Co-Formulation of Recombinant Porcine IL-18 Enhances the Onset of Immune Response in a New Lawsonia intracellularis Vaccine
Abstract
1. Introduction
2. Materials and Methods
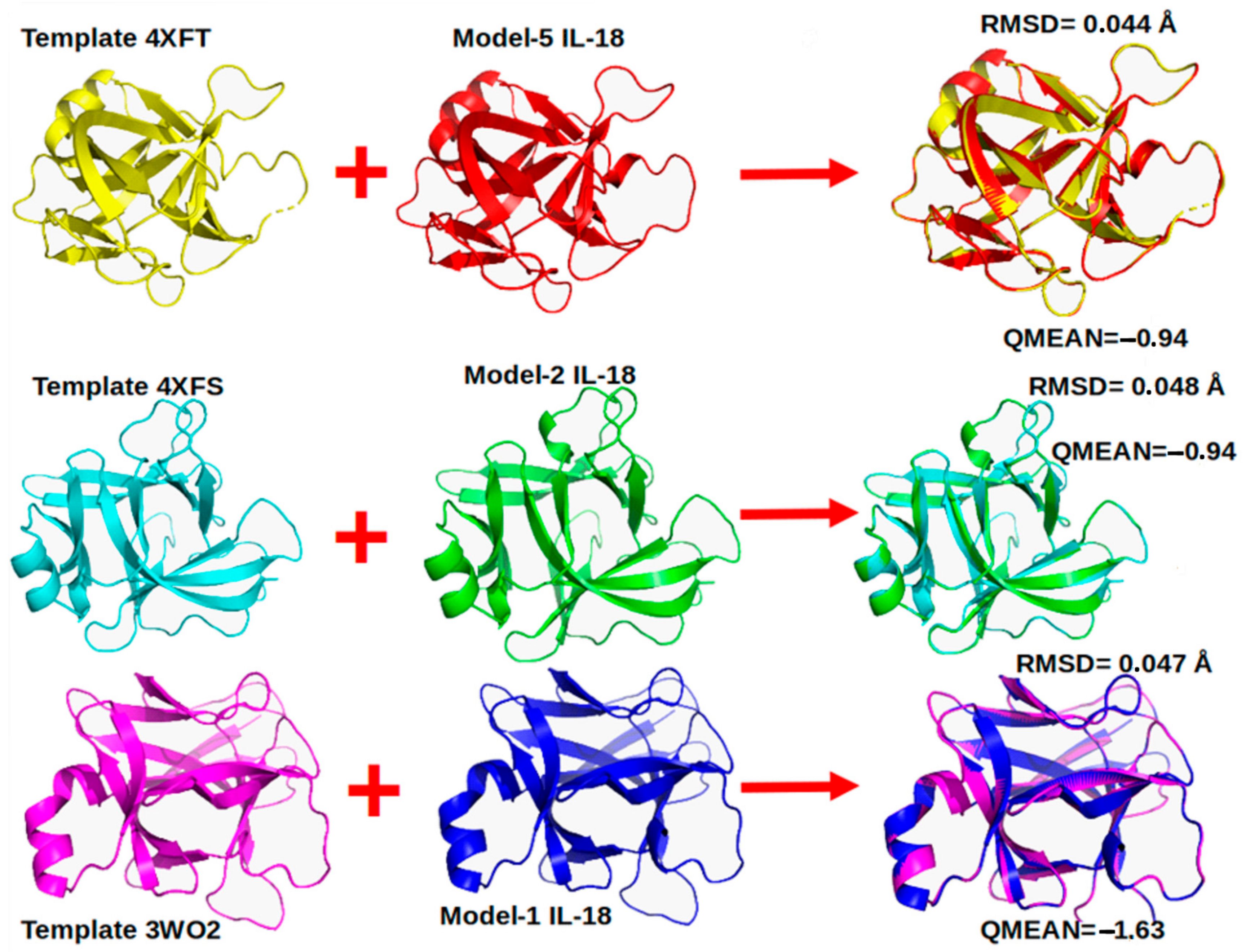
2.1. Porcine IL-18 Modeling by Homology
2.2. E. coli Rupture and pIL-18 Expression
2.3. Cloning of pIL-18 in pPS10
2.4. pIL-18 Yeast Expression
2.5. SDS-PAGE and Western Blot
2.6. epIL-18 Purification by Immobilized Metal Affinity Chromatography (IMAC)
2.7. Pig Peripheral Blood Lymphocyte Extraction
2.8. Lymphocyte Proliferation Assay
2.9. Pro-Inflammatory Cytokines Analysis In Vitro by Real-Time PCR
2.10. Pro-Inflammatory Cytokines Analysis In Vivo by Real-Time PCR
2.11. Immunization Assay in Pigs
2.12. Detection of Cellular Immune Response in Pigs
2.13. Humoral Immune Response Evaluation
2.14. Statistical Analysis
3. Results
3.1. pIL-18 Modeling
3.2. Porcine IL-18 Expression in E. coli and P. pastoris
3.3. Evaluation of the Biological Activity of IL-18
3.4. Analysis of Immunostimulation of IL-18 in Pigs
3.5. Analysis of ppIL-18 as an Adjuvant in a Recombinant Vaccine against L. intracellularis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Maes, D.G.D.; Dewulf, J.; Pineiro, C.; Edwards, S.; Kyriazakis, I. A critical reflection on intensive pork production with an emphasis on animal health and welfare. J. Anim. Sci. 2020, 98 (Suppl. S1), S15–S26. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.G.; Collins, A.M.; Donahoo, M.; Emery, D. Immunological responses to vaccination following experimental Lawsonia intracellularis virulent challenge in pigs. Vet. Microbiol. 2013, 164, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.J.; Roof, M.B.; Hoffman, L.J.; Dickson, J.S.; Harris, D.L. Proliferative enteropathy: A global enteric disease of pigs caused by Lawsonia intracellularis. Anim. Health Res. Rev. 2005, 6, 173–197. [Google Scholar] [CrossRef]
- Musse, S.L.; Nielsen, G.B.; Stege, H.; Weber, N.R.; Houe, H. Effect of intramuscular vaccination against Lawsonia intracellularis on production parameters, diarrhea occurrence, antimicrobial treatment, bacterial shedding, and lean meat percentage in two Danish naturally infected finisher pig herds. Prev. Vet. Med. 2023, 212, 105837. [Google Scholar] [CrossRef]
- Riber, U.; Heegaard, P.M.; Cordes, H.; Stahl, M.; Jensen, T.K.; Jungersen, G. Vaccination of pigs with attenuated Lawsonia intracellularis induced acute phase protein responses and primed cell-mediated immunity without reduction in bacterial shedding after challenge. Vaccine 2015, 33, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Barna, P.; Bilkei, G. Effect of gilt seropositivity to Lawsonia intracellularis (LI) on their offspring’s seropositivity to LI and on diarrhoea after a pure-culture challenge. Prev. Vet. Med. 2003, 61, 71–78. [Google Scholar] [CrossRef]
- Obradovic, M.R.; Wilson, H.L. Immune response and protection against Lawsonia intracellularis infections in pigs. Vet. Immunol. Immunopathol. 2020, 219, 109959. [Google Scholar] [CrossRef]
- Wattanaphansak, S.; Pereira, C.E.R.; Kaenson, W.; Assavacheep, P.; Tantilertcharoen, R.; Resende, T.P.; Barrera-Zarate, J.A.; de Oliveira-Lee, J.S.V.; Klein, U.; Gebhart, C.J.; et al. Isolation and in vitro antimicrobial susceptibility of porcine Lawsonia intracellularis from Brazil and Thailand. BMC Microbiol. 2019, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Cordes, H.; Riber, U.; Jensen, T.K.; Jungersen, G. Cell-mediated and humoral immune responses in pigs following primary and challenge-exposure to Lawsonia intracellularis. Vet. Res. 2012, 43, 9. [Google Scholar] [CrossRef]
- Nogueira, M.G.; Collins, A.M.; Dunlop, R.H.; Emery, D. Effect of the route of administration on the mucosal and systemic immune responses to Lawsonia intracellularis vaccine in pigs. Aust. Vet. J. 2015, 93, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016, 120, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef] [PubMed]
- Nanishi, E.; Dowling, D.J.; Levy, O. Toward precision adjuvants: Optimizing science and safety. Curr. Opin. Pediatr. 2020, 32, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Martinez, D.T.; Carlos, I.Z. Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed. Pharmacother. 2018, 105, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Dubensky, T.W., Jr.; Reed, S.G. Adjuvants for cancer vaccines. Semin. Immunol. 2010, 22, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Bertholet, S.; Coler, R.N.; Friede, M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009, 30, 23–32. [Google Scholar] [CrossRef]
- García-Hernández, M.; Guerrero-Ramírez, G.; Castro Corona, M.D.L.Á.; Medina de la Garza, C.E. Inmunomoduladores como terapia adyuvante en la enfermedad infecciosa. Rev. Med. Univ. 2009, 11, 247–259. [Google Scholar]
- Kayamuro, H.; Yoshioka, Y.; Abe, Y.; Arita, S.; Katayama, K.; Nomura, T.; Yoshikawa, T.; Kubota-Koketsu, R.; Ikuta, K.; Okamoto, S.; et al. Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus. J. Virol. 2010, 84, 12703–12712. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Gracie, J.A.; Robertson, S.E.; McInnes, I.B. Interleukin-18. J. Leukoc. Biol. 2003, 73, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Montesino, R.; Gutierrez, N.; Camacho, F.; Farnos, O.; Andrades, S.; Gonzalez, A.; Acosta, J.; Cortez-San Martin, M.; Sanchez, O.; Ruiz, A.; et al. Multi-antigenic recombinant subunit vaccine against Lawsonia intracellularis: The etiological agent of porcine proliferative enteropathy. Vaccine 2019, 37, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Chikhale, R.V.; Gupta, V.K.; Eldesoky, G.E.; Wabaidur, S.M.; Patil, S.A.; Islam, M.A. Identification of potential anti-TMPRSS2 natural products through homology modelling, virtual screening and molecular dynamics simulation studies. J. Biomol. Struct. Dyn. 2020, 39, 6660–6675. [Google Scholar] [CrossRef]
- Fiser, A.; Do, R.K.; Sali, A. Modeling of loops in protein structures. Protein Sci. 2000, 9, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Conformational sampling in template-free protein loop structure modeling: An overview. Comput. Struct. Biotechnol. J. 2013, 5, e201302003. [Google Scholar] [CrossRef] [PubMed]
- Oduselu, G.O.; Ajani, O.O.; Ajamma, Y.U.; Brors, B.; Adebiyi, E. Homology Modelling and Molecular Docking Studies of Selected Substituted Benzo[d]imidazol-1-yl)methyl)benzimidamide Scaffolds on Plasmodium falciparum Adenylosuccinate Lyase Receptor. Bioinform. Biol. Insights 2019, 13, 1177932219865533. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Boratyn, G.M.; Schaffer, A.A.; Agarwala, R.; Altschul, S.F.; Lipman, D.J.; Madden, T.L. Domain enhanced lookup time accelerated BLAST. Biol. Direct. 2012, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Kuma, K.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Remmert, M.; Biegert, A.; Hauser, A.; Soding, J. HHblits: Lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat. Methods 2011, 9, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B., 3rd; de Bakker, P.I.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Calpha geometry: Phi, psi and Cbeta deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Benkert, P.; Kunzli, M.; Schwede, T. QMEAN server for protein model quality estimation. Nucleic Acids Res. 2009, 37, W510–W514. [Google Scholar] [CrossRef]
- Bairoch, A.; Apweiler, R. The SWISS-PROT protein sequence data bank and its new supplement TREMBL. Nucleic Acids Res. 1996, 24, 21–25. [Google Scholar] [CrossRef]
- Lill, M.A.; Danielson, M.L. Computer-aided drug design platform using PyMOL. J. Comput. Aided Mol. Des. 2011, 25, 13–19. [Google Scholar] [CrossRef]
- Schrödinger, L. The PyMOL Molecular Graphics System, Version~1.3r1. 2010. Available online: https://pymol.sourceforge.net/overview/index.htm (accessed on 8 February 2021).
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Baeshen, M.N.; Al-Hejin, A.M.; Bora, R.S.; Ahmed, M.M.; Ramadan, H.A.; Saini, K.S.; Baeshen, N.A.; Redwan, E.M. Production of Biopharmaceuticals in E. coli: Current Scenario and Future Perspectives. J. Microbiol. Biotechnol. 2015, 25, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Shukla, P. Advanced technologies for improved expression of recombinant proteins in bacteria: Perspectives and applications. Crit. Rev. Biotechnol. 2016, 36, 1089–1098. [Google Scholar] [CrossRef]
- Ozturk, S.; Ergun, B.G.; Calik, P. Double promoter expression systems for recombinant protein production by industrial microorganisms. Appl. Microbiol. Biotechnol. 2017, 101, 7459–7475. [Google Scholar] [CrossRef]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein expression in Pichia pastoris: Recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef]
- Cregg, J.M.; Cereghino, J.L.; Shi, J.; Higgins, D.R. Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 2000, 16, 23–52. [Google Scholar] [CrossRef]
- Panula-Perala, J.; Vasala, A.; Karhunen, J.; Ojamo, H.; Neubauer, P.; Mursula, A. Small-scale slow glucose feed cultivation of Pichia pastoris without repression of AOX1 promoter: Towards high throughput cultivations. Bioprocess Biosyst. Eng. 2014, 37, 1261–1269. [Google Scholar] [CrossRef]
- Weinacker, D.; Rabert, C.; Zepeda, A.B.; Figueroa, C.A.; Pessoa, A.; Farias, J.G. Applications of recombinant Pichia pastoris in the healthcare industry. Braz. J. Microbiol. 2013, 44, 1043–1048. [Google Scholar] [CrossRef]
- Vogl, T.; Glieder, A. Regulation of Pichia pastoris promoters and its consequences for protein production. New Biotechnol. 2013, 30, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Hartner, F.S.; Glieder, A. New opportunities by synthetic biology for biopharmaceutical production in Pichia pastoris. Curr. Opin. Biotechnol. 2013, 24, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Oem, J.K.; Song, H.J.; Kang, S.W.; Jeong, W.S. Cloning, sequencing, and expression of porcine interleukin-18 in Escherichia coli. Mol. Cells 2000, 10, 343–347. [Google Scholar]
- Sorensen, H.P.; Mortensen, K.K. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J. Biotechnol. 2005, 115, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Kosobokova, E.N.; Skrypnik, K.A.; Kosorukov, V.S. Overview of Fusion Tags for Recombinant Proteins. Biochemistry 2016, 81, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; Sharma, A.; Upadhyay, A.K.; Singh, A.; Garg, L.C.; Panda, A.K. Solubilization of inclusion body proteins using n-propanol and its refolding into bioactive form. Protein Expr. Purif. 2012, 81, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ciofalo, V.; Barton, N.; Kreps, J.; Coats, I.; Shanahan, D. Safety evaluation of a lipase enzyme preparation, expressed in Pichia pastoris, intended for use in the degumming of edible vegetable oil. Regul. Toxicol. Pharmacol. 2006, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Cui, B.A.; Xia, P.A.; Li, X.S.; Hu, G.Z.; Yang, M.F.; Zhang, H.Y.; Wang, X.B.; Cao, S.F.; Zhang, L.X.; et al. Cloning, in vitro expression and bioactivity of duck interleukin-18. Vet. Immunol. Immunopathol. 2008, 123, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Zheng, L.L.; Li, X.S.; Wei, Z.Y.; Cui, B.A.; Li, X.K.; Liu, J.P.; Yin, H.Z.; Meng, J.T.; Zhang, Y.; et al. Cloning, in vitro expression, and bioactivity of interleukin-18 isolated from a domestic porcine breed found in Henan. FEMS Immunol. Med. Microbiol. 2009, 57, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Puren, A.J.; Fantuzzi, G.; Gu, Y.; Su, M.S.; Dinarello, C.A. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J. Clin. Investig. 1998, 101, 711–721. [Google Scholar] [CrossRef]
- Ushio, S.; Namba, M.; Okura, T.; Hattori, K.; Nukada, Y.; Akita, K.; Tanabe, F.; Konishi, K.; Micallef, M.; Fujii, M.; et al. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J. Immunol. 1996, 156, 4274–4279. [Google Scholar] [CrossRef]
- Neighbors, M.; Xu, X.; Barrat, F.J.; Ruuls, S.R.; Churakova, T.; Debets, R.; Bazan, J.F.; Kastelein, R.A.; Abrams, J.S.; O’Garra, A. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on Interferon gamma production. J. Exp. Med. 2001, 194, 343–354. [Google Scholar] [CrossRef]
- Robinson, C.M.; Jung, J.Y.; Nau, G.J. Interferon-gamma, tumor necrosis factor, and interleukin-18 cooperate to control growth of Mycobacterium tuberculosis in human macrophages. Cytokine 2012, 60, 233–241. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Liong, K.H.; Gunalan, M.G.; Li, N.; Lim, D.S.; Fisher, D.A.; MacAry, P.A.; Leo, Y.S.; Wong, S.C.; Puan, K.J.; et al. Type I IFNs and IL-18 regulate the antiviral response of primary human gammadelta T cells against dendritic cells infected with Dengue virus. J. Immunol. 2015, 194, 3890–3900. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Ueda, H.; Hosohara, K.; Tsuji, R.; Nagata, Y.; Kashiwamura, S.; Okamura, H. Interleukin-18 stimulates hematopoietic cytokine and growth factor formation and augments circulating granulocytes in mice. Blood 2001, 98, 2101–2107. [Google Scholar] [CrossRef]
- Riber, U.; Boesen, H.T.; Jakobsen, J.T.; Nguyen, L.T.; Jungersen, G. Co-incubation with IL-18 potentiates antigen-specific IFN-gamma response in a whole-blood stimulation assay for measurement of cell-mediated immune responses in pigs experimentally infected with Lawsonia intracellularis. Vet. Immunol. Immunopathol. 2011, 139, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, G.; Gao, L.; Mu, L.; Zhang, L.; Cong, Y.; Ding, Z. Positive inductive effect of IL-18 on virus-specific immune responses induced by PRRSV-GP5 DNA vaccine in swine. Res. Vet. Sci. 2013, 94, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Mu, L.; Ding, Z. Immune responses in pigs induced by recombinant DNA vaccine co-expressing swine IL-18 and membrane protein of porcine reproductive and respiratory syndrome virus. Int. J. Mol. Sci. 2012, 13, 5715–5728. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. S2), 49. [Google Scholar] [CrossRef]
- Ogawa, Y.; Minagawa, Y.; Shi, F.; Eguchi, M.; Muneta, Y.; Shimoji, Y. Immunostimulatory effects of recombinant Erysipelothrix rhusiopathiae expressing porcine interleukin-18 in mice and pigs. Clin. Vaccine Immunol. 2012, 19, 1393–1398. [Google Scholar] [CrossRef]
- Gao, X.; Xu, K.; Yang, G.; Shi, C.; Huang, H.; Wang, J.; Yang, W.; Liu, J.; Liu, Q.; Kang, Y.; et al. Construction of a novel DNA vaccine candidate targeting F gene of genotype VII Newcastle disease virus and chicken IL-18 delivered by Salmonella. J. Appl. Microbiol. 2019, 126, 1362–1372. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Zhang, C.; Wu, T.; Li, Y.; Cheng, X. A eukaryotic expression plasmid carrying chicken interleukin-18 enhances the response to newcastle disease virus vaccine. Clin. Vaccine Immunol. 2015, 22, 56–64. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Sun, C.; Zhao, X.; Sun, M.; Gao, X.; Jia, F.; Zhang, T.; Ge, C.; Zhang, X.; et al. Protection against genotype VII Newcastle disease virus challenge by a minicircle DNA vaccine coexpressing F protein and chicken IL-18 adjuvant. Vet. Microbiol. 2022, 270, 109474. [Google Scholar] [CrossRef]
- Yang, Y.; Teng, Z.; Lu, Y.; Luo, X.; Mu, S.; Ru, J.; Zhao, X.; Guo, H.; Ran, X.; Wen, X.; et al. Enhanced immunogenicity of foot and mouth disease DNA vaccine delivered by PLGA nanoparticles combined with cytokine adjuvants. Res. Vet. Sci. 2021, 136, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.L.; Guo, X.Q.; Zhu, Q.L.; Chao, A.J.; Fu, P.F.; Wei, Z.Y.; Wang, S.J.; Chen, H.Y.; Cui, B.A. Construction and immunogenicity of a recombinant pseudorabies virus co-expressing porcine circovirus type 2 capsid protein and interleukin 18. Virus Res. 2015, 201, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, H.B.; Jiang, Y.L.; Liu, J.; Gao, X.; Liu, Y.; Yang, W.T.; Shi, C.W.; Wang, D.; Wang, J.Z.; et al. Immunological evaluation of invasive Lactobacillus plantarum co-expressing EtMIC2 and chicken interleukin-18 against Eimeria tenella. Parasitol. Res. 2020, 119, 2885–2895. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Nemattalab, M.; Mahjoob, M.; Hasan-Alizadeh, E.; Zamani, N.; Nikokar, I.; Evazalipour, M.; Soltani Tehrani, B.; Shenagari, M. Toward a universal influenza virus vaccine: Some cytokines may fulfill the request. Cytokine 2021, 148, 155703. [Google Scholar] [CrossRef]
- Fourie, K.R.; Choudhary, P.; Ng, S.H.; Obradovic, M.; Brownlie, R.; Anand, S.K.; Wilson, H.L. Evaluation of immunogenicity and protection mediated by Lawsonia intracellularis subunit vaccines. Vet. Immunol. Immunopathol. 2021, 237, 110256. [Google Scholar] [CrossRef]
- Park, S.; Won, G.; Lee, J.H. An attenuated Salmonella vaccine secreting Lawsonia intracellularis immunogenic antigens confers dual protection against porcine proliferative enteropathy and salmonellosis in a murine model. J. Vet. Sci. 2019, 20, e24. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Gajardo, A.; Gutiérrez, N.; Lamazares, E.; Espinoza, F.; Escobar-Riquelme, F.; Leiva, M.J.; Villavicencio, C.; Mena-Ulecia, K.; Montesino, R.; Altamirano, C.; et al. Co-Formulation of Recombinant Porcine IL-18 Enhances the Onset of Immune Response in a New Lawsonia intracellularis Vaccine. Vaccines 2023, 11, 1788. https://doi.org/10.3390/vaccines11121788
Hidalgo-Gajardo A, Gutiérrez N, Lamazares E, Espinoza F, Escobar-Riquelme F, Leiva MJ, Villavicencio C, Mena-Ulecia K, Montesino R, Altamirano C, et al. Co-Formulation of Recombinant Porcine IL-18 Enhances the Onset of Immune Response in a New Lawsonia intracellularis Vaccine. Vaccines. 2023; 11(12):1788. https://doi.org/10.3390/vaccines11121788
Chicago/Turabian StyleHidalgo-Gajardo, Angela, Nicolás Gutiérrez, Emilio Lamazares, Felipe Espinoza, Fernanda Escobar-Riquelme, María J. Leiva, Carla Villavicencio, Karel Mena-Ulecia, Raquel Montesino, Claudia Altamirano, and et al. 2023. "Co-Formulation of Recombinant Porcine IL-18 Enhances the Onset of Immune Response in a New Lawsonia intracellularis Vaccine" Vaccines 11, no. 12: 1788. https://doi.org/10.3390/vaccines11121788
APA StyleHidalgo-Gajardo, A., Gutiérrez, N., Lamazares, E., Espinoza, F., Escobar-Riquelme, F., Leiva, M. J., Villavicencio, C., Mena-Ulecia, K., Montesino, R., Altamirano, C., Sánchez, O., Rivas, C. I., Ruíz, Á., & Toledo, J. R. (2023). Co-Formulation of Recombinant Porcine IL-18 Enhances the Onset of Immune Response in a New Lawsonia intracellularis Vaccine. Vaccines, 11(12), 1788. https://doi.org/10.3390/vaccines11121788







