First Investigation of the Optimal Timing of Vaccination of Nile Tilapia (Oreochromis niloticus) Larvae against Streptococcus agalactiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Husbandry
2.2. Bacterial Cultivation and Preparation of the Formalin-Killed S. agalactiae Vaccine (FKV-SA)
2.2.1. Bacterial Preparation
2.2.2. Formalin-Killed S. agalactiae Vaccine (FKV-SA) Preparation
2.3. Efficacy of the S. agalactiae Immersion Vaccine (FKV-SA) in Nile Tilapia Larvae
2.3.1. Experimental Design
2.3.2. Immersion Vaccination
2.3.3. Sample Collection
2.3.4. Measurement of Fish Weight and Size Prior to and Post-Vaccination
2.3.5. Quantification of Specific IgMs against FKV-SA via ELISA
2.3.6. Determination of Immune-Related Gene Expression in Tilapia Larvae after Immersion Vaccination
2.3.7. Immunohistochemical Analysis
2.4. Effects of Vaccination in Different Nile Tilapia Larval Stages (DAYCs 21, 28, 35, and 42) on Disease Resistance against S. agalactiae
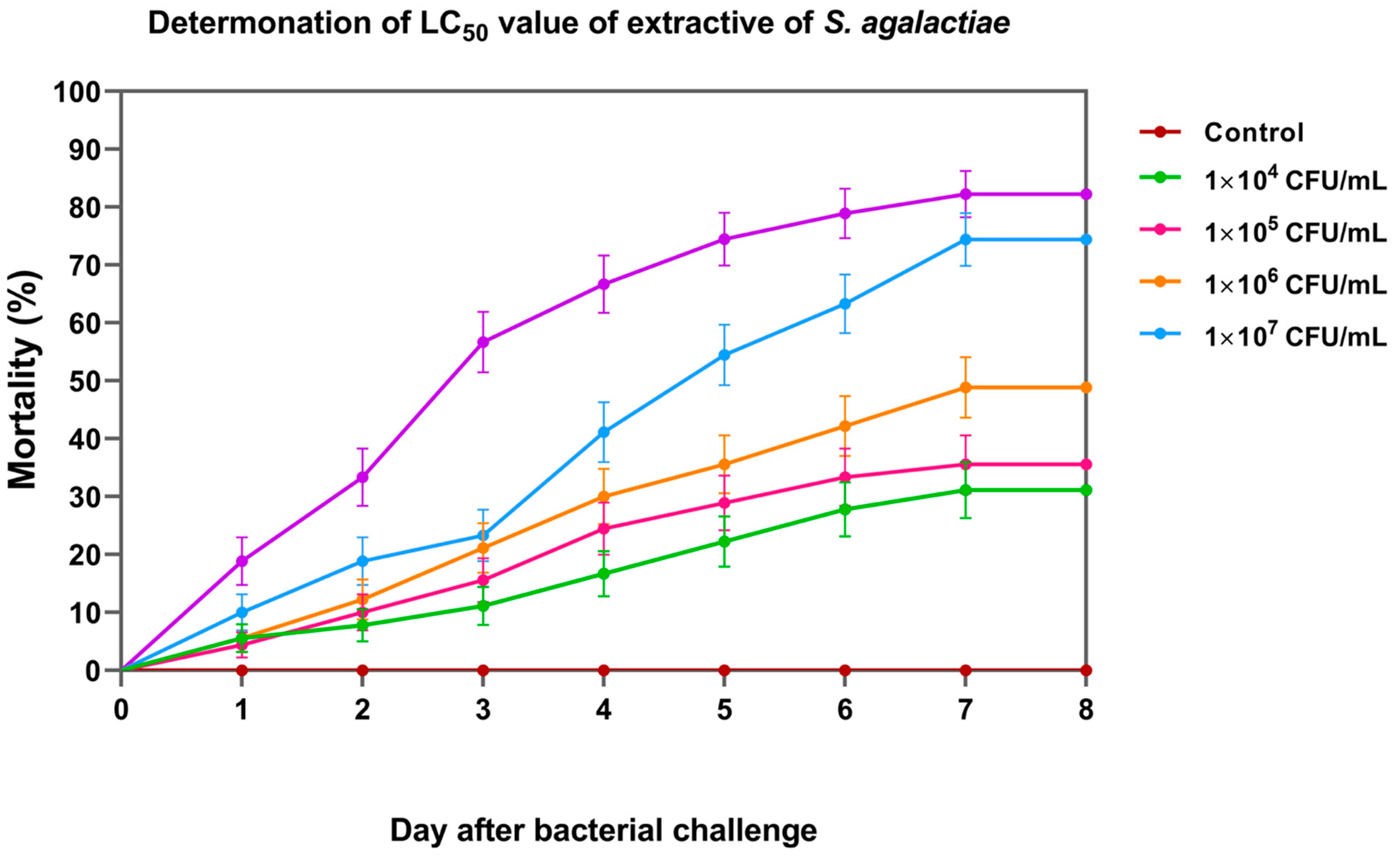
2.4.1. Assessment of the Time-Dependent Toxicity of the Lethal Concentration (LC50) of S. agalactiae in Nile Tilapia
2.4.2. Vaccination of Fish Larvae
2.4.3. Challenge Test
2.5. Statistical Analysis
3. Results
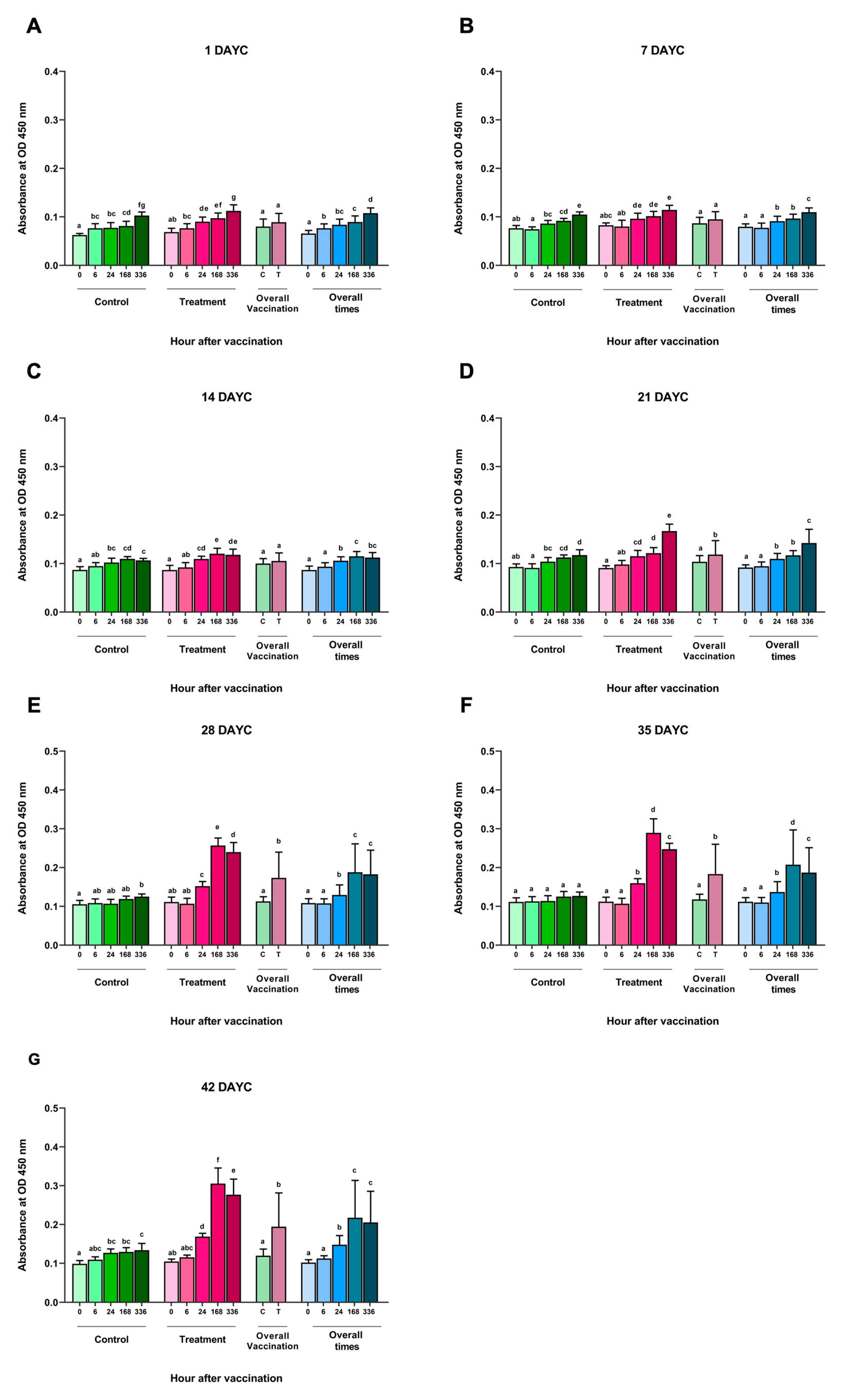
3.1. Determination of the Levels of Serum IgM Specific to S. agalactiae via ELISA
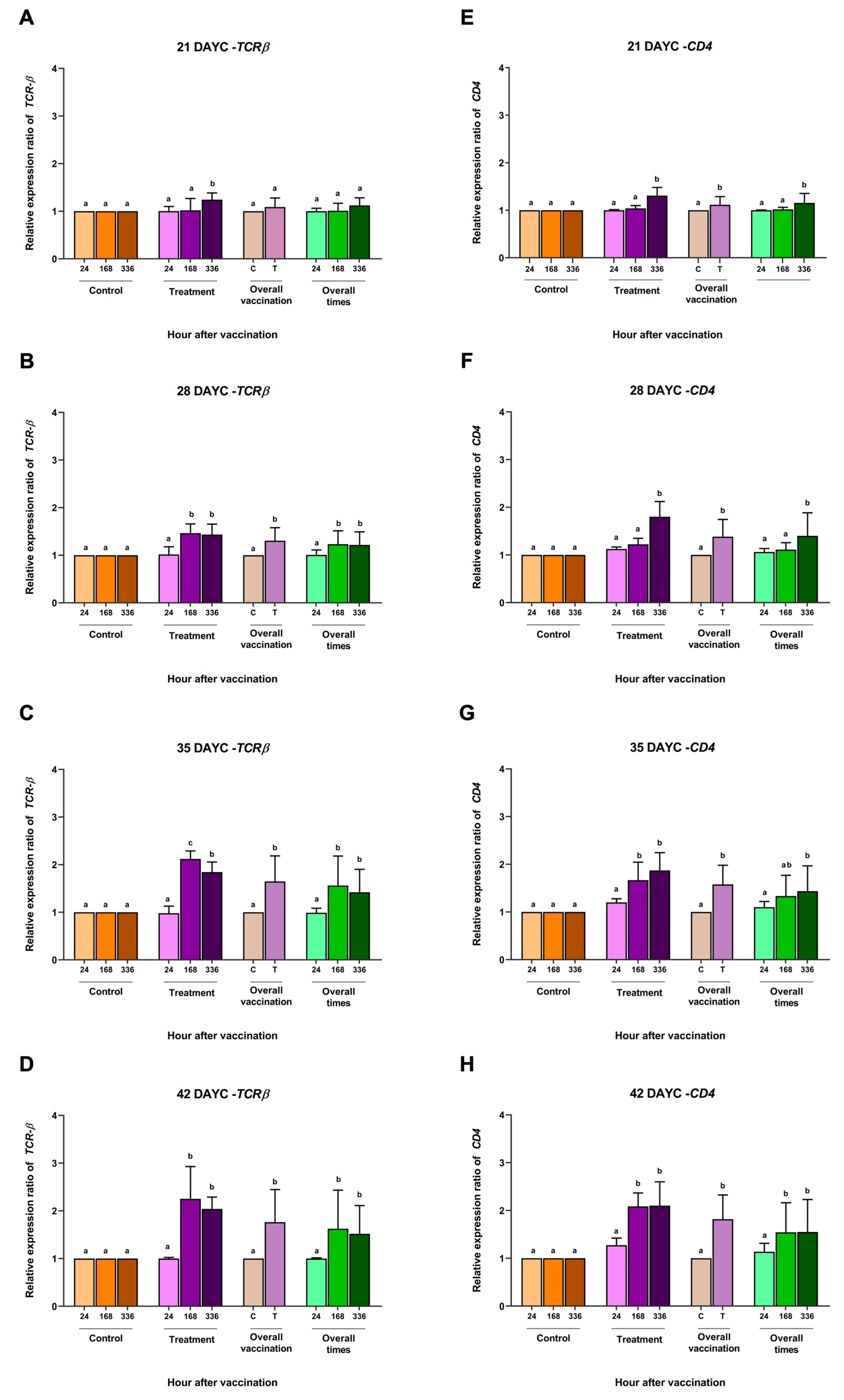
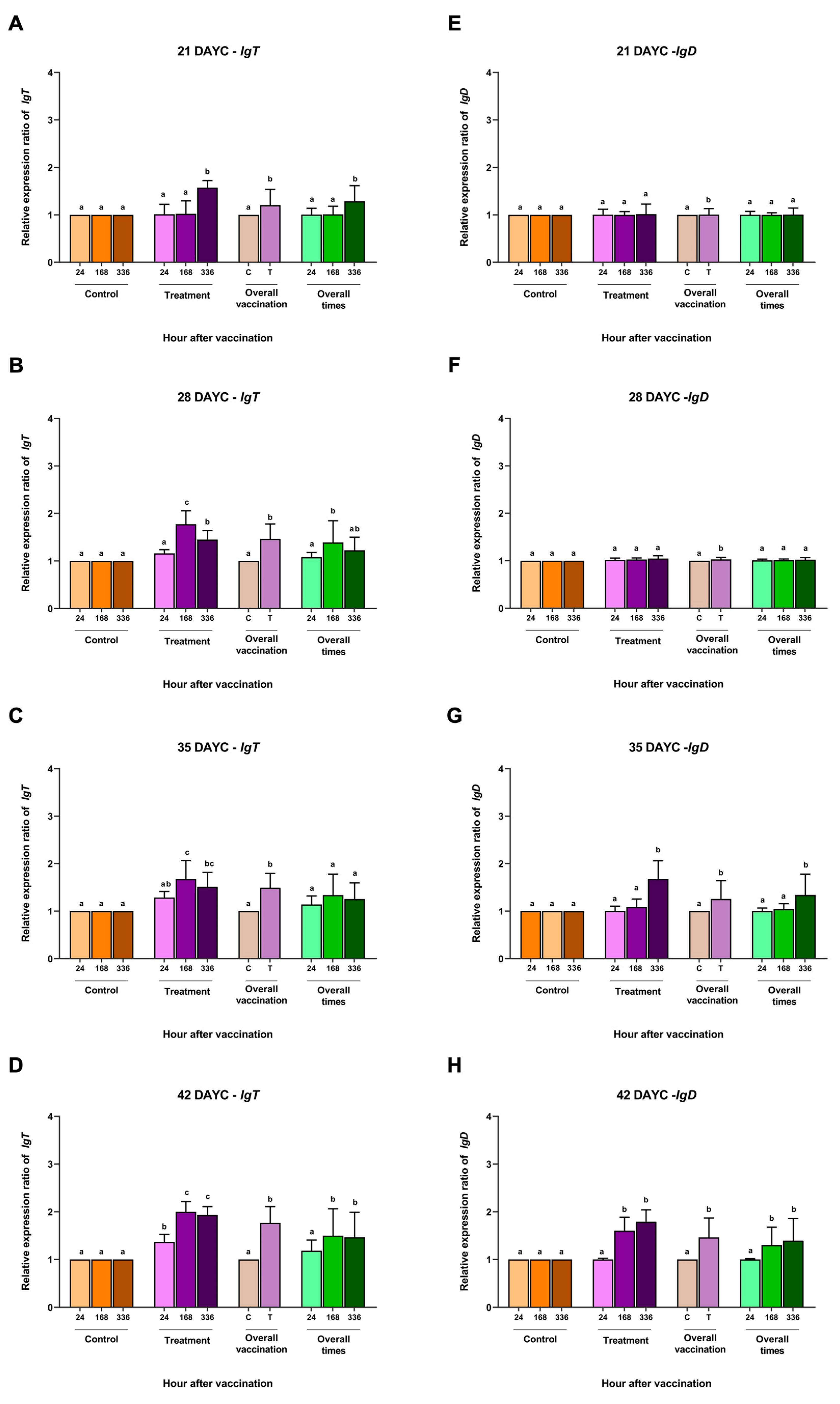
3.2. Immune-Related Gene Expression
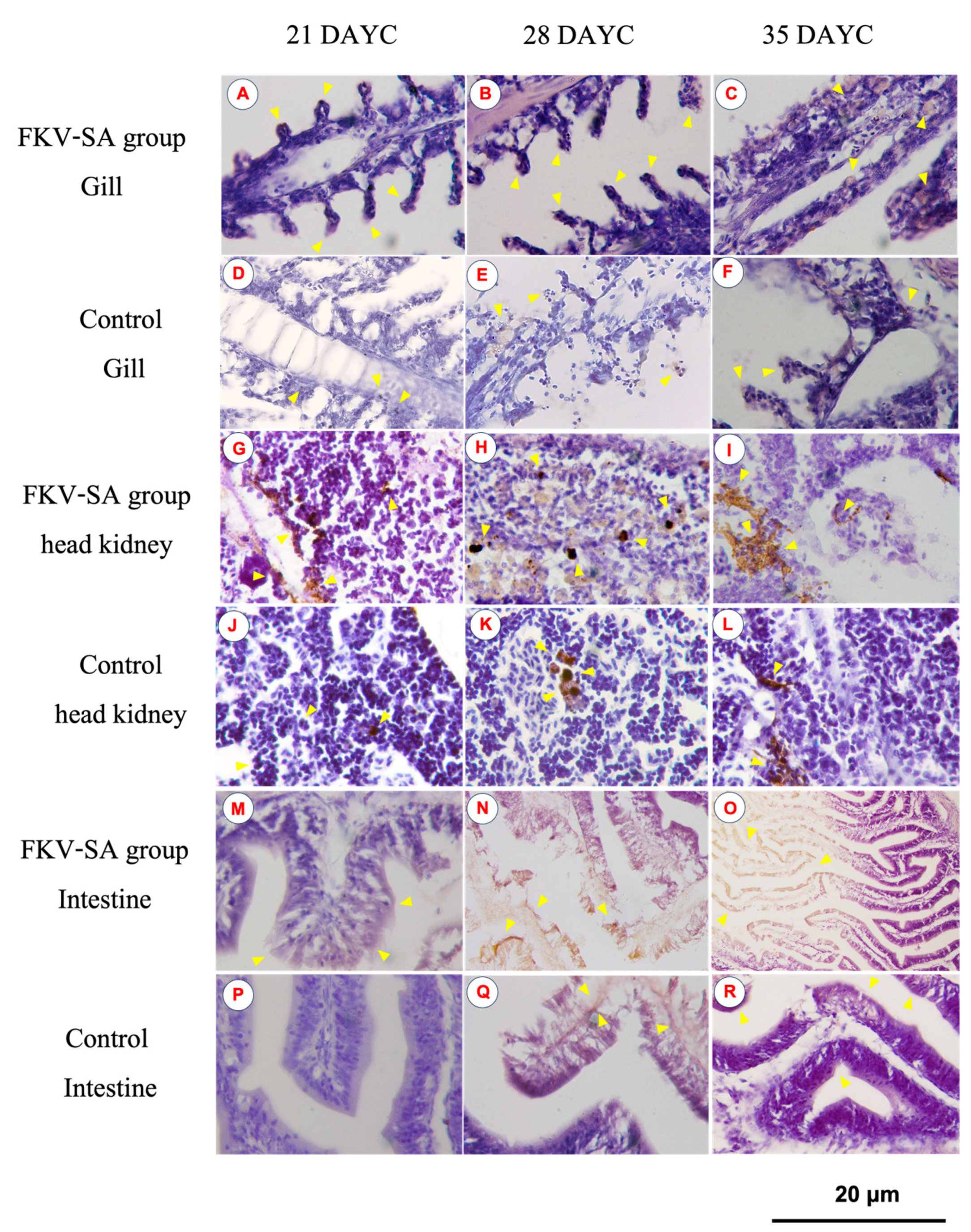
3.3. IgM Distribution in Nile Tilapia Larvae after Immersion Vaccination

3.4. Weight and Length Measurements
3.5. Lethal Concentration (LC50) of S. agalactiae
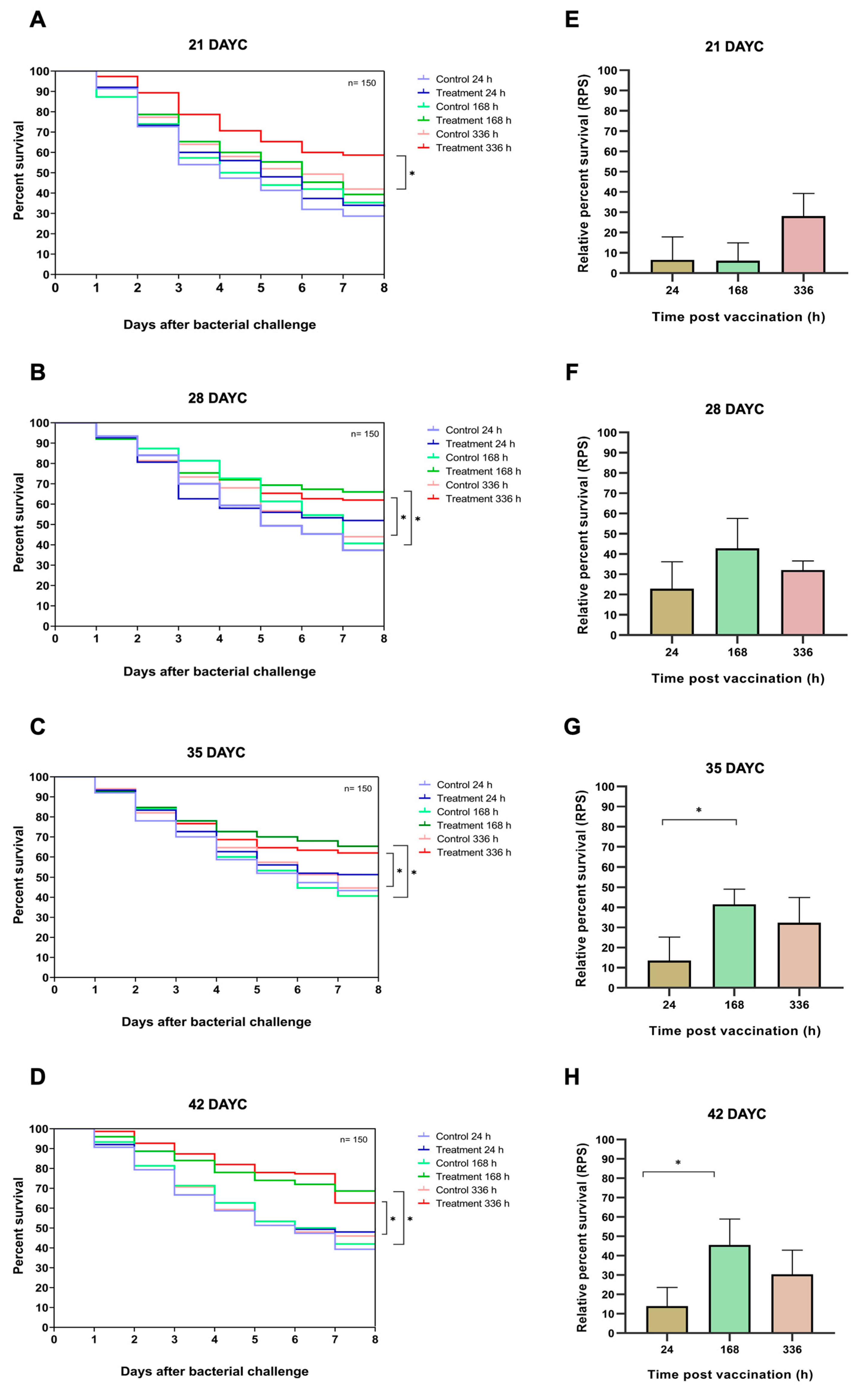
3.6. Survival Analysis and RPS of Nile Tilapia Challenged with S. agalactiae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Fishery and Aquaculture Statistics. Global Aquaculture Production 1950–2021 (FishStatJ). In FAO Fisheries and Aquaculture Division; Updated 2023; FAO: Rome, Italy, 2023; Available online: www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 2 March 2023).
- Zarkadis, I.K.; Mastellos, D.; Lambris, J.D. Phylogenetic aspects of the complement system. Dev. Comp. Immunol. 2001, 25, 745–762. [Google Scholar] [CrossRef]
- Jantrakajorn, S.; Maisak, H.; Wongtavatchai, J. Comprehensive investigation of streptococcosis outbreaks in cultured Nile tilapia, Oreochromis niloticus, and red tilapia, Oreochromis sp., of Thailand. J. World Aquac. Soc. 2014, 45, 392–402. [Google Scholar] [CrossRef]
- Bunnoy, A.; Thangsunan, P.; Chokmangmeepisarn, P.; Yata, T.; Klongklaew, N.; Pirarat, N.; Rodkhum, C. Mucoadhesive cationic lipid-based Flavobacterium oreochromis nanoencapsulation enhanced the efficacy of mucoadhesive immersion vaccination against columnaris disease and strengthened immunity in Asian sea bass (Lates calcarifer). Fish Shellfish Immunol. 2022, 127, 633–646. [Google Scholar] [CrossRef]
- Maisak, H.; Patamalai, B.; Amonsin, A.; Wongtavatchai, J. Streptococcosis in Thai culture tilapia Oreochromis niloticus. Thai J. Vet. Med. 2008, 38, 85–86. [Google Scholar]
- Kayansamruaj, P.; Pirarat, N.; Katagiri, T.; Hirono, I.; Rodkhum, C. Molecular characterization and virulence gene profiling of pathogenic Streptococcus agalactiae populations from tilapia (Oreochromis sp.) farms in Thailand. J. Vet. Diagn. Investig. 2014, 26, 488–495. [Google Scholar] [CrossRef]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef]
- Pal, S. Phage Therapy an alternate disease control in Aquaculture: A review on recent advancements. J. Agric. Vet. Sci. 2015, 8, 68–81. [Google Scholar]
- Shefat, S.H.T. Vaccines for infectious bacterial and viral diseases of fish. J. Bacteriol. Infec. Dis. 2018, 2, 1–5. [Google Scholar]
- Shoemaker, C.A.; Klesius, P.H.; Evans, J.J.; Arias, C.R. Use of modified live vaccines in aquaculture. J. World Aquac. Soc. 2009, 40, 573–585. [Google Scholar] [CrossRef]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert. Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef]
- Kitiyodom, S.; Yata, T.; Thompson, K.D.; Costa, J.; Elumalai, P.; Katagiri, T.; Pirarat, N. Immersion vaccination by a biomimetic-mucoadhesive nanovaccine induces humoral immune response of red tilapia (Oreochromis sp.) against Flavobacterium columnare challenge. Vaccines 2021, 9, 1253. [Google Scholar] [CrossRef]
- Ma, Y.; Hao, L.; Liang, Z.; Ma, J.; Ke, H.; Kang, H.; Liu, Z. Characterization of novel antigenic vaccine candidates for Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae infection. Fish Shellfish Immunol. 2020, 105, 405–414. [Google Scholar] [CrossRef]
- Heckman, T.I.; Shahin, K.; Henderson, E.E.; Griffin, M.J.; Soto, E. Development and efficacy of Streptococcus iniae live attenuated vaccines in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2022, 121, 152–162. [Google Scholar]
- Dhar, A.K.; Allnutt, F. Challenges and opportunities in developing oral vaccines against viral diseases of fish. J. Mar. Sci. Res. Dev. 2011, 1, 7. [Google Scholar] [CrossRef]
- Vinitnantharat, S.; Gravningen, K.; Greger, E. Fish vaccines. Adv. Anim. Vet. Sci. 1999, 41, 539–550. [Google Scholar]
- Bøgwald, J.; Dalmo, R.A. Review on immersion vaccines for fish: An update 2019. Microorganisms 2019, 7, 627. [Google Scholar] [CrossRef]
- Plant, K.P.; LaPatra, S.E. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef]
- Bunnoy, A.; Thompson, K.D.; Thangsunan, P.; Chokmangmeepisarn, P.; Yata, T.; Pirarat, N.; Rodkhum, C. Development of a bivalent mucoadhesive nanovaccine to prevent francisellosis and columnaris diseases in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2023, 138, 108813. [Google Scholar] [CrossRef]
- Saengrung, J.; Bunnoy, A.; Du, X.; Huang, L.; An, R.; Liang, X.; Srisapoome, P. Effects of ribonucleotide supplementation in modulating the growth of probiotic Bacillus subtilis and the synergistic benefits for improving the health performance of Asian seabass (Lates calcarifer). Fish Shellfish Immunol. 2023, 140, 108983. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nithikulworawong, N.; Yakupitiyage, A.; Rakshit, S.K.; Srisapoome, P. Molecular characterization and increased expression of the Nile tilapia, Oreochromis niloticus (L.), T-cell receptor beta chain in response to Streptococcus agalactiae infection. J. Fish Dis. 2012, 35, 343–358. [Google Scholar] [CrossRef]
- Wang, B.; Wang, P.; Wu, Z.H.; Lu, Y.S.; Wang, Z.L.; Jian, J.C. Molecular cloning and expression analysis of IgD in Nile tilapia (Oreochromis niloticus) in response to Streptococcus agalactiae stimulus. Int. J. Mol. Sci. 2016, 17, 348. [Google Scholar] [CrossRef]
- Finney, D.J. Statistical aspects of monitoring for dangers in drug therapy. Methods Inf. Med. 1971, 10, 1–8. [Google Scholar] [CrossRef]
- Litchfield, J.J.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Suanyuk, N.; Kong, F.; Ko, D.; Gilbert, G.L.; Supamattaya, K. Occurrence of rare genotypes of Streptococcus agalactiae in cultured red tilapia Oreochromis sp. and Nile tilapia O. niloticus in Thailand—Relationship to human isolates? Aquaculture 2008, 284, 35–40. [Google Scholar] [CrossRef]
- Ye, X.; Li, J.; Lu, M.; Deng, G.; Jiang, X.; Tian, Y.; Jian, Q. Identification and molecular typing of Streptococcus agalactiae isolated from pond-cultured tilapia in China. Fish. Sci. 2011, 77, 623–632. [Google Scholar] [CrossRef]
- Dangwetngam, M.; Suanyuk, N.; Kong, F.; Phromkunthong, W. Serotype distribution and antimicrobial susceptibilities of Streptococcus agalactiae isolated from infected cultured tilapia (Oreochromis niloticus) in Thailand: Nine-year perspective. Int. J. Med. Microbiol. 2016, 65, 247–254. [Google Scholar] [CrossRef]
- Barato, P.; Martins, E.R.; Melo Cristino, J.; Iregui, C.A.; Ramirez, M. Persistence of a single clone of Streptococcus agalactiae causing disease in tilapia (Oreochromis sp.) cultured in Colombia over 8 years. J. Fish Dis. 2015, 38, 1083–1087. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, Y.; Li, Q.; Ke, X.; Liu, Z.; Lu, M.; Shi, C. An effective live attenuated vaccine against Streptococcus agalactiae infection in farmed Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 98, 853–859. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, J.; Liu, G.; Du, H.; Liu, T.; Wang, G.; Wang, E. Exploring the immunoprotective potential of a nanocarrier immersion vaccine encoding sip against Streptococcus infection in tilapia (Oreochromis niloticus). Vaccines 2023, 11, 1262. [Google Scholar] [CrossRef]
- Suwanbumrung, D.; Wongkhieo, S.; Keaswejjareansuk, W.; Dechbumroong, P.; Kamble, M.T.; Yata, T.; Pirarat, N. Oral delivery of a Streptococcus agalactiae vaccine to Nile tilapia (Oreochromis niloticus) using a novel cationic-based nanoemulsion containing bile salts. Fish Shellfish Immunol. 2023, 139, 108913. [Google Scholar] [CrossRef]
- Mo, X.B.; Wang, J.; Guo, S.; Li, A.X. Potential of naturally attenuated Streptococcus agalactiae as a live vaccine in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 518, 734774. [Google Scholar] [CrossRef]
- Li, L.P.; Wang, R.; Liang, W.W.; Huang, T.; Huang, Y.; Luo, F.G.; Gan, X. Development of live attenuated Streptococcus agalactiae vaccine for tilapia via continuous passage in vitro. Fish Shellfish Immunol. 2015, 45, 955–963. [Google Scholar] [CrossRef]
- Guo, W.L.; Deng, H.W.; Wang, F.; Wang, S.F.; Zhong, Z.H.; Sun, Y.; Zhou, Y.C. In vitro and in vivo screening of herbal extracts against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 503, 412–421. [Google Scholar] [CrossRef]
- Huang, L.Y.; Wang, K.Y.; Xiao, D.; Chen, D.F.; Geng, Y.; Wang, J.; Xiao, G.Y. Safety and immunogenicity of an oral DNA vaccine encoding Sip of Streptococcus agalactiae from Nile tilapia Oreochromis niloticus delivered by live attenuated Salmonella typhimurium. Fish Shellfish Immunol. 2014, 38, 34–41. [Google Scholar] [CrossRef]
- Linh, N.V.; Dien, L.; Dong, H.T.; Khongdee, N.; Hoseinifar, S.H.; Musthafa, M.S.; Van Doan, H. Efficacy of different routes of formalin-killed vaccine administration on immunity and disease resistance of Nile tilapia (Oreochromis niloticus) challenged with Streptococcus agalactiae. Fishes 2022, 7, 398. [Google Scholar]
- Pretto-Giordano, L.G.; Müller, E.E.; Klesius, P.; Da Silva, V.G. Efficacy of an experimentally inactivated Streptococcus agalactiae vaccine in Nile tilapia (Oreochromis niloticus) reared in Brazil. Aquaculture Res. 2010, 41, 1539–1544. [Google Scholar]
- Evans, J.J.; Klesius, P.H.; Shoemaker, C.A. Efficacy of Streptococcus agalactiae (group B) vaccine in tilapia (Oreochromis niloticus) by intraperitoneal and bath immersion administration. Vaccine 2004, 22, 3769–3773. [Google Scholar] [CrossRef]
- Ramos-Espinoza, F.C.; Cueva-Quiroz, V.A.; Yunis-Aguinaga, J.; Alvarez-Rubio, N.C.; de Mello, N.P.; de Moraes, J.R.E. Efficacy of two adjuvants administrated with a novel hydrogen peroxide-inactivated vaccine against Streptococcus agalactiae in Nile tilapia fingerlings. Fish Shellfish Immunol. 2020, 105, 350–358. [Google Scholar] [CrossRef]
- Abotaleb, M.M.; Soliman, H.M.; Tawfik, R.G.; Mourad, A.; Khalil, R.H.; Abdel-Latif, H.M. Efficacy of combined inactivated vaccines against Vibrio alginolyticus and Streptococcus agalactiae infections in Nile tilapia in Egypt. Aquac. Int. 2023, 31, 1–18. [Google Scholar] [CrossRef]
- Laith, A.A.; Abdullah, M.A.; Nurhafizah, W.W.I.; Hussein, H.A.; Aya, J.; Effendy, A.W.M.; Najiah, M. Efficacy of live attenuated vaccine derived from the Streptococcus agalactiae on the immune responses of Oreochromis niloticus. Fish Shellfish Immunol. 2019, 90, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Su, J. Cyprinid viral diseases and vaccine development. Fish Shellfish Immunol. 2018, 83, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.D.; Ototake, M.; Nakanishi, T. Particulate antigen uptake during immersion immunisation of fish: The effectiveness of prolonged exposure and the roles of skin and gill. Fish Shellfish Immunol. 1998, 8, 393–408. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ototake, M. Antigen uptake and immune responses after immersion vaccination. Dev. Biol. Stand. 1997, 90, 59–68. [Google Scholar] [PubMed]
- Adams, A.; Subasinghe, R. Fish vaccines. In Veterinary Vaccines: Principles and Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 113–118. [Google Scholar]
- Cao, J.; Chen, Q.; Lu, M.; Hu, X.; Wang, M. Histology and ultrastructure of the thymus during development in tilapia, Oreochromis niloticus. J. Anat. 2017, 230, 720–733. [Google Scholar] [CrossRef]
- Fujimura, K.; Okada, N. Development of the embryo, larva and early juvenile of Nile tilapia Oreochromis niloticus (Pisces: Cichlidae). Developmental saging system. Dev. Growth Differ. 2007, 49, 301–324. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.M.; Miyake, T.; Wright, J.R., Jr. Histological study of the development of the embryo and early larva of Oreochromis niloticus (Pisces: Cichlidae). J. Morphol. 2001, 247, 172–195. [Google Scholar] [CrossRef]
- Pasaribu, W.; Sukenda, S.; Nuryati, S. The efficacy of Nile tilapia (Oreochromis niloticus) broodstock and larval immunization against Streptococcus agalactiae and Aeromonas hydrophila. Fishes 2018, 3, 16. [Google Scholar] [CrossRef]
- Magnadottir, B. Immunological control of fish diseases. Mar. Biotechnol. 2010, 12, 361–379. [Google Scholar] [CrossRef]
- Kjørsvik, E.; Galloway, T.F.; Estevez, A.; Sæle, Ø.; Moren, M. Effects of larval nutrition on development. In Larval Fish Nutrition; Holt, G.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 219–248. [Google Scholar]
- Mulero, I.; García-Ayala, A.; Meseguer, J.; Mulero, V. Maternal transfer of immunity and ontogeny of autologous immunocompetence of fish: A minireview. Aquaculture 2007, 268, 244–250. [Google Scholar] [CrossRef]
- Tatner, M.F.; Horne, M.T. Susceptibility and immunity to Vibrio anguillarum in post-hatching rainbow trout fry, Salmogairdneri richardson 1836. Dev. Comp. Immunol. 1983, 7, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Liu, Z.; Chen, S.; Chen, Z.; Zhang, D.; Gao, F.; Lu, M. The immune efficacy of a Streptococcus agalactiae immersion vaccine for different sizes of young tilapia. Aquaculture 2021, 534, 736289. [Google Scholar] [CrossRef]
- Patrie-Hanson, L.; Ainsworth, A.J. Humoral immune responses of channel catfish (Ictalurus punctatus) fry and fingerlings exposed to Edwardsiella ictaluri. Fish Shellfish Immunol. 1999, 9, 579–589. [Google Scholar] [CrossRef]
- Vinh, N.T.; Dong, H.T.; Lan, N.G.T.; Sangsuriya, P.; Salin, K.R.; Chatchaiphan, S.; Senapin, S. Immunological response of 35 and 42 days old Asian seabass (Lates calcarifer, Bloch 1790) fry following immersion immunization with Streptococcus iniae heat-killed vaccine. Fish Shellfish Immunol. 2023, 138, 108802. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A. Exosomes-based cell-free cancer therapy: A novel strategy for targeted therapy. Immunol. Med. 2021, 44, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Smart, C.; Pardi, R. T lymphocytes. Int. J. Biochem. Cell Biol. 2003, 35, 1004–1008. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Tian, J.; Gao, J.; Chen, J.; LI, F.; Xie, X.; Du, J. Efficacy and safety of the combined treatment with intravenous immunoglobulin and oral glucocorticoid in the elderly with dermatomyositis. Chin. J. Geriatr. 2008, 12, 588–590. [Google Scholar]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef]
- Abós, B.; Bailey, C.; Tafalla, C. Adaptive immunity. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Springer: Berlin/Heidelberg, Germany, 2022; pp. 105–140. [Google Scholar]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, Ø. A review of the immunological mechanisms following mucosal vaccination of finfish. Front. Immunol. 2015, 6, 427. [Google Scholar] [CrossRef]
- Saha, N.R.; Suetake, H.; Suzuki, Y. Analysis and characterization of the expression of the secretory and membrane forms of IgM heavy chains in the pufferfish, Takifugu rubripes. Mol. Immunol. 2005, 42, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Chen, X.; Ao, J. The up-regulation of large yellow croaker secretory IgM heavy chain at early phase of immune response. Fish Physiol. Biochem. 2010, 36, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yuan, X.; Mu, P.; Li, Q.; Ao, J.; Chen, X. Development of monoclonal antibody against IgM of large yellow croaker (Larimichthys crocea) and characterization of IgM+ B cells. Fish Shellfish Immunol. 2019, 91, 216–222. [Google Scholar] [CrossRef]
- Xu, Z.; Takizawa, F.; Parra, D.; Gómez, D.; von Gersdorff Jørgensen, L.; LaPatra, S.E.; Sunyer, J.O. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 2016, 7, 10728. [Google Scholar] [CrossRef]
- Rességuier, J.; Dalum, A.S.; Du Pasquier, L.; Zhang, Y.; Koppang, E.O.; Boudinot, P.; Wiegertjes, G.F. Lymphoid tissue in teleost gills: Variations on a theme. Biology 2020, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.T.; Yu, Y.Y.; Xu, H.Y.; Huang, Z.Y.; Liu, X.; Cao, J.F.; Xu, Z. Prevailing role of mucosal Igs and B cells in teleost skin immune responses to bacterial infection. J. Immunol. 2021, 206, 1088–1101. [Google Scholar] [CrossRef]
- Dixon, B.; Barreda, D.R.; Sunyer, J.O. Perspective on the development and validation of Ab reagents to fish immune proteins for the correct assessment of immune function. Front. Immunol. 2018, 9, 2957. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Ballesteros, N.A.; Castro, R.; Abos, B.; Rodríguez Saint-Jean, S.S.; Pérez-Prieto, S.I.; Tafalla, C. The pyloric caeca area is a major site for IgM+ and IgT+ B cell recruitment in response to oral vaccination in rainbow trout. PLoS ONE 2013, 8, e66118. [Google Scholar] [CrossRef]
- Salinas, I.; Parra, D. Fish mucosal immunity: Intestine. In Mucosal Health in Aquaculture; Beck, B.H., Peatman, E., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 135–170. [Google Scholar]
- Ellis, A.E. General principles of fish vaccination. In Fish Vaccination; Academic Press: London, UK, 1988; pp. 1–19. [Google Scholar]
- Kitiyodom, S.; Kaewmalun, S.; Nittayasut, N.; Suktham, K.; Surassmo, S.; Namdee, K.; Yata, T. The potential of mucoadhesive polymer in enhancing efficacy of direct immersion vaccination against Flavobacterium columnare infection in tilapia. Fish Shellfish Immunol. 2019, 86, 635–640. [Google Scholar] [CrossRef]
| Gene | Primer Name | Nucleotide Sequences (5′ → 3′) | Amplicon Size (bp) | Tm (°C) | References |
|---|---|---|---|---|---|
| β-Actin | On_β-actin | F: ACAGGATGCAGAAGGAGATCACAG R: GTACTCCTGCTTGCTGATCCACAT | 155 | 60 | [19] |
| T-cell receptor β-chain (constant region) | On_TCRβ | F: GGACCTTCAGAACATGAGTGCAG R: TCTTCACGCGCAGCTTCATCTGT | 164 | 60 | [22] |
| Cluster of differentiation 4 (CD4) | On_CD4 | F: GCTCCAGTGTGACGTGAAA R: TACAGGTTTGAGTTGAGCTG | 150 | 60 | [19] |
| Major histocompatibility complex (MHC) class IIα molecules | On_MHC-IIα | F: CAGTGTTTGATGTGTTTTCAG R: CTCTTCACCATCCAGTCCA | 100 | 60 | [19] |
| Immunoglobulin M heavy chain (IgHM) | On_IgM | F: GGATGACGAGGAAGCAGACT R: CATCATCCCTTTGCCACTGG | 122 | 60 | [19] |
| Immunoglobulin T heavy chain (IgHT) | On_IgT | F: TGACCAGAAATGGCGAAGTCTG R: GTTATAGTCACATTCTTTAGAATTACC | 136 | 60 | [19] |
| Immunoglobulin D heavy chain (IgHD) | On_IgD | F: AACACCACCCTGTCCCTGAAT R: GGGTGAAAACCACATTCCAAC | 127 | 60 | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumwan, B.; Bunnoy, A.; Chatchaiphan, S.; Kayansamruaj, P.; Dong, H.T.; Senapin, S.; Srisapoome, P. First Investigation of the Optimal Timing of Vaccination of Nile Tilapia (Oreochromis niloticus) Larvae against Streptococcus agalactiae. Vaccines 2023, 11, 1753. https://doi.org/10.3390/vaccines11121753
Kumwan B, Bunnoy A, Chatchaiphan S, Kayansamruaj P, Dong HT, Senapin S, Srisapoome P. First Investigation of the Optimal Timing of Vaccination of Nile Tilapia (Oreochromis niloticus) Larvae against Streptococcus agalactiae. Vaccines. 2023; 11(12):1753. https://doi.org/10.3390/vaccines11121753
Chicago/Turabian StyleKumwan, Benchawan, Anurak Bunnoy, Satid Chatchaiphan, Pattanapon Kayansamruaj, Ha Thanh Dong, Saengchan Senapin, and Prapansak Srisapoome. 2023. "First Investigation of the Optimal Timing of Vaccination of Nile Tilapia (Oreochromis niloticus) Larvae against Streptococcus agalactiae" Vaccines 11, no. 12: 1753. https://doi.org/10.3390/vaccines11121753








