and future potential. Sci. China Life Sci. 2023, 66, 12–30. [Google Scholar] [CrossRef]
- Majhen, D. Human adenovirus type 26 basic biology and its usage as vaccine vector. Rev. Med. Virol. 2022, 32, e2338. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef]
- Ma, L.; Li, H.; Lan, J.; Hao, X.; Liu, H.; Wang, X.; Huang, Y. Comprehensive analyses of bioinformatics applications in the fight against COVID-19 pandemic. Comput. Biol. Chem. 2021, 95, 107599. [Google Scholar] [CrossRef]
- Rosa, S.S.; Prazeres, D.; Azevedo, A.M.; Marques, M. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Dolgin, E. The tangled history of mRNA vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef]
- Syyam, A.; Nawaz, A.; Ijaz, A.; Sajjad, U.; Fazil, A.; Irfan, S.; Muzaffar, A.; Shahid, M.; Idrees, M.; Malik, K.; et al. Adenovirus vector system: Construction, history and therapeutic applications. Biotechniques 2022, 73, 297–305. [Google Scholar] [CrossRef]
- Buss, N.; Henderson, S.J.; McFarlane, M.; Shenton, J.M.; Haan, L. Monoclonal antibody therapeutics: History and future. Curr. Opin. Pharmacol. 2012, 12, 615–622. [Google Scholar] [CrossRef]
- Wirth, T.; Parker, N.; Yla-Herttuala, S. History of gene therapy. Gene 2013, 525, 162–169. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 19, 101. [Google Scholar] [CrossRef]
- Beck, J.D.; Reidenbach, D.; Salomon, N.; Sahin, U.; Tureci, O.; Vormehr, M.; Kranz, L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef]
- Okuyama, R. Nurturing deep tech to solve social problems: Learning from COVID-19 mRNA vaccine development. Pathogens 2022, 11, 1469. [Google Scholar] [CrossRef]
- Development of the ChAdOx Vaccine Platform. Available online: https://www.jenner.ac.uk/about/the-oxford-astrazeneca-covid-19-vaccine/ChAdOx-platform (accessed on 12 September 2023).
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Ankomah, A.A.; Moa, A.; Chughtai, A.A. The long road of pandemic vaccine development to rollout: A systematic review on the lessons learnt from the 2009 H1N1 influenza pandemic. Am. J. Infect. Control 2022, 50, 735–742. [Google Scholar] [CrossRef]
- Bayani, F.; Hashkavaei, N.S.; Arjmand, S.; Rezaei, S.; Uskokovic, V.; Alijanianzadeh, M.; Uversky, V.N.; Siadat, S.O.R.; Mozaffari-Jovin, S.; Sefidbakht, Y. An overview of the vaccine platforms to combat COVID-19 with a focus on the subunit vaccines. Prog. Biophys. Mol. Biol. 2023, 178, 32–49. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Wang, Y. Recent advances in the production of recombinant subunit vaccines in Pichia pastoris. Bioengineered 2016, 7, 155–165. [Google Scholar] [CrossRef]
- Vartak, A.; Sucheck, S.J. Recent Advances in Subunit Vaccine Carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef]
- Kheirvari, M.; Liu, H.; Tumban, E. Virus-like Particle Vaccines and Platforms for Vaccine Development. Viruses 2023, 15, 1109. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–134. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Mottaghi-Dastjerdi, N.; Raad, M.S.R. A Review of Virus-Like Particle-Based SARS-CoV-2 Vaccines in Clinical Trial Phases. Iran. J. Pharm. Res. 2022, 21, e127042. [Google Scholar] [CrossRef]
- Takeyama, N.; Kiyono, H.; Yuki, Y. Plant-based vaccines for animals and humans: Recent advances in technology and clinical trials. Ther. Adv. Vaccines 2015, 3, 139–154. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammade-Samani, S.; Firouzabadi, N.; Dehshahri, A.; Vazin, A. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. Int. Immunopharmacol. 2021, 100, 108162. [Google Scholar] [CrossRef]
- Kumar, P.; Bird, C.; Holland, D.; Joshi, S.B.; Volkin, D.B. Current and next-generation formulation strategies for inactivated polio vaccines to lower costs, increase coverage, and facilitate polio eradication. Hum. Vaccin. Immunother. 2022, 18, 2154100. [Google Scholar] [CrossRef]
- Donaldson, B.; Lateef, Z.; Walker, G.; Young, S.L.; Ward, V.K. Virus-like particle vaccines: Immunology and formulation for clinical translation. Expert. Rev. Vaccines 2018, 17, 833–849. [Google Scholar] [CrossRef]
- Vasireddy, D.; Atluri, P.; Malayala, S.V.; Vanaparthy, R.; Mohan, G. Review of COVID-19 Vaccines Approved in the United States of America for Emergency Use. J. Clin. Med. Res. 2021, 13, 204–213. [Google Scholar] [CrossRef]
- Joe, C.C.; Jiang, J.; Linke, T.; Li, Y.; Fedosyuk, S.; Gupta, G.; Berg, A.; Segireddy, R.R.; Mainwaring, D.; Joshi, A.; et al. Manufacturing a chimpanzee adenovirus-vectored SARS-CoV-2 vaccine to meet global needs. Biotechnol. Bioeng. 2022, 119, 48–58. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Lore, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The use of viral vectors in vaccine development. NPJ Vaccines 2022, 7, 75. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef]
- Stephenson, J.R. Genetically modified viruses: Vaccines by design. Curr. Pharm. Biotechnol. 2001, 2, 47–76. [Google Scholar] [CrossRef]
- Moderna Announces First Participant Dosed in NIH-Led Phase 1 Study of mRNA Vaccine (mRNA-1273) against Novel Coronavirus. Available online: https://www.businesswire.com/news/home/20200316005666/en/Moderna-Announces-First-Participant-Dosed-in-NIH-led-Phase-1-Study-of-mRNA-Vaccine-mRNA-1273-Against-Novel-Coronavirus (accessed on 12 September 2023).
- Pather, S.; Madhi, S.A.; Cowling, B.J.; Moss, P.; Kamil, J.P.; Ciesek, S.; Muik, A.; Tureci, O. SARS-CoV-2 Omicron variants: Burden of disease, impact on vaccine effectiveness and need for variant-adapted vaccines. Front. Immunol. 2023, 14, 1130539. [Google Scholar] [CrossRef]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef]
- NIKKEI. 7 May 2022. Available online: https://www.nikkei.com/article/DGKKZO60565180W2A500C2TB0000/ (accessed on 14 September 2023).
- Japanese Science and Technology Indicators 2022. Available online: https://www.nistep.go.jp/research/science-and-technology-indicators-and-scientometrics/indicators (accessed on 14 September 2023).
- Okuyama, R. Academia’s contribution to drug discovery: Current status and perspectives in Japan. Translat. Regulat. Sci. 2023, 5. in press. [Google Scholar] [CrossRef]
- Jain, S.; Venkataraman, A.; Wechsler, M.E.; Peppas, N. Messenger RNA-based vaccines: Past, present, and future directions in the context of the COVID-19 pandemic. Adv. Drug Deliv. Rev. 2021, 179, 114000. [Google Scholar] [CrossRef]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef]
- Hornung, V.; Ellegast, J.; Kim, S.; Brzózka, K.; Jung, A.; Kato, H.; Poeck, H.; Akira, S.; Conzelmann, K.K.; Schlee, M.; et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 2006, 314, 994–997. [Google Scholar] [CrossRef]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Bernard, M.C.; Bazin, E.; Petiot, N.; Lemdani, K.; Commandeur, S.; Verdelet, C.; Margot, S.; Perkov, V.; Ripoll, M.; Garinot, M.; et al. The impact of nucleoside base modification in mRNA vaccine is influenced by the chemistry of its lipid nanoparticle delivery system. Mol. Ther. Nucleic Acids 2023, 32, 794–806. [Google Scholar] [CrossRef]
- Nance, K.D.; Meier, J.L. Modifications in an Emergency: The Role of N1-Methylpseudouridine in COVID-19 Vaccines. ACS Cent. Sci. 2021, 7, 748–756. [Google Scholar] [CrossRef]
- Stepinski, J.; Waddell, C.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3′-O-methyl)GpppG and 7-methyl (3′-deoxy)GpppG. RNA 2001, 7, 1486–1495. [Google Scholar]
- Shanmugasundaram, M.; Senthilvelan, A.; Kore, A.R. Recent Advances in Modified Cap Analogs: Synthesis, Biochemical Properties, and mRNA Based Vaccines. Chem. Rec. 2022, 22, e202200005. [Google Scholar] [CrossRef]
- Kim, S.C.; Sekhon, S.S.; Shin, W.R.; Ahn, G.; Cho, B.K.; Ahn, J.Y.; Kim, Y.H. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol. Cell Toxicol. 2022, 18, 1–8. [Google Scholar] [CrossRef]
- Kudla, G.; Lipinski, L.; Caffin, F.; Helwak, A.; Zylicz, M. High guanine and cytosine content increases mRNA levels in mammalian cells. PLoS Biol. 2006, 4, e180. [Google Scholar] [CrossRef]
- Pollard, C.; Rejman, J.; De Haes, W.; Verrier, B.; Van Gulck, E.; Naessens, T.; De Smedt, S.; Bogaert, P.; Grooten, J.; Vanham, G.; et al. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol. Ther. 2013, 21, 251–259. [Google Scholar] [CrossRef]
- Kariko, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef]
- Weissman, D.; Pardi, N.; Muramatsu, H.; Kariko, K. HPLC purification of in vitro transcribed long RNA. Methods Mol. Biol. 2013, 969, 43–54. [Google Scholar]
- Baiersdörfer, M.; Boros, G.; Muramatsu, H.; Mahiny, A.; Vlatkovic, I.; Sahin, U.; Karikó, K. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol. Ther. Nucleic Acids 2019, 15, 26–35. [Google Scholar] [CrossRef]
- Hama, S.; Akita, H.; Iida, S.; Mizuguchi, H.; Harashima, H. Quantitative and mechanism-based investigation of post-nuclear delivery events between adenovirus and lipoplex. Nucleic Acids Res. 2007, 35, 1533–1543. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8529–8533. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; Smedt, S.C.D.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Nelson, J.; Sorensen, E.W.; Mintri, S.; Rabideau, A.E.; Zheng, W.; Besin, G.; Khatwani, N.; Su, S.V.; Miracco, E.J.; Issa, W.J.; et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 2020, 6, eaaz6893. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 5, 237. [Google Scholar] [CrossRef]
- Milicic, A.; Rollier, C.S.; Tang, C.K.; Longley, R.; Hill, A.V.S.; Reyes-Sandoval, A. Adjuvanting a viral vectored vaccine against pre-erythrocytic malaria. Sci. Rep. 2017, 7, 7284. [Google Scholar] [CrossRef]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.-G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef]
- Pham, J.; Su, L.D.; Hanson, K.E.; Hogan, C.A. Sequence-based diagnostics and precision medicine in bacterial and viral infections: From bench to bedside. Curr. Opin. Infect. Dis. 2023, 36, 228–234. [Google Scholar] [CrossRef]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef]
- Tatsis, N.; Ertl, H.C. Adenoviruses as vaccine vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023? Viruses 2023, 15, 698. [Google Scholar] [CrossRef]
- Wilson, J.M. Gendicine: The first commercial gene therapy product. Hum. Gene Ther. 2005, 16, 1014–1015. [Google Scholar] [CrossRef]
- Liang, M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr. Cancer Drug Targets 2018, 18, 171–176. [Google Scholar] [CrossRef]
- Mendonga, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 2021, 6, 97. [Google Scholar] [CrossRef]
- Dicks, M.D.; Spencer, A.J.; Edwards, N.J.; Wadell, G.; Bojang, K.; Gilbert, S.C.; Hill, A.V.; Cottingham, M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: Improved systems for vector derivation and comparative immunogenicity. PLoS ONE 2012, 7, e40385. [Google Scholar] [CrossRef]
- Graham, S.P.; McLean, R.K.; Spencer, A.J.; Belij-Rammerstorfer, S.; Wright, D.; Ulaszewska, M.; Edwards, J.C.; Hayes, J.W.; Martini, V.; Thakur, N.; et al. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ Vaccines 2020, 5, 69. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Wold, W.S.; Gooding, L.R. Adenovirus region E3 proteins that prevent cytolysis by cytotoxic T cells and tumor necrosis factor. Mol. Biol. Med. 1989, 6, 433–452. [Google Scholar]
- Sullivan, N.J.; Hensley, L.; Asiedu, C.; Geisbert, T.W.; Stanley, D.; Johnson, J.; Honko, A.; Olinger, G.; Bailey, M.; Geisbert, J.B.; et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat. Med. 2011, 17, 1128–1131. [Google Scholar] [CrossRef]
- Pereira, I.R.; Vilar-Pereira, G.; Marques, V.; da Silva, A.A.; Caetano, B.; Moreira, O.C.; Machado, A.V.; Bruna-Romero, O.; Rodrigues, M.M.; Gazzinelli, R.T.; et al. A human type 5 adenovirus-based Trypanosoma cruzi therapeutic vaccine re-programs immune response and reverses chronic cardiomyopathy. PLoS Pathog. 2015, 11, e1004594. [Google Scholar] [CrossRef]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A.; et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef]
- Barough, D.H. Challenges in the development of an HIV-1 vaccine. Nature 2008, 455, 613–619. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Guerrero, M.L.; Navarro, S.R.M.; et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: An international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2022, 399, 237–248. [Google Scholar]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Bailey, M.; Hensley, L.; Asiedu, C.; Geisbert, J.; Stanley, D.; Honko, A.; Johnson, J.; Mulangu, S.; Pau, M.G.; et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J. Virol. 2011, 85, 4222–4233. [Google Scholar] [CrossRef]
- Custers, J.; Kim, D.; Leyssen, M.; Gurwith, M.; Tomaka, F.; Robertson, J.; Heijnen, E.; Condit, R.; Shukarev, G.; Heerwegh, D.; et al. Vaccines based on replication incompetent Ad26 viral vectors: Standardized template with key considerations for a risk/benefit assessment. Vaccine 2021, 39, 3081–3101. [Google Scholar] [CrossRef]
- Bos, R.; Rutten, L.; van der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020, 5, 91. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Tomas-Grau, R.H.; Maldonado-Galdeano, C.; López, M.A.; Pingitore, E.V.; Aznar, P.; Alcorta, M.E.; del Mar Vélez, E.M.; Stagnetto, A.; Soliz-Santander, S.E.; Ávila, C.L.; et al. Humoral immunoresponse elicited against an adenoviral-based SARS-CoV-2 coronavirus vaccine in elderly patients. Aging 2022, 14, 7193–7205. [Google Scholar] [CrossRef]
- Freeman, C. The ‘National System of Innovation’ in historical perspective. Camb. J. Econ. 1995, 19, 5–24. [Google Scholar]
- An Interview with Dr. Derrick Rossi, Co-Founder of Moderna Therapeutics. Available online: http://www.simplyblood.org/2021/03/an-interview-with-dr-derrick-rossi-co.html (accessed on 4 October 2023).
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef]
- Person of the Year 2010. Available online: https://content.time.com/time/specials/packages/article/0,28804,2036683_2036767_2037437,00.html (accessed on 4 October 2023).
- Dolgin, E. Business: The billion-dollar biotech. Nature 2015, 522, 26–28. [Google Scholar] [CrossRef]
- Flagship Pioneering, HP. Available online: https://www.flagshippioneering.com/people/noubar-afeyan/ (accessed on 4 October 2023).
- Interview: Moderna Co-founder Robert Ranger. Available online: https://www.theguardian.com/society/2022/mar/12/moderna-co-founder-robert-langer-covid-19-coronavirus-interview (accessed on 4 October 2023).
- AstraZeneca Press Release. Available online: https://www.astrazeneca.com/media-centre/press-releases/2013/astrazeneca-moderna-therapeutics-cardiometabolic-diseases-cancer-treatment-21032013.html# (accessed on 6 October 2023).
- Alexion Press Release. Available online: https://media.alexion.com/news-releases/news-release-details/alexion-pharmaceuticals-and-moderna-therapeutics-announce (accessed on 6 October 2023).
- Merck Press Release. Available online: https://www.merck.com/news/merck-and-moderna-announce-strategic-collaboration-to-advance-novel-mrna-based-personalized-cancer-vaccines-with-keytruda-pembrolizumab-for-the-treatment-of-multiple-types-of-cancer/ (accessed on 6 October 2023).
- Merck Press Release. Available online: https://www.merck.com/news/moderna-and-merck-expand-mrna-cancer-vaccines-collaboration/ (accessed on 6 October 2023).
- Moran, N. AstraZeneca juggernaut heads for Cambridge. Nat. Biotechnol. 2013, 31, 476–478. [Google Scholar] [CrossRef]
- Moderna Homepage. Available online: https://www.modernatx.com/partnerships/strategic-collaborators (accessed on 6 October 2023).
- Moderna feud with NIH over COVID vaccine. Nat. Biotechnol. 2021, 39, 1481.
- NIAID News Release. Available online: https://www.niaid.nih.gov/news-events/statement-nih-and-barda-fda-emergency-use-authorization-moderna-covid-19-vaccine (accessed on 6 October 2023).
- New York Times. 23 February 2023. Available online: https://www.nytimes.com/2023/02/23/science/moderna-covid-vaccine-patent-nih.html (accessed on 6 October 2023).
- Moderna Homepage. Available online: https://www.modernatx.com/power-of-mrna/modernas-mrna-platform (accessed on 7 October 2023).
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- The Woman Developing the Next Generation of Cancer Immunotherapy. Available online: https://www.labiotech.eu/interview/ozlem-tureci-cancer-immunotherapy (accessed on 15 August 2023).
- Wöll, S.; Schlitter, A.M.; Dhaene, K.; Roller, M.; Esposito, I.; Sahin, U.; Tuereci, O. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int. J. Cancer 2014, 134, 731–739. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Schuler, M.H.; Zvirbule, Z.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnyk, Y.; Vynnychenko, I.; Fadeeva, N.; Nechaeva, M.; et al. FAST: An international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without IMAB362, a first-in-class anti-CLDN18.2 antibody, as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Ganymed’s Claudin win. Nat. Biotechnol. 2017, 35, 5.
- Boczkowski, D.; Nair, S.K.; Snyder, D.; Gilboa, E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 1996, 184, 465–472. [Google Scholar] [CrossRef]
- Pascolo, S. The messenger’s great message for vaccination. Expert Rev. Vaccines 2015, 14, 153–156. [Google Scholar] [CrossRef]
- Orosz, M. German Billionaire Thomas Struengmann Says a Coronavirus Vaccine from His Biotech Firm Would Be a Dream Come True. Forbes, 1 April 2020. [Google Scholar]
- BioNTech Homepage. Available online: https://www.biontech.com/int/en/home/about/who-we-are/history.html (accessed on 7 October 2023).
- Pfizer Press Release. Available online: https://www.pfizer.com/news/press-release/press-release-detail/biontech-signs-collaboration-agreement-pfizer-develop-mrna (accessed on 7 October 2023).
- Their Coronavirus Vaccine Candidate Has Made Them Billionaires. This Modest German Turkish Couple Doesn’t Own a Car. The Washington Post, 12 November 2020.
- COVID-19 and Cancer Vaccines with Immunotherapy Pioneers Ugur Şahin and Özlem Türeci. Available online: https://www.cancerresearch.org/blog/december-2021/mrna-covid-cancer-vaccines-ugur-sahin-ozlem-tureci (accessed on 8 October 2023).
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Nair, P. QnAs with Katalin Karikó. Proc. Natl. Acad. Sci. USA 2021, 118, e2119757118. [Google Scholar] [CrossRef]
- Neill, U.S. A conversation with Katalin Karikó. J. Clin. Investig. 2021, 131, e155559. [Google Scholar] [CrossRef]
- O’Hara, G.A.; Duncan, C.J.; Ewer, K.J.; Collins, K.A.; Elias, S.C.; Halstead, F.D.; Goodman, A.L.; Edwards, N.J.; Reyes-Sandoval, A.; Bird, P.; et al. Clinical assessment of a recombinant simian adenovirus ChAd63: A potent new vaccine vector. J. Infect. Dis. 2012, 205, 772–781. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Jenkin, D.; Morris, S.; Gilbert, S.; Kim, D.; Robertson, J.S.; Smith, E.R.; Martin, E.; Gurwith, M.; Chen, R.T. Vaccines based on the replication-deficient simian adenoviral vector ChAdOx1: Standardized template with key considerations for a risk/benefit assessment. Vaccine 2022, 40, 5248–5262. [Google Scholar] [CrossRef]
- The Jenner Institute Press Release. Available online: https://www.jenner.ac.uk/about/newsletter/jenner-institute-newsletter-autumn-2016/vaccitech-spin-out-kick-starts-with-ps10m-from-oxford-sciences-innovation (accessed on 8 October 2023).
- Vaccitech Homepage. Available online: https://www.vactitech.co.uk/about/ (accessed on 8 October 2023).
- Oxford University Innovation Press Release. Available online: https://innovation.ox.ac.uk/news/vaccitech-20m-series-a/ (accessed on 8 October 2023).
- Vaccitech Completes $168 Million Series B Financing to Advance Three Clinical Programs through Phase 2 Results. Bloomberg 17 March 2021. Available online: https://www.bloomberg.com/press-releases/2021-03-17/vaccitech-completes-168-million-series-b-financing-to-advance-three-clinical-programs-through-phase-2-results (accessed on 8 October 2023).
- UK Research and Innovation. The Story behind the Oxford-AstraZeneca COVID-19 Vaccine Success. Available online: https://www.ukri.org/news-and-events/tackling-the-impact-of-covid-19/vaccines-and-treatments/the-story-behind-the-oxford-astrazeneca-covid-19-vaccine-success/ (accessed on 9 October 2023).
- ChAdOx1: More than a Coronavirus Vaccine. Available online: https://www.nature.com/articles/d42473-021-00625-2 (accessed on 9 October 2023).
- IQVIA. The Global Use of Medicines 2022. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/library/publications/the-global-use-of-medicines-2022.pdf (accessed on 9 October 2023).
- Kneller, R. The importance of new companies for drug discovery: Origins of a decade of new drugs. Nat. Rev. Drug Discov. 2010, 9, 867–882. [Google Scholar] [CrossRef]
- Okuyama, R. Chronological Analysis of First-in-Class Drugs Approved from 2011 to 2022: Their Technological Trend and Origin. Pharmaceutics 2023, 15, 1794. [Google Scholar] [CrossRef]
- Okuyama, R. Strengthening the Competitiveness of Japan’s Pharmaceutical Industry: Analysis of Country Differences in the Origin of New Drugs and Japan’s Highly Productive Firm. Biol. Pharm. Bull. 2023, 46, 718–724. [Google Scholar] [CrossRef]
- Mainichi Newspaper. 7 May 2022. Available online: https://mainichi.jp/articles/20220507/ddm/002/010/126000c (accessed on 9 October 2023).
- NIKKEI Newspaper. 10 May 2021. Available online: https://www.nikkei.com/article/DGKKZO71685240Y1A500C2TL5000/ (accessed on 9 October 2023).
- NIKKEI Newspaper. 2 August 2023. Available online: https://www.nikkei.com/article/DGXZQOUC026GZ0S3A800C2000000/ (accessed on 9 October 2023).
- Ministry of Health, Labour and Welfare Homepage. Available online: https://www.cov19-vaccine.mhlw.go.jp/qa/0137.html (accessed on 9 October 2023).
- Ministry of Health, Labour and Welfare News Release. Available online: https://www.mhlw.go.jp/stf/newpage_34457.html (accessed on 9 October 2023).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 October 2023).
- Saville, M.; Cramer, J.P.; Downham, M.; Hacker, A.; Lurie, N.; Van der Veken, L.; Whelan, M.; Hatchett, R. Delivering Pandemic Vaccines in 100 Days—What Will It Take? N. Engl. J. Med. 2022, 387, e3. [Google Scholar] [CrossRef]
- Handel, A.S.; Muller, W.J.; Planet, P.J. Metagenomic Next-Generation Sequencing (mNGS): SARS-CoV-2 as an Example of the Technology’s Potential Pediatric Infectious Disease Applications. J. Pediatr. Infect. Dis. Soc. 2021, 10 (Suppl. S4), S69–S70. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Quer, J.; Colomer-Castell, S.; Campos, C.; Andrés, C.; Piñana, M.; Cortese, M.F.; González-Sánchez, A.; Garcia-Cehic, D.; Ibáñez, M.; Pumarola, T. Next-Generation Sequencing for Confronting Virus Pandemics. Viruses 2022, 14, 600. [Google Scholar] [CrossRef]
- Kallel, H.; Kamen, A.A. Large-scale adenovirus and poxvirus-vectored vaccine manufacturing to enable clinical trials. Biotechnol. J. 2015, 10, 741–747. [Google Scholar] [CrossRef]
- Slaoui, M.; Hepburn, M. Developing Safe and Effective Covid Vaccines—Operation Warp Speed’s Strategy and Approach. N. Engl. J. Med. 2020, 383, 1701–1703. [Google Scholar] [CrossRef]
- Winch, G.M.; Dao, D.; Maytorea-Sanchez, E.; Pinto, J.; Sergeeva, N.; Zhang, S. Operation Warp Speed: Projects responding to the COVID-19 pandemic. Proj. Leadersh. Soc. 2021, 2, 100019. [Google Scholar] [CrossRef]
- Ho, R.J.Y. Warp-Speed COVID-19 Vaccine Development: Beneficiaries of Maturation in Biopharmaceutical Technologies and Public-Private Partnerships. J. Pharm. Sci. 2021, 110, 615–618. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Husby, A.; Hansen, J.V.; Fosbøl, E.; Thiesson, E.M.; Madsen, M.; Thomsen, R.W.; Sørensen, H.T.; Andersen, M.; Wohlfahrt, J.; Gislason, G.; et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: Population based cohort study. BMJ 2021, 375, e068665. [Google Scholar] [CrossRef]
- Lai, F.T.T.; Li, X.; Peng, K.; Huang, L.; Ip, P.; Tong, X.; Chui, C.S.L.; Wan, E.Y.F.; Wong, C.K.H.; Chan, E.W.Y.; et al. Carditis After COVID-19 Vaccination with a Messenger RNA Vaccine and an Inactivated Virus Vaccine: A Case-Control Study. Ann. Intern. Med. 2022, 175, 362–370. [Google Scholar] [CrossRef]
- Andrews, N.J.; Stowe, J.; Ramsay, M.E.; Miller, E. Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: A national cohort study in England. Lancet Reg. Health Eur. 2022, 13, 100260. [Google Scholar] [CrossRef]
- de Gregorio, C.; Colarusso, L.; Calcaterra, G.; Bassareo, P.P.; Ieni, A.; Mazzeo, A.T.; Ferrazzo, G.; Noto, A.; Koniari, I.; Mehta, J.L.; et al. Cerebral Venous Sinus Thrombosis following COVID-19 Vaccination: Analysis of 552 Worldwide Cases. Vaccines 2022, 10, 232. [Google Scholar] [CrossRef]
- Global Entrepreneurship Monitor. Entrepreneurial Behavior and Attitudes. Available online: https://gemconsortium.org/economy-profiles/japan-2 (accessed on 14 October 2023).
| Vaccine Type | Product Name | Originator |
|---|---|---|
| RNA vaccine | GEMCOVAC-19 | Gennova Biopharmaceuticals Limited |
| Spikevax | Moderna | |
| Spikevax Bivalent Original/Omicron BA.1 | ||
| Spikevax Bivalent Original/Omicron BA.4/BA.5 | ||
| Comirnaty | BioNTech | |
| Comirnaty Bivalent Original/Omicron BA.1 | ||
| Comirnaty Bivalent Original/Omicron BA.4/BA.5 | ||
| AWcorna | Walvax | |
| Viral vector vaccine | iNCOVACC | Washington University/Bharat Biotech |
| Convidecia (inhaled type of Convidecia: Convidecia Air) | CanSino | |
| Gam-COVID-Vac (two-dose regimen: Sputnik V one-dose regimen: Sputnik Light) | Gamaleya | |
| Jcovden | Janssen (Johnson & Johnson) | |
| Vaxzevria | University of Oxford | |
| DNA vaccine | ZyCoV-D | Zydus Cadila |
| Inactivated vaccine | Covaxin | Bharat Biotech |
| KoviVac | Chumakov Center | |
| Turkovac | Health Institutes of Turkey | |
| FAKHRAVAC (MIVAC) | Organization of Defensive Innovation and Research | |
| QazVac | Research Institute for Biological Safety Problems (RIBSP) | |
| KCONVAC | Shenzhen Kangtai Biological Products Co | |
| COVIran Barekat | Shifa Pharmed Industrial Co | |
| Covilo | Sinopharm | |
| CoronaVac | Sinovac | |
| VLA2001 | Valneva | |
| Protein subunit vaccine | Zifivax | Anhui Zhifei Longcom/ Chinese Academy of Sciences |
| Noora vaccine | Baqiyatallah University of Medical Sciences | |
| Corbevax | Baylor College of Medicine/Texas Children’s Hospital Center/Dynavax technologies | |
| Abdala | Center for Genetic Engineering and Biotechnology | |
| Soberana 02 | Finlay Institute | |
| Soberana Plus | ||
| V-01 | Livzon Mabpharm Inc | |
| MVC-COV1901 | Medigen | |
| Recombinant SARS-CoV-2 Vaccine (CHO Cell) | National Vaccine and Serum Institute | |
| Nuvaxovid | Novavax | |
| IndoVac | PT Bio Farma/Baylor College of Medicine | |
| Razi Cov Pars | Razi Vaccine and Serum Research Institute | |
| VidPrevtyn Beta | Sanofi/GSK | |
| SKYCovione | SK Bioscience/University of Washington | |
| SpikoGen | Vaxine/CinnaGen Co. | |
| Aurora-CoV | Vector State Research Center of Virology and Biotechnology | |
| EpiVacCorona | ||
| Virus-like particles vaccine | Covifenz | Medicago |
| Type of Resource | Moderna | BioNTech | University of Oxford/Vaccitech |
|---|---|---|---|
| Technology seed/scientific background | -Pseudouridine discovery by Kariko et al. at University of Pennsylvania -Rossi’s research of cell transformation by mRNA at Boston Children’s Hospital | -mRNA cancer vaccine research by Gilboa at Duke University -Cancer immunotherapy research by Şahin and Türeci at University of Mainz | In-house research for novel chimpanzee adenovirus by Gilbert, Hill etc. at Jenner Institute |
| Entrepreneurial scientist | Derrick J. Rossi | Uğur Şahin, Özlem Türeci | Sarah Gilbert, Adrian Hill |
| Entrepreneur | Robert S. Langer | ||
| Investor | Noubar Afeyan at Flagship Pioneering | Thomas Strüngmann, Redmile Group | Oxford Sciences Innovation, GV, M&G Investment Management |
| Incumbent company | AstraZeneca, Alexion, Merck | Pfizer | AstraZeneca |
| Government agency | DARPA, NIAID | DSC, MRC, BBSRC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuyama, R. mRNA and Adenoviral Vector Vaccine Platforms Utilized in COVID-19 Vaccines: Technologies, Ecosystem, and Future Directions. Vaccines 2023, 11, 1737. https://doi.org/10.3390/vaccines11121737
Okuyama R. mRNA and Adenoviral Vector Vaccine Platforms Utilized in COVID-19 Vaccines: Technologies, Ecosystem, and Future Directions. Vaccines. 2023; 11(12):1737. https://doi.org/10.3390/vaccines11121737
Chicago/Turabian StyleOkuyama, Ryo. 2023. "mRNA and Adenoviral Vector Vaccine Platforms Utilized in COVID-19 Vaccines: Technologies, Ecosystem, and Future Directions" Vaccines 11, no. 12: 1737. https://doi.org/10.3390/vaccines11121737
APA StyleOkuyama, R. (2023). mRNA and Adenoviral Vector Vaccine Platforms Utilized in COVID-19 Vaccines: Technologies, Ecosystem, and Future Directions. Vaccines, 11(12), 1737. https://doi.org/10.3390/vaccines11121737





