Immunogenicity and Therapeutic Efficacy of a Sendai-Virus-Vectored HSV-2 Vaccine in Mouse and Guinea Pig Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses, Cells, and Animals
2.2. Study Design
2.3. Methods
3. Results
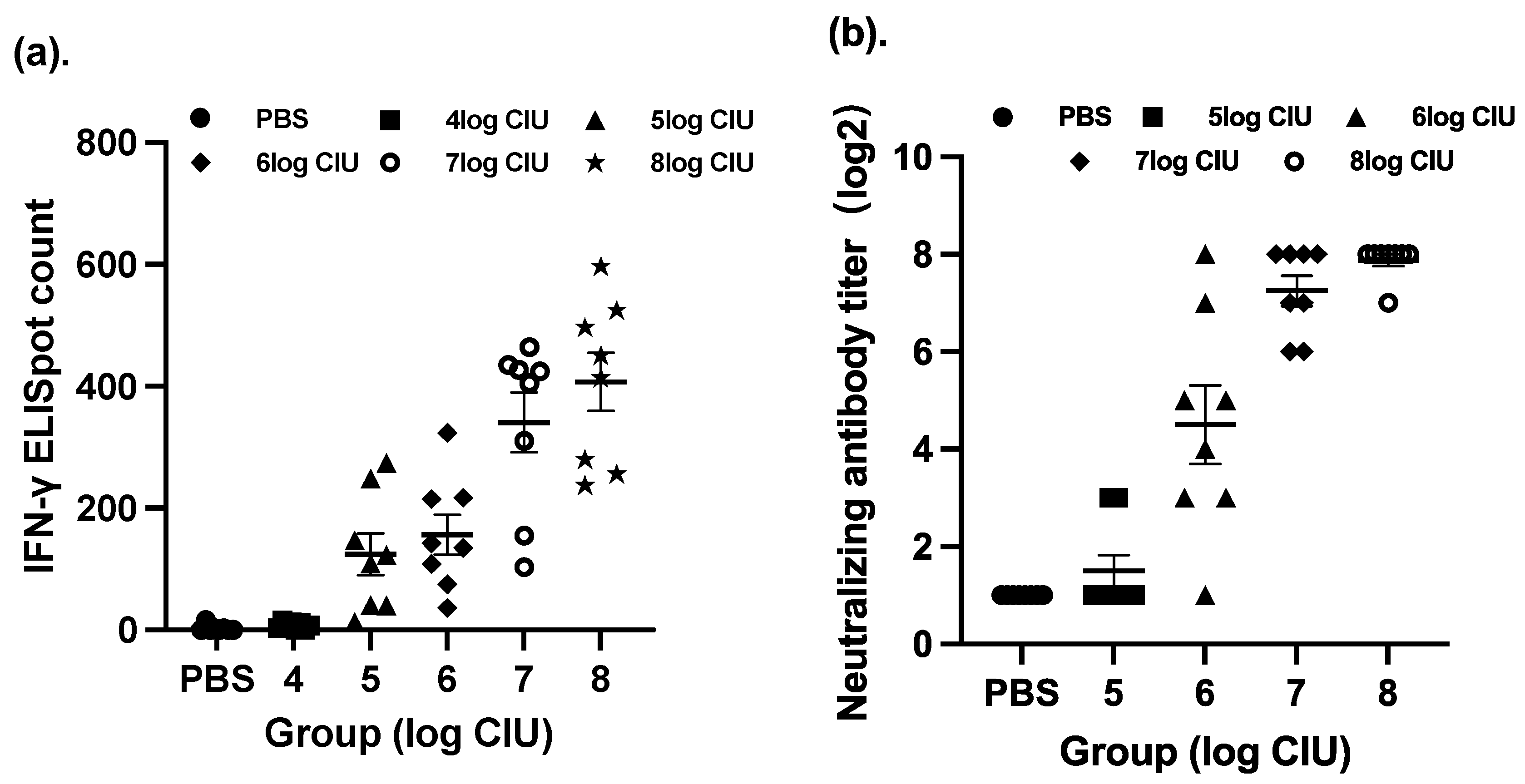
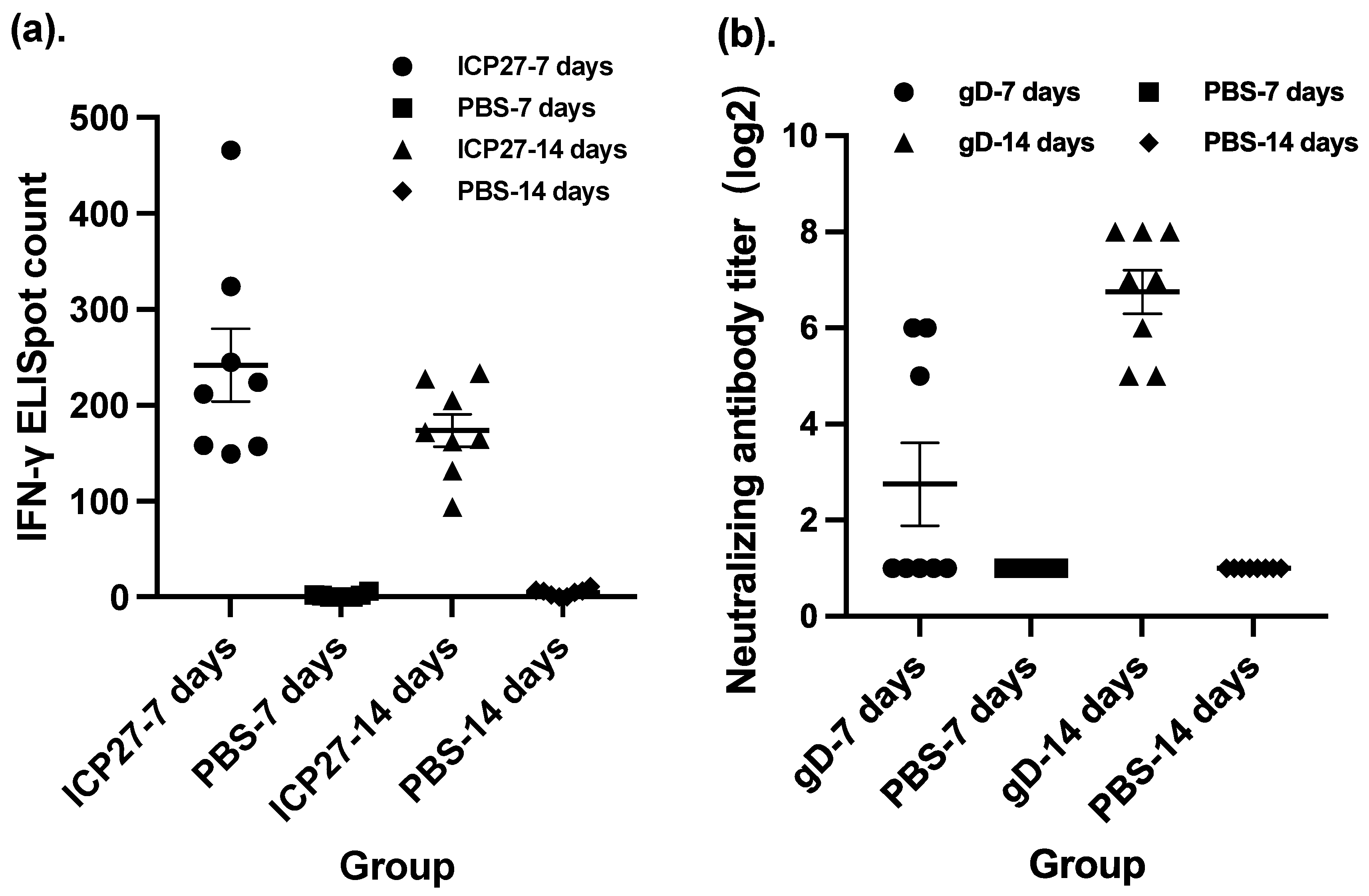
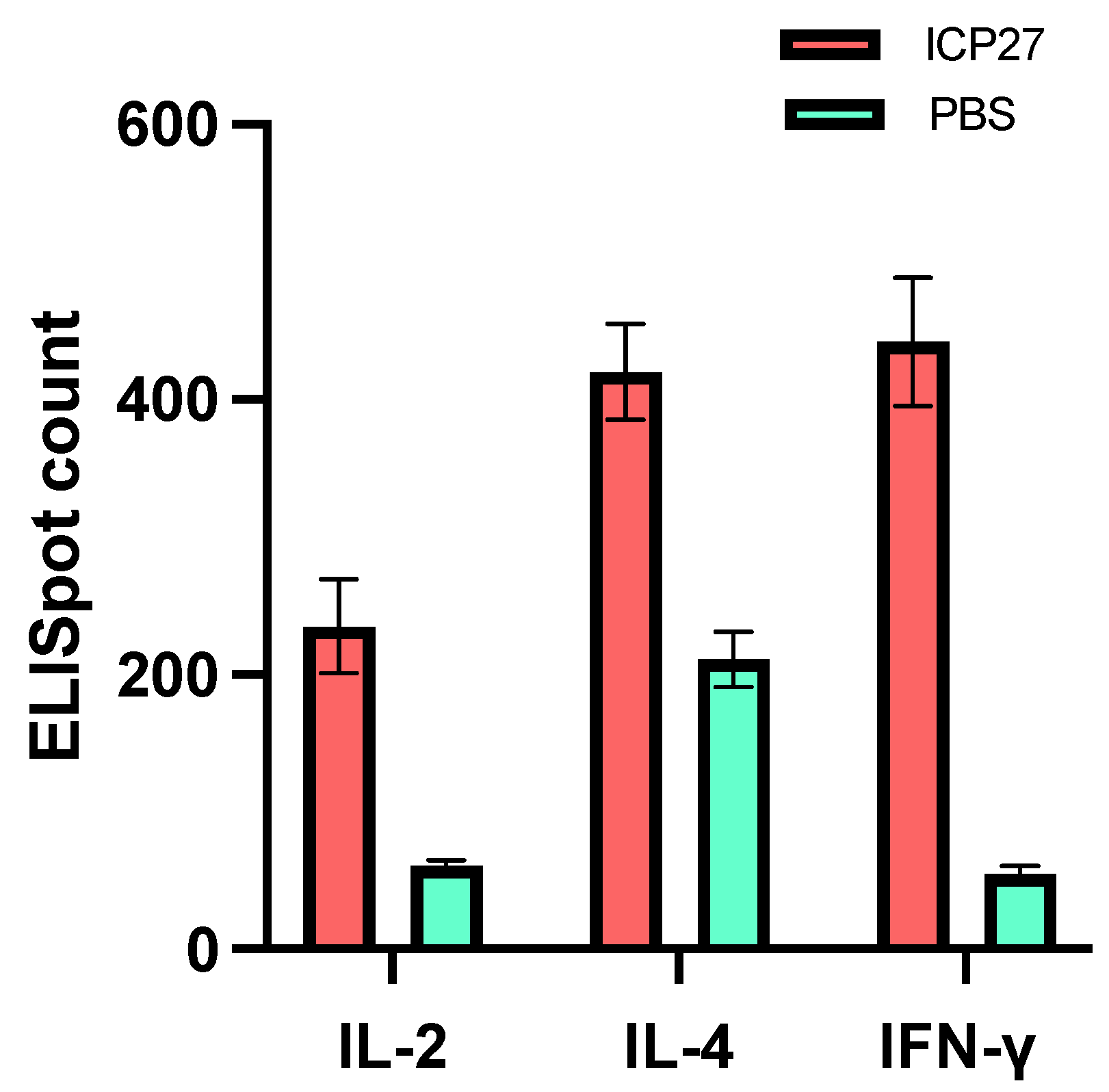
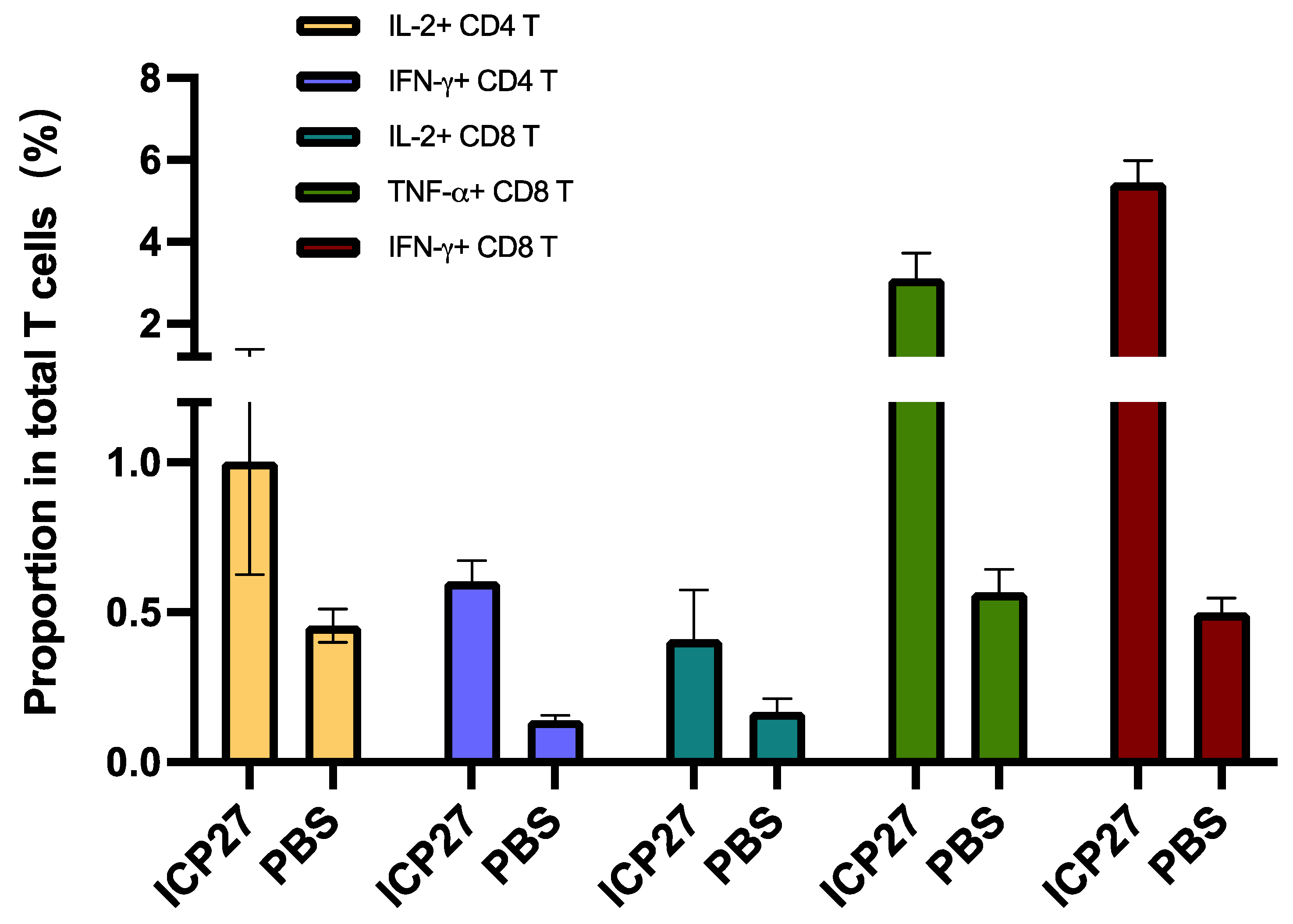
3.1. Immunogenicity in Mice
3.2. Vaccine Evaluation in a Guinea Pig Model of Recurrent HSV-2 Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, A.C.; Mohr, I. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol. 2012, 20, 604–611. [Google Scholar] [CrossRef]
- Gupta, R.; Warren, T.; Wald, A. Genital herpes. Lancet 2007, 370, 2127–2137. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; BenMohamed, L. Future viral vectors for the delivery of asymptomatic herpes epitope-based immunotherapeutic vaccines. Future Virol. 2010, 5, 525–528. [Google Scholar] [CrossRef]
- De Rose, D.U.; Bompard, S.; Maddaloni, C.; Bersani, I.; Martini, L.; Santisi, A.; Longo, D.; Ronchetti, M.P.; Dotta, A.; Auriti, C. Neonatal herpes simplex virus infection: From the maternal infection to the child outcome. J. Med. Virol. 2023, 95, e29024. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W. Neonatal herpes simplex infection. Clin. Microbiol. Rev. 2004, 17, 1–13. [Google Scholar] [CrossRef]
- Freeman, E.E.; Weiss, H.A.; Glynn, J.R.; Cross, P.L.; Whitworth, J.A.; Hayes, R.J. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and metaanalysis of longitudinal studies. AIDS 2006, 20, 73–83. [Google Scholar] [CrossRef]
- Schacker, T.; Ryncarz, A.J.; Goddard, J.; Diem, K.; Shaughnessy, M.; Corey, L. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA 1998, 280, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Thurman, A.R.; Doncel, G.F. Herpes simplex virus and HIV: Genital infection synergy and novel approaches to dual prevention. Int. J. STD AIDS 2012, 23, 613–619. [Google Scholar] [CrossRef]
- Sausen, D.G.; Shechter, O.; Gallo, E.S.; Dahari, H.; Borenstein, R. Herpes Simplex Virus, Human Papillomavirus, and Cervical Cancer: Overview, Relationship, and Treatment Implications. Cancers 2023, 15, 3692. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Kreisel, K.M.; Spicknall, I.H.; Gargano, J.W.; Lewis, F.M.T.; Lewis, R.M.; Markowitz, L.E.; Roberts, H.; Johnson, A.S.; Song, R.; Cyr, S.B.S.; et al. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2018. Sex. Transm. Dis. 2021, 48, 208–214. [Google Scholar] [CrossRef]
- Chesson, H.W.; Spicknall, I.H.; Bingham, A.; Brisson, M.; Eppink, S.T.; Farnham, P.G.; Kreisel, K.M.; Kumar, S.; Laprise, J.-F.; Peterman, T.A.; et al. The Estimated Direct Lifetime Medical Costs of Sexually Transmitted Infections Acquired in the United States in 2018. Sex. Transm. Dis. 2021, 48, 215–221. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bolan, G.A. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1–137. [Google Scholar]
- Sharma, D.; Sharma, S.; Akojwar, N.; Dondulkar, A.; Yenorkar, N.; Pandita, D.; Prasad, S.K.; Dhobi, M. An Insight into Current Treatment Strategies, Their Limitations, and Ongoing Developments in Vaccine Technologies against Herpes Simplex Infections. Vaccines 2023, 11, 206. [Google Scholar] [CrossRef]
- Johnston, C.; Gottlieb, S.L.; Wald, A. Status of vaccine research and development of vaccines for herpes simplex virus. Vaccine 2016, 34, 2948–2952. [Google Scholar] [CrossRef]
- Awasthi, S.; Shaw, C.; Friedman, H. Improving immunogenicity and efficacy of vaccines for genital herpes containing herpes simplex virus glycoprotein D. Expert Rev. Vaccines 2014, 13, 1475–1488. [Google Scholar] [CrossRef]
- Zhu, X.-P.; Muhammad, Z.S.; Wang, J.-G.; Lin, W.; Guo, S.-K.; Zhang, W. HSV-2 Vaccine: Current Status and Insight into Factors for Developing an Efficient Vaccine. Viruses 2014, 6, 371–390. [Google Scholar] [CrossRef]
- Johnston, C.; Koelle, D.M.; Wald, A. HSV-2: In pursuit of a vaccine. J. Clin. Investig. 2011, 121, 4600–4609. [Google Scholar] [CrossRef]
- Bernstein, D.I. Use of the Guinea pig model of genital herpes to evaluate vaccines and antivirals: Review. Antivir. Res. 2020, 180, 104821. [Google Scholar] [CrossRef]
- Stanberry, L.R.; Kern, E.R.; Richards, J.T.; Abbott, T.M.; Overall, J.C., Jr. Genital Herpes in Guinea Pigs: Pathogenesis of the Primary Infection and Description of Recurrent Disease. J. Infect. Dis. 1982, 146, 397–404. [Google Scholar] [CrossRef]
- Skoberne, M.; Cardin, R.; Lee, A.; Kazimirova, A.; Zielinski, V.; Garvie, D.; Lundberg, A.; Larson, S.; Bravo, F.J.; Bernstein, D.I.; et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs. J. Virol. 2013, 87, 3930. [Google Scholar] [CrossRef]
- Smith, J.B.; Herbert, J.J.; Truong, N.R.; Cunningham, A.L. Cytokines and chemokines: The vital role they play in herpes simplex virus mucosal immunology. Front. Immunol. 2022, 13, 936235. [Google Scholar] [CrossRef]
- McBride, J.A.; Striker, R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef]
- Hensel, J.A.; Khattar, V.; Ashton, R.; Ponnazhagan, S. Characterization of immune cell subtypes in three commonly used mouse strains reveals gender and strain-specific variations. Lab. Investig. 2019, 99, 93–106. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; Dhanushkodi, N.R.; Srivastava, R.; Prakash, S.; Coulon, P.-G.A.; Zayou, L.; Vahed, H.; Chentoufi, H.A.; Hormi-Carver, K.K.; BenMohamed, L. Combinatorial Herpes Simplex Vaccine Strategies: From Bedside to Bench and Back. Front. Immunol. 2022, 13, 849515. [Google Scholar] [CrossRef]
- Mitra, S.; Leonard, W.J. Biology of IL-2 and its therapeutic modulation: Mechanisms and strategies. J. Leukoc. Biol. 2018, 103, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, C.; Trivedi, S.; Wijesundara, D.K.; Jackson, R.J. IL-4 and IL-13 receptors: Roles in immunity and powerful vaccine adjuvants. Cytokine Growth Factor Rev. 2014, 25, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B. IFN-γ in tissue-immune homeostasis and antitumor immunity. Cell. Mol. Immunol. 2018, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Khanna, K.M.; Carriere, B.N.; Hendricks, R.L. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 2001, 75, 11178–11184. [Google Scholar] [CrossRef]
- Mikloska, Z.; Ruckholdt, M.; Ghadiminejad, I.; Dunckley, H.; Denis, M.; Cunningham, A.L. Monophosphoryl lipid A and QS21 increase CD8 T lymphocyte cytotoxicity to herpes simplex virus-2 infected cell proteins 4 and 27 through IFN-γ and IL-12 production. J. Immunol. 2000, 164, 5167–5176. [Google Scholar] [CrossRef] [PubMed]
- Harandi, A.M.; Svennerholm, B.; Holmgren, J.; Eriksson, K. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. Gen. Virol. 2001, 82, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Verjans, G.M.; Hintzen, R.Q.; van Dun, J.M.; Poot, A.; Millikan, J.C.; Laman, J.D.; Langerak, A.W.; Kinchington, P.R.; Osterhaus, A.D. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. USA 2007, 104, 3496–3501. [Google Scholar] [CrossRef] [PubMed]
- Preda, M.; Manolescu, L.S.C.; Chivu, R.D. Advances in Alpha Herpes Viruses Vaccines for Human. Vaccines 2023, 11, 1094. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, R.J.; Atanasiu, D.; Cairns, T.M.; Gallagher, J.R.; Krummenacher, C.; Cohen, G.H. Herpes virus fusion and entry: A story with many characters. Viruses 2012, 4, 800–832. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.H.; Dietzschold, B.; de Leon, M.P.; Long, D.; Golub, E.; Varrichio, A.; Pereira, L.; Eisenberg, R.J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984, 49, 102–108. [Google Scholar] [CrossRef]
- Van Wagoner, N.; Fife, K.; Leone, P.A.; Bernstein, D.I.; Warren, T.; Panther, L.; Novak, R.M.; Beigi, R.; Kriesel, J.; Tyring, S.; et al. Effects of Different Doses of GEN-003, a Therapeutic Vaccine for Genital HSV-2, on Viral Shedding and Lesions: Results of a Randomized Placebo-controlled Trial. J. Infect. Dis. 2018, 218, 1890–1899. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Wald, A.; Warren, T.; Fife, K.; Tyring, S.; Lee, P.; Van Wagoner, N.; Magaret, A.; Flechtner, J.B.; Tasker, S.; et al. Therapeutic Vaccine for Genital Herpes Simplex Virus-2 Infection: Findings from a Randomized Tria. J. Infect. Dis. 2017, 215, 856–864. [Google Scholar] [CrossRef]
- Dutton, J.L.; Li, B.; Woo, W.-P.; Marshak, J.O.; Xu, Y.; Huang, M.-L.; Dong, L.; Frazer, I.H.; Koelle, D.M. A Novel DNA Vaccine Technology Conveying Protection against a Lethal Herpes Simplex Viral Challenge in Mice. PLoS ONE 2013, 8, e76407. [Google Scholar] [CrossRef]
- Dutton, J.L.; Woo, W.-P.; Chandra, J.; Xu, Y.; Li, B.; Finlayson, N.; Griffin, P.; Frazer, I.H. An escalating dose study to assess the safety, tolerability and immunogenicity of a Herpes Simplex Virus DNA vaccine, COR-1. Hum. Vaccines 2016, 12, 3079–3088. [Google Scholar] [CrossRef]
- Corey, L.; Langenberg, A.G.; Ashley, R.; Sekulovich, R.E.; Izu, A.E.; Douglas, J.M., Jr.; Handsfield, H.H.; Warren, T.; Marr, L.; Tyring, S.; et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: Two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 1999, 282, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Stanberry, L.R.; Spruance, S.L.; Cunningham, A.L.; Bernstein, D.I.; Mindel, A.; Sacks, S.; Tyring, S.; Aoki, F.Y.; Slaoui, M.; Denis, M.; et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 2002, 347, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.W.; Malik, P.; Clements, J.B. The herpes simplex virus ICP27 protein: A multifunctional post-transcriptional regulator of gene expression. Biochem. Soc. Trans. 2005, 33, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Sandri-Goldin, R.M. The many roles of the regulatory protein ICP27 during herpes simplex virus infection. Front. Biosci. 2008, 13, 5241–5256. [Google Scholar] [CrossRef] [PubMed]
- Manickan, E.; Francotte, M.; Kuklin, N.; Dewerchin, M.; Molitor, C.; Gheysen, D.; Slaoui, M.; Rouse, B.T. Vaccination with recombinant vaccinia viruses expressing ICP27 induces protective immunity against herpes simplex virus through CD4+ Th1+ T cells. J. Virol. 1995, 69, 4711. [Google Scholar] [CrossRef] [PubMed]
- Bright, H.; Perez, D.L.; Christy, C.; Cockle, P.; Eyles, J.E.; Hammond, D.; Khodai, T.; Lang, S.; West, K.; Loudon, P.T. The efficacy of HSV-2 vaccines based on gD and gB is enhanced by the addition of ICP27. Vaccine 2012, 30, 7529–7535. [Google Scholar] [CrossRef]
- Latt, R.J.; Khodai, T.; Townend, T.J.; Bright, H.H.; Cockle, P.; Perez-Tosar, L.; Webster, R.; Champion, B.; Hickling, T.P.; Mirza, F. CD8+ T Lymphocyte Epitopes from The Herpes Simplex Virus Type 2 ICP27, VP22 and VP13/14 Proteins To Facilitate Vaccine Design And Characterization. Cells 2013, 2, 19–42. [Google Scholar]
- Bitzer, M.; Armeanu, S.; Lauer, U.M.; Neubert, W.J. Sendai virus vectors as an emerging negative-strand RNA viral vector system. J. Gene Med. 2003, 5, 543–553. [Google Scholar] [CrossRef]
- Slobod, K.S.; Shenep, J.L.; Luján-Zilbermann, J.; Allison, K.; Brown, B.; Scroggs, R.A.; Portner, A.; Coleclough, C.; Hurwitz, J.L. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 2004, 22, 3182–3186. [Google Scholar] [CrossRef]
- Adderson, E.; Branum, K.; Sealy, R.E.; Jones, B.G.; Surman, S.L.; Penkert, R.; Freiden, P.; Slobod, K.S.; Gaur, A.H.; Hayden, R.T.; et al. Safety and immunogenicity of an intranasal Sendai virus-based human parainfluenza virus type 1 vaccine in 3- to 6-year-old children. Clin. Vaccine Immunol. 2015, 22, 298–303. [Google Scholar] [CrossRef]
- Lyn, D.; Gill, D.S.; Scroggs, R.A.; Portner, A. The nucleoproteins of human parainfluenza virus type 1 and Sendai virus share amino acid sequences and antigenic and structural determinants. J. Gen. Virol. 1991, 72 Pt 4, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Curran, J.; Pelet, T.; Kolakofsky, D.; Ray, R.; Compans, R.W. The P gene of human parainfluenza virus type 1 encodes P and C proteins but not a cysteine-rich V protein. J. Virol. 1991, 65, 3406–3410. [Google Scholar] [CrossRef]
- Power, U.F.; Ryan, K.W.; Portner, A. Sequence characterization and expression of the matrix protein gene of human parainfluenza virus type 1. Virology 1992, 191, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Merson, J.R.; Hull, R.A.; Estes, M.K.; Kasel, J.A. Molecular cloning and sequence determination of the fusion protein gene of human parainfluenza virus type 1. Virology 1988, 167, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gorman, W.L.; Gill, D.S.; Scroggs, R.A.; Portner, A. The hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus type 1 and Sendai virus have high structure-function similarity with limited antigenic cross-reactivity. Virology 1990, 175, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, T.; Bousse, T.; Portner, A. Molecular cloning and expression of human parainfluenza virus type 1 L gene. Virus Res. 2000, 70, 45–53. [Google Scholar] [CrossRef]
- Hara, H.; Hara, H.; Hironaka, T.; Inoue, M.; Iida, A.; Shu, T.; Hasegawa, M.; Nagai, Y.; Falsey, A.R.; Kamali, A.; et al. Prevalence of specific neutralizing antibodies against Sendai virus in populations from different geographic areas: Implications for AIDS vaccine development using Sendai virus vectors. Hum. Vaccines 2011, 7, 639–645. [Google Scholar] [CrossRef]
- Nyombayire, J.; Anzala, O.; Gazzard, B.; Karita, E.; Bergin, P.; Hayes, P.; Kopycinski, J.; Omosa-Manyonyi, G.; Jackson, A.; Bizimana, J.; et al. First-in-Human Evaluation of the Safety and Immunogenicity of an Intranasally Administered Replication-Competent Sendai Virus–Vectored HIV Type 1 Gag Vaccine: Induction of Potent T-Cell or Antibody Responses in Prime-Boost Regimens. J. Infect. Dis. 2017, 215, 95–104. [Google Scholar] [CrossRef]
- Moriya, C.; Horiba, S.; Kurihara, K.; Kamada, T.; Takahara, Y.; Inoue, M.; Iida, A.; Hara, H.; Shu, T.; Hasegawa, M.; et al. Intranasal Sendai viral vector vaccination is more immunogenic than intramuscular under pre-existing anti-vector antibodies. Vaccine 2011, 29, 8557–8563. [Google Scholar] [CrossRef]



| Factors | Grouping (−logCIU) | Vaccination at | ||||
|---|---|---|---|---|---|---|
| Day0 | D7 | D14 | D21 | D35 | ||
| Dosage (SeV-dF/HSV-2-ICP27) | 4, 5, 6, 7, 8, PBS | √ | √ | / | / | / |
| Dosage (SeV-dF/HSV-2-gD) | 5, 6, 7, 8, PBS | √ | √ | / | √ | √ |
| Time interval: 7 days (SeV-dF/HSV-2-ICP27) | 8, PBS | √ | √ | / | / | / |
| Time interval: 14 days (SeV-dF/HSV-2-ICP27) | 8, PBS | √ | / | √ | / | / |
| Time interval: 7 days (SeV-dF/HSV-2-gD) | 8, PBS | √ | √ | √ | √ | / |
| Time interval: 14 days (SeV-dF/HSV-2-gD) | 8, PBS | √ | √ | / | √ | √ |
| Strategy: Simultaneous vaccination | 8, PBS | √ | √ | / | √ | √ |
| Strategy: Sequential vaccination | 8, PBS | √ | √ | / | √ | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Su, W.; Li, S.; Zhao, T.; Huang, Q.; Wang, Y.; Wang, X.; Zhang, X.; Wei, J. Immunogenicity and Therapeutic Efficacy of a Sendai-Virus-Vectored HSV-2 Vaccine in Mouse and Guinea Pig Models. Vaccines 2023, 11, 1752. https://doi.org/10.3390/vaccines11121752
Ren X, Su W, Li S, Zhao T, Huang Q, Wang Y, Wang X, Zhang X, Wei J. Immunogenicity and Therapeutic Efficacy of a Sendai-Virus-Vectored HSV-2 Vaccine in Mouse and Guinea Pig Models. Vaccines. 2023; 11(12):1752. https://doi.org/10.3390/vaccines11121752
Chicago/Turabian StyleRen, Xiuxiu, Wenhao Su, Shishi Li, Tingting Zhao, Qiufang Huang, Yinan Wang, Xiaojie Wang, Xiaohuan Zhang, and Jiangbo Wei. 2023. "Immunogenicity and Therapeutic Efficacy of a Sendai-Virus-Vectored HSV-2 Vaccine in Mouse and Guinea Pig Models" Vaccines 11, no. 12: 1752. https://doi.org/10.3390/vaccines11121752





