Analysis of the Economic Burden of COVID-19 on the Workers of a Teaching Hospital in the Centre of Italy: Changes in Productivity Loss and Healthcare Costs Pre and Post Vaccination Campaign
Abstract
:1. Introduction
1.1. Studying the Socio-Economic Burden of COVID-19
1.1.1. Health-Related Productivity Loss
- i.
- Absenteeism: being absent from work;
- ii.
- Presenteeism: working while being sick;
- iii.
- Inability to do unpaid work due to illness [12].
- i.
- Temporary time off work: individuals taking time off after the diagnosis;
- ii.
- Premature mortality: years of life lost because of the diagnosis.
1.1.2. Hospitalisation and At-Home Treatments
1.1.3. Contact Tracing and Screening
1.2. Perspective of the Study
1.3. Objective of the Study
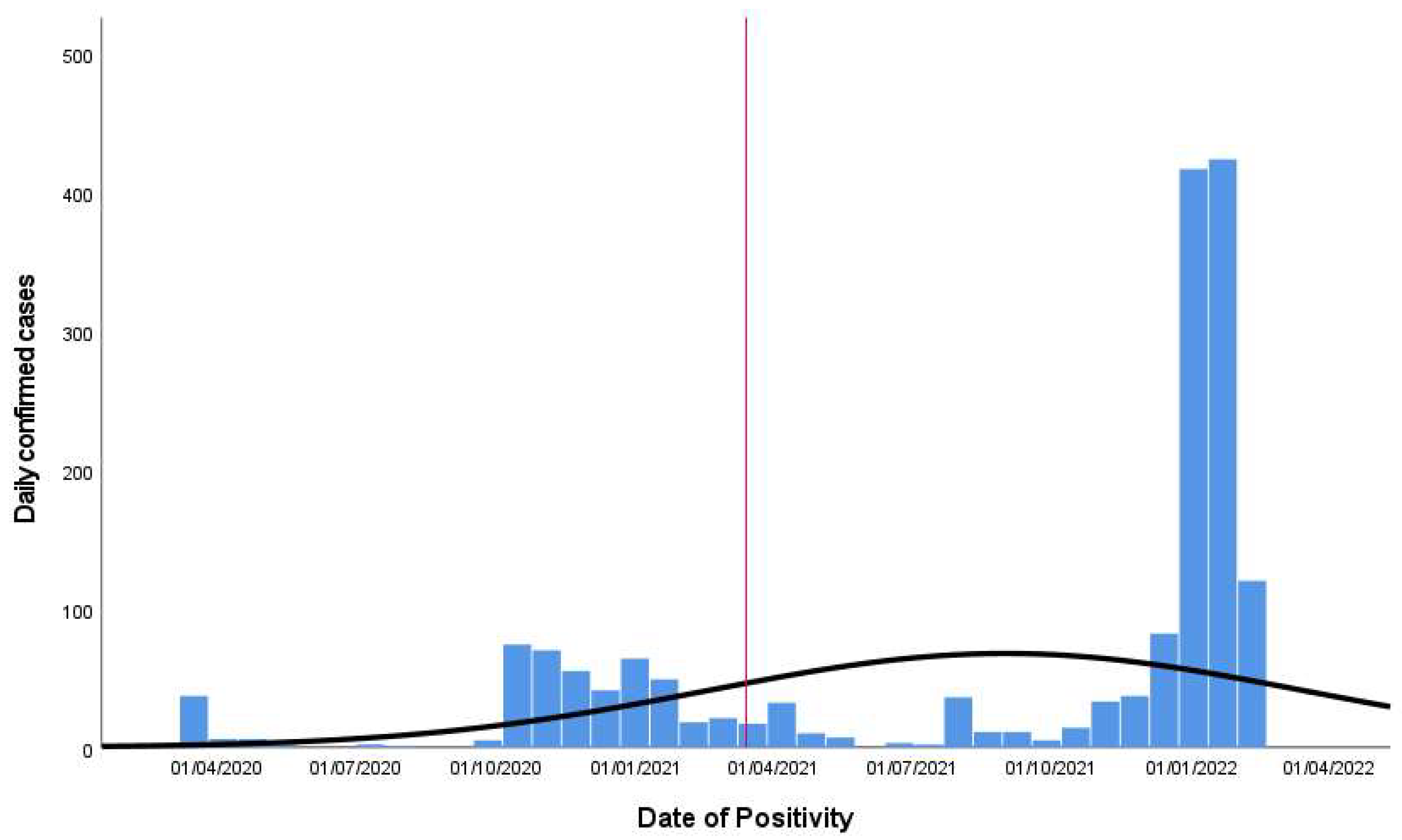
- First period: from the first registered case among the hospital workforce on 1 March 2020 until 9 February 2021.
- Second period: from 10 February 2021 until 31 March 2022, the day when the Italian state of emergency ended.
2. Materials and Methods
2.1. Study Data
2.1.1. Individual Data
2.1.2. Cost Data
- i.
- Hourly salaries were used to estimate the productivity loss with a human capital approach;
- ii.
- Medical expenses were used to estimate the cost of the disease for the society. The medical expenses refer to the cost of hospitalisation, the cost of treatments when not hospitalised, and the hospital cost of performing screening and contact tracing tests.
- Gross hourly salary from the national contracts of hospital workers;
- Net hourly salary from previous micro-costing analysis conducted within the hospital, derived from interviews with hospital workers;
- Online job portals and reports from recruiting companies.
2.2. Methods
2.2.1. Estimation of the Cost of Productivity Loss
2.2.2. Estimation of the Cost of Hospital Admission
2.2.3. Estimation of the Distribution of Confirmed Cases per Health State
2.2.4. Estimation of the Cost of At-Home Treatment Options
2.2.5. Estimation of the Cost of Contact Tracing and Screening Activities
3. Results
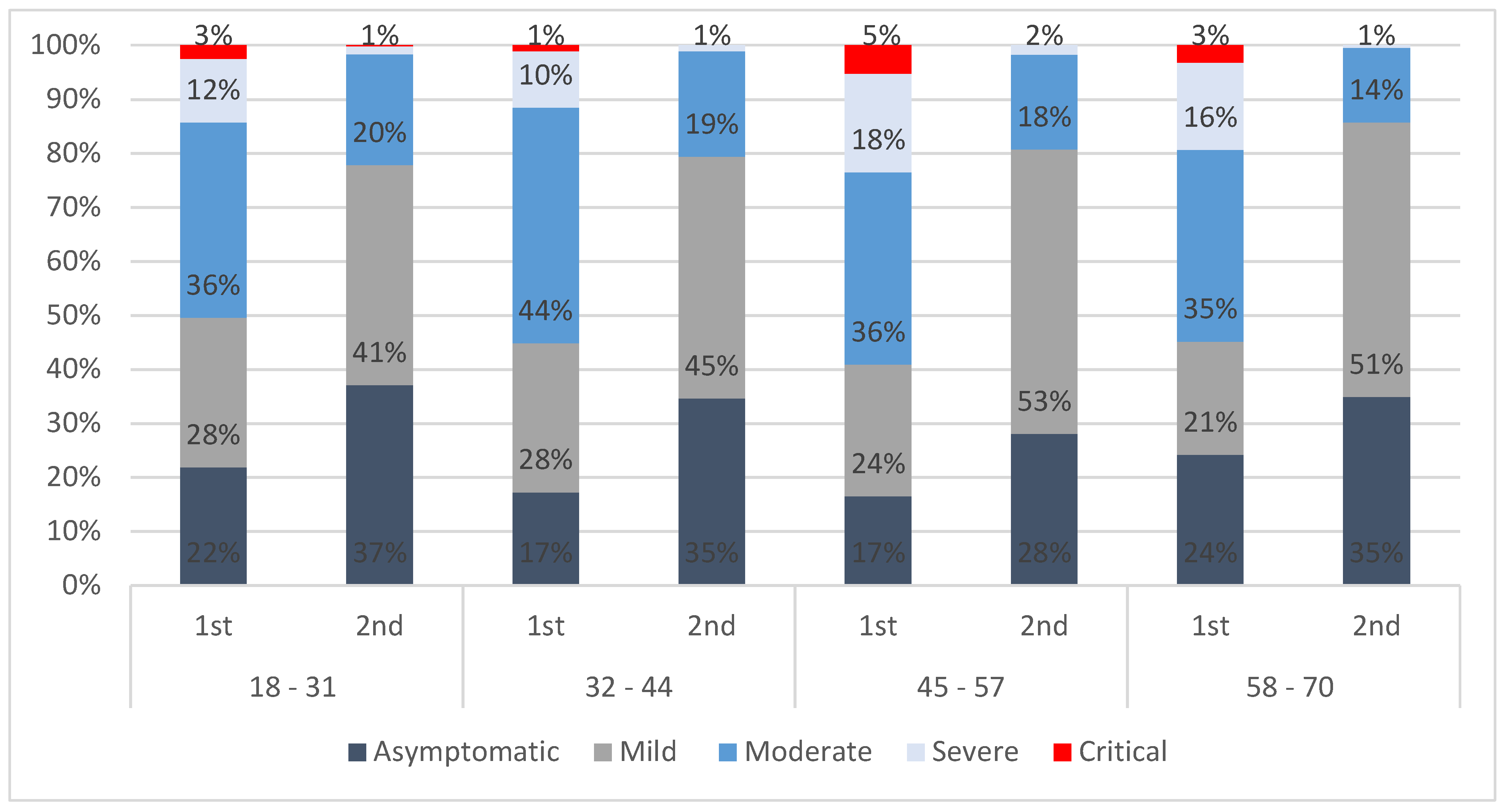
3.1. Distribution of Confirmed Cases and the Health States Model
- i.
- Those with a positive test result, i.e., confirmed cases;
- ii.
- Those with a negative test result who have been in contact with a person testing positive for the infection, i.e., potential cases;
3.2. Productivity Losses
3.3. Hospital Admissions
3.4. At-Home Treatments
3.5. Contact Tracing and Screening Tests
3.6. Total Economic Burden of COVID-19 on the HW
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salinas-Escudero, G.; Toledano-Toledano, F.; García-Peña, C.; Parra-Rodríguez, L.; Granados-García, V.; Carrillo-Vega, M.F. Disability-Adjusted Life Years for the COVID-19 Pandemic in the Mexican Population. Front Public Health 2021, 9, 686700. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Go, D.; Kim, R.; Lee, S.W.; Ock, M.; Kim, Y.; Oh, I.; Yoon, S.; Park, H. The Burden of Disease due to COVID-19 in Korea Using Disability-Adjusted Life Years. J. Korean Med Sci. 2020, 35, e199. [Google Scholar] [CrossRef] [PubMed]
- Quast, T.; Andel, R.; Gregory, S.; Storch, E.A. Years of life lost associated with COVID-19 deaths in the United States. J. Public Health 2020, 42, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.P. Estimating total morbidity burden of COVID-19: Relative importance of death and disability. J. Clin. Epidemiol. 2021, 142, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Gianino, M.M.; Savatteri, A.; Politano, G.; Nurchis, M.C.; Pascucci, D.; Damiani, G. Burden of COVID-19: Disability-Adjusted Life Years (DALYs) across 16 European countries. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5529–5541. [Google Scholar] [CrossRef] [PubMed]
- Rommel, A.; von der Lippe, E.; Plass, D.; Ziese, T.; Diercke, M.; an der Heiden, M.; Haller, S.; Wengler, A.; on behalf of the BURDEN 2020 Study Group. The COVID-19 disease burden in Germany in 2020—Years of life lost to death and disease over the course of the pandemic. Dtsch. Arztebl. Int. 2021, 118, 145. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S.; Calleja, N.; Devleesschauwer Wyper, G.M.A. Estimating the direct COVID-19 disability-adjusted life years impact on the Malta population for the first full year. BMC Public Health 2021, 21, 1827. [Google Scholar] [CrossRef]
- Nurchis, M.C.; Pascucci, D.; Sapienza, M.; Villani, L.; D’Ambrosio, F.; Castrini, F.; Specchia, M.L.; Laurenti, P.; Damiani, G. Impact of the Burden of COVID-19 in Italy: Results of Disability-Adjusted Life Years (DALYs) and Productivity Loss. Int. J. Environ. Res. Public Health 2020, 17, 4233. [Google Scholar] [CrossRef]
- John, D.; Narassima, M.S.; Menon, J.; Rajesh, J.G.; Banerjee, A. Estimation of the economic burden of COVID-19 using disability-adjusted life years (DALYs) and productivity losses in Kerala, India: A model-based analysis. BMJ Open 2021, 11, e049619. [Google Scholar] [CrossRef]
- Ishimaru, T.; Tsuno, K.; Hori, A.; Okawara, M.; Yasuda, Y.; Fujino, Y.; Tabuchi, T. Disrupted care during the COVID-19 state of emergency and productivity loss attributed to presenteeism in workers: A nationwide cross sectional study. BMJ Open 2021, 11, e050068. [Google Scholar] [CrossRef]
- Hanly, P.; Ahern, M.; Sharp, L.; Ursul, D.; Loughnane, G. The cost of lost productivity due to premature mortality associated with COVID-19: A Pan-European study. Eur. J. Health Econ. 2022, 23, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, M.; Salimi, M.; Meskarpour-Amiri, M. Systematic review of productivity loss among healthcare workers due to COVID-19. Int. J. Health Plan. Manag. 2021, 37, 94–111. [Google Scholar] [CrossRef]
- Burdorf, A.; Porru, F.; Rugulies, R. COVID-19 (Coronavirus) pandemic: Consequences for occupational health. Scand. J. Work Environ. Health 2020, 46, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, A.; Javan-Noughabi, J.; Tabatabaee, S.S.; Najafpoor, A.A.; Rezapour, A. The lost productivity cost of absenteeism due to COVID-19 in health care workers in Iran: A case study in the hospitals of Mashhad University of Medical Sciences. BMC Health Serv. Res. 2021, 21, 1169. [Google Scholar] [CrossRef] [PubMed]
- Presidenza del Consiglio dei Ministri—Dipartimento della Protezione Civile. COVID-19 Dashboard. 2020. Available online: https://opendatadpc.maps.arcgis.com/apps/dashboards/b0c68bce2cce478eaac82fe38d4138b1 (accessed on 31 October 2022).
- Wyper, G.A.M.; Assunção, R.M.A.; Colzani, E.; Grant, I.; Haagsma, J.A.; Lagerweij, G.; Von der Lippe, E.; McDonald, S.A.; Pires, S.M.; Porst, M.; et al. Burden of disease methods: A guide to calculate COVID-19 Disability-Adjusted life years. Int. J. Public Health 2021, 66, 619011. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.M.; Hanly, P.; Timmons, A.; Walsh, P.M.; O’Neill, C.; O’Sullivan, E.; Gooberman-Hill, R.; Thomas, A.A.; Gallagher, P.; Sharp, L. Productivity Losses Associated with Head and Neck Cancer Using the Human Capital and Friction Cost Approaches. Appl. Health Econ. Health Policy 2015, 13, 359–367. [Google Scholar] [CrossRef]
- Agenzia Nazionale per i Servizi Sanitari Regionali. Criteri di Appropriatezza Per i Setting Assistenziali di Gestione Ospedaliera dei Pazienti Affetti da COVID-19. Version 2.0. 2021. Available online: https://www.agenas.gov.it/images/agenas/covid-19/Appropriatezza_setting_ospedalieri__COVID_2.0_17_6_2021.pdf (accessed on 31 October 2022).
- Istituto Superiore di Sanità. Piano Nazionale di Vaccinazione COVID-19. 2021. Available online: https://www.epicentro.iss.it/vaccini/covid-19-piano-vaccinazione#:~:text=Il%2027%20dicembre%202020%2C%20il,%C3%A8%20iniziata%20il%2031%20dicembre (accessed on 17 February 2022).
- Quotidiano Sanità. Vaccino Pfizer. Ema: “La Seconda dose va Somministrata dopo tre Settimane dalla Prima”. 2021. Available online: https://www.quotidianosanita.it/scienza-e-farmaci/articolo.php?articolo_id=91954 (accessed on 15 April 2022).
- Giorgi, G. Una Dose Vale L’altra? Efficacia, Richiami, Costi, Effetti Collaterali: Le Differenze Tra I vaccini Anti-Covid. Open. 2021. Available online: https://www.open.online/2021/01/15/covid-19-vaccini-confronto-efficacia-costo/ (accessed on 31 October 2022).
- Istituto Nazionale di Statistica—ISTAT. Life Tables of the Resident Population. 2015. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCIS_MORTALITA1&Lang=en (accessed on 31 October 2022).
- Vekaria, B.; Overton, C.; Wiśniowski, A.; Ahmad, S.; Aparicio-Castro, A.; Curran-Sebastian, J.; Eddleston, J.; Hanley, N.A.; House, T.; Kim, J.; et al. Hospital length of stay for COVID-19 patients: Data-driven methods for forward planning. BMC Infect. Dis. 2021, 21, 700. [Google Scholar] [CrossRef]
- Uzun, O.; Akpolat, T.; Varol, A.; Turan, S.; Bektas, S.G.; Cetinkaya, P.D.; Dursun, M.; Bakan, N.; Ketencioglu, B.B.; Bayrak, M.; et al. COVID-19: Vaccination vs. hospitalization. Infection 2022, 50, 747–752. [Google Scholar] [CrossRef]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The impact of vaccination on COVID-19 outbreaks in the United States. Natl. Libr. Med. 2021. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Omicron: Cosa Sappiamo. 2022. Available online: https://www.iss.it/web/guest/cov19-omicron-cosa-sappiamo (accessed on 20 May 2022).
- Istituto Superiore di Sanità. Variante Delta Sempre Dominante in Italia. 2021. Available online: https://www.iss.it/web/guest/cov19-cosa-fa-iss-varianti/-/asset_publisher/yJS4xO2fauqM/content/variante-delta-sempre-dominante-in-italia?_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_assetEntryId=5881453&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_redirect=https%3A%2F%2Fwww.iss.it%2Fweb%2Fguest%2Fcov19-cosa-fa-iss-varianti%3Fp_p_id%3Dcom_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_assetEntryId%3D5881453%26_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_cur%3D0%26p_r_p_resetCur%3Dfalse (accessed on 20 May 2022).
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Lopez Bernal, J.; Kall, M.; Bhatt, S. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Laxminarayan RWahl, B.; Dudala, S.R.; Mohan, G.B.; Neelima, S.; Jawahar Reddy KSRadhakrishnan, J.; Lewnard, J.A. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science 2020, 370, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.C.; Wu, P.; Wong, J.Y.; Lau, E.H.Y.; Tsang, T.K.; Cauchemez, S.; Leung, G.M.; Cowling, B.J. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat. Med. 2020, 26, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- IRCCS Ospedale San Raffaele. COVID-19: Come Avviene Il Contagio. 2020. Available online: https://www.hsr.it/news/2020/dicembre/covid-come-dove-contagia#:~:text=Nella%20pandemia%20da%20Covid%2D19,dell’80%25%20dei%20contagi (accessed on 14 April 2022).
- Wadman, M.; Hidden Immune Weakness Found in 14% of Gravely ill COVID-19 Patients. Science. 2020. Available online: https://www.science.org/content/article/hidden-immune-weakness-found-14-gravely-ill-covid-19-patients (accessed on 14 April 2022).
- Maltezou, H.C.; Giannouchos, T.V.; APavli Tsonou, P.; Dedoukou, X.; Tseroni, M.; Papadima, K.; Hatzigeorgiou, D.; Sipsas, N.V.; Souliotis, K. Costs associated with COVID-19 in healthcare personnel in Greece: A cost-of-illness analysis. J. Hosp. Infect. 2021, 114, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Camera dei Deputati. Misure Per Il Rafforzamento Del Personale Sanitario Nell’emergenza Coronavirus. 2022. Available online: https://www.camera.it/temiap/documentazione/temi/pdf/1215313.pdf?_1592529612251 (accessed on 31 October 2022).
- Payán, D.D.; Frehn, J.L.; Garcia, L.; Tierney, A.A.; Rodriguez, H.P. Telemedicine implementation and use in community health centers during COVID-19: Clinic personnel and patient perspectives. Qual. Res. Health 2022, 2, 100054. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco. Gestione degli studi clinici in Italia in corso di emergenza COVID-19. 2020. Available online: https://www.aifa.gov.it/-/gestione-degli-studi-clinici-in-italia-in-corso-di-emergenza-covid-19-aggiornamento-del-17-settembre-2020- (accessed on 31 October 2022).




| ANALYSIS | n1 | n2 | DESCRIPTION |
|---|---|---|---|
| TOTAL SAMPLES | 654 | 2621 | All registered confirmed cases |
| ESTIMATION OF THE COST OF PRODUCTIVITY LOSS | 598 | 1709 1 | Confirmed cases for which the days of missed work were available |
| ESTIMATION OF THE COST OF HOSPITALISATION | 38 | 11 | Confirmed cases for which data on hospitalisation were available |
| ESTIMATION OF HEALTH STATES | 380 | 1287 | Confirmed cases for which data on experienced symptoms were available |
| ESTIMATION OF THE COST OF TREATMENT | 144 | 237 | Confirmed cases in the moderate health state |
| GROUP | AVERAGE GROSS SALARY (EUR) | ||
|---|---|---|---|
| Monthly | Daily | Hourly | |
| A | 1523.78 | 58.61 | 9.77 |
| B | 1656.26 | 63.70 | 10.62 |
| C | 1706.85 | 65.65 | 10.94 |
| D | 1798.35 | 69.17 | 11.53 |
| DS | 1892.27 | 72.78 | 12.13 |
| E | 1845.31 | 70.97 | 11.83 |
| A |
|
|
|
| |
| ||
| B |
|
|
|
| |
|
| |
|
| |
| ||
| C |
|
|
|
| |
|
| |
| ||
| D |
|
|
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| ||
| DS |
|
|
|
| |
| E |
|
|
|
| |
|
| TYPE OF HOSPITAL ADMISSION | COST PER DAY (EUR) |
|---|---|
| Ordinary hospital bed | 1000.00 |
| Cost of intensive care w/o automatic ventilation | 1315.00 |
| Cost of intensive care w automatic ventilation | 1654.00 |
| Average cost of hospital stay per day | 1323.00 |
| NAME | DESCRIPTION | CLINICAL MANIFESTATIONS | SETTING FOR ASSISTANCE |
|---|---|---|---|
| ASYMPTOMATIC | Positive test result for SARS-CoV-2 without symptoms or signs of the disease. | Absence of symptoms | Home and potentially activate local services |
| MILD | Has a low fever and mild discomfort but no difficulty with daily activities. Does not require hospitalisation. | Presence of 1 or 2 symptoms with no need of medical attention | Home without activation of local services |
| MODERATE | Has a fever and aches, and feels weak, which causes some difficulty with daily activities. Home quarantining or just admitted to hospital. | Presence of 3–5 symptoms, in need of medical attention but not hospitalised | Home with activation of local services |
| SEVERE | Has a high fever and pain and feels very weak, which causes great difficulty with daily activities. Requires hospitalisation. | Presence of 6–8 symptoms, in need of hospitalisation | Hospital, ordinary bed, or semi-intensive care unit |
| CRITICAL | Positive test result for SARS-CoV-2 with clear signs and symptoms of the disease that require intensive care unit admission (with or without respiratory support). | Presence of 9 or more symptoms, in need of intensive care unit | Hospital, intensive care unit |
| MEDICINAL PRODUCT | DOSE | PRICE/UNIT | UNITS | TOTAL PRICE | |
|---|---|---|---|---|---|
| Treatment 1 1 Mar 2020–9 Feb 2021 | Zitromax or generic | 3 pills/day per 6 days | EUR 1.50 | 2 | EUR 3.00 |
| Deltacortene | 25 mg/day per 3 days and then reduced dose | EUR 1.29 | 1 | EUR 1.29 | |
| Total price per treatment | EUR 4.29 | ||||
| Treatment 2 10 Feb 2021–31 Mar 2022 | Eparina-Clexane | 4000 units/day per 12–18 days | - | 3 | - |
| Deltacortene | 25 mg/day per 3 days and then reduced dose | EUR 1.29 | 1 | EUR 1.29 | |
| Tachipirina/Brufen | 2 pills/day per 5 days | - | 1 | - | |
| Total price per treatment | EUR 1.29 | ||||
| SARS-CoV-2 TEST | COST/UNIT | QUANTITY/DAY | TOTAL COST/DAY |
|---|---|---|---|
| Cost of molecular test for contact tracing | EUR 19.00 | 150 | EUR 2850.00 |
| Cost of molecular test for screening (in pooling) | EUR 2.00 | 500 | EUR 1000.00 |
| Cost of rapid test | EUR 7.00 | 100 | EUR 700.00 |
| (a) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AGE CLUSTER | Males | Females | Total Cases | |||||||||
| Confirmed Cases | % of Total Cases | Deaths | % of Total Deaths | Confirmed Cases | % of Total Cases | Deaths | % of Total Deaths | Confirmed Cases | % of Cases per Age Cluster | Deaths | % of Death per Age Cluster | |
| 18–31 | 46 | 38.7% | 0 | - | 73 | 61.3% | 0 | - | 119 | 18.2% | 0 | - |
| 32–44 | 63 | 36.6% | 0 | - | 109 | 63.4% | 0 | - | 172 | 26.3% | 0 | - |
| 45–57 | 74 | 36.3% | 1 | 100% | 130 | 63.7% | 0 | - | 204 | 31.2% | 1 | 100% |
| 58–70 | 72 | 46.5% | 0 | - | 83 | 53.5% | 0 | - | 155 | 23.7% | 0 | - |
| NOT AVAILABLE | 2 | 66.7% | 0 | - | 1 | 33.3% | 0 | - | 3 | 0.5% | 0 | - |
| TOTAL | 257 | 39.4% | 1 | 100% | 396 | 60.6% | 0 | - | 653 | 100% | 1 | 100% |
| (b) | ||||||||||||
| 18–31 | 302 | 33.0% | 0 | - | 613 | 67.0% | 0 | - | 915 | 34.9% | 0 | - |
| 32–44 | 198 | 34.3% | 0 | - | 379 | 65.7% | 0 | - | 577 | 22.0% | 0 | - |
| 45–57 | 182 | 30.9% | 0 | - | 407 | 69.1% | 0 | - | 589 | 22.5% | 0 | - |
| 58–70 | 196 | 43.9% | 0 | - | 250 | 56.1% | 0 | - | 446 | 17.0% | 0 | - |
| NOT AVAILABLE | 42 | 44.7% | 0 | - | 52 | 55.3% | 0 | - | 94 | 3.6% | 0 | - |
| TOTAL | 920 | 35.1% | 0 | - | 1701 | 64.9% | 0 | - | 2621 | 100% | 0 | - |
| PERIOD | COST OF TEMPORARY PL | COST OF PERMANENT PL | COST OF TOTAL PL | AVERAGE TPL PER PERSON |
|---|---|---|---|---|
| 1 Mar 2020–9 Feb 2021 | EUR 1,802,866.04 | EUR 317,329.68 | EUR 2,120,195.72 | EUR 3545.48 |
| 10 Feb 2021–31 Mar 2022 | EUR 2,348,841.18 | - | EUR 2,348,841.18 | EUR 1374.40 |
| 1 March 2020–9 February 2021 | |||||||
| Row Labels | Count of Age Cluster | Sum of Days of Missed Work | Sum of Gross Individual TPL (EUR) | Avg. TPL per Age Cluster (EUR) | % of TPL per Gender and Age | Avg. Individual TPL (EUR) | Days of Missed Work per Person |
| F | 366 | 11,121.6 | 1,153,810.78 | 64% | 3152.49 | 30.4 | |
| 18–31 | 65 | 1541.1 | 139,855.78 | 2151.63 | 7.8% | 2151.63 | 23.7 |
| 32–44 | 102 | 3103.4 | 329,608.21 | 3231.45 | 18.3% | 3231.45 | 30.4 |
| 45–57 | 122 | 4229.7 | 418,980.78 | 3434.27 | 23.2% | 3434.27 | 34.7 |
| 58–70 | 77 | 2247.3 | 265,366.01 | 3446.31 | 14.7% | 3446.31 | 29.2 |
| M | 231 | 6070.0 | 649,055.26 | 36.0% | 2809.76 | 26.3 | |
| 18–31 | 41 | 909.9 | 90,808.25 | 2214.84 | 5.0% | 2214.84 | 22.2 |
| 32–44 | 59 | 1309.4 | 125,728.71 | 2131.00 | 7.0% | 2131.00 | 22.2 |
| 45–57 | 63 | 1922.3 | 198,063.43 | 3143.86 | 11.0% | 3143.86 | 30.5 |
| 58–70 | 68 | 1928.4 | 234,454.87 | 3447.87 | 13.0% | 3447.87 | 28.4 |
| GRAND TOTAL | 597 | 17,191.6 | 1,802,866.04 | 100.0% | 3019.88 | 28.8 | |
| 10 February 2021–31 March 2022 | |||||||
| Row Labels | Count of Age Cluster | Sum of Days of Missed Work | Sum of Gross Individual TPL (EUR) | Avg. TPL per Age Cluster (EUR) | % of TPL per Gender and Age | Avg. Individual TPL (EUR) | Days of Missed Work per Person |
| F | 1104 | 15,045.6 | 1,516,907.14 | 64.6% | 1374.01 | 13.6 | |
| 18–31 | 343 | 3820.0 | 362,415.05 | 1056.60 | 15.4% | 1056.60 | 11.1 |
| 32–44 | 284 | 3726.4 | 377,726.01 | 1330.02 | 16.1% | 1330.02 | 13.1 |
| 45–57 | 296 | 4439.1 | 448,347.41 | 1514.69 | 19.1% | 1514.69 | 15.0 |
| 58–70 | 181 | 3060.0 | 328,418.67 | 1814.47 | 14.0% | 1814.47 | 16.9 |
| M | 605 | 7485.3 | 831,934.04 | 35.4% | 1375.10 | 12.4 | |
| 18–31 | 195 | 2081.3 | 210,780.09 | 1080.92 | 9.0% | 1080.92 | 10.7 |
| 32–44 | 131 | 1550.9 | 167,317.60 | 1277.23 | 7.1% | 1277.23 | 11.8 |
| 45–57 | 133 | 1852.0 | 193,608.15 | 1455.70 | 8.2% | 1455.70 | 13.9 |
| 58–70 | 146 | 2001.1 | 260,228.20 | 1782.38 | 11.1% | 1782.38 | 13.7 |
| GRAND TOTAL | 1709 | 22,530.9 | 2348,841.18 | 100.0% | 1374.40 | 13.2 | |
| 1 Mar 2020–9 Feb 2021 | ||||||
| Row Labels | Count of Age Cluster | Days of Hospital Stay | Sum of Cost of Hospital Stay (EUR) | Avg. Cost of Hospital Stay per Age Cluster (EUR) | Avg. Cost of Hospital Stay per Gender (EUR) | Avg. Tot Cost of Hospital Stay per Person (EUR) |
| F | 23 | 12 | 275,900.00 | 11,995.65 | ||
| 18–31 | 2 | 13 | 25,180.00 | 12,590.00 | ||
| 32–44 | 3 | 11 | 34,180.00 | 11,393.33 | ||
| 45–57 | 10 | 14 | 139,180.00 | 13,918.00 | ||
| 58–70 | 8 | 10 | 77,360.00 | 9670.00 | ||
| M | 14 | 13 | 187,540.00 | 13,395.71 | ||
| 18–31 | - | - | - | - | ||
| 32–44 | 2 | 12 | 23,590.00 | 11,795.00 | ||
| 45–57 | 4 | 16 | 63,770.00 | 15,942.50 | ||
| 58–70 | 9 | 13 | 100,180.00 | 12,522.50 | ||
| GRAND TOTAL | 38 | 13 | 463,440.00 | 12,590.00 | 12,195.79 | |
| 10 Feb 2021–31 Mar 2022 | ||||||
| Row Labels | Count of Age Cluster | Days of Hospital Stay | Sum of Cost of Hospital Stay (EUR) | Avg. Cost of Hospital Stay per Age Cluster (EUR) | Avg. Cost of Hospital Stay per Gender (EUR) | Avg. Tot Cost of Hospital Stay per Person (EUR) |
| F | 7 | 12 | 82,180.00 | 11,740.00 | ||
| 18–31 | - | - | - | - | ||
| 32–44 | - | - | - | - | ||
| 45–57 | 6 | 10 | 60,180.00 | 10,030.00 | ||
| 58–70 | 1 | 22 | 22,000.00 | 22,000.00 | ||
| M | 4 | 17 | 69,590.00 | 17,397.50 | ||
| 18–31 | 1 | 15 | 15,000.00 | 15,000.00 | ||
| 32–44 | - | - | - | |||
| 45–57 | - | - | - | - | ||
| 58–70 | 3 | 18 | 54,590.00 | 18,196.67 | ||
| GRAND TOTAL | 11 | 14 | 151,770.00 | 13,797.27 | ||
| PERIOD 1 | PERIOD 2 | |
|---|---|---|
| Cost of at-home treatment per person | EUR 4.29 | EUR 1.29 |
| Moderate confirmed cases | 144 | 237 |
| Total cost of at-home treatment | EUR 617.76 | EUR 305.73 |
| FIRST PERIOD | SECOND PERIOD | TOTAL | ||||
|---|---|---|---|---|---|---|
| Type of test | N. of Tests | Expenditure (EUR) | N. of Tests | Expenditure (EUR) | Total Tests | Total Expenditure (EUR) |
| Cost of molecular test for contact tracing | 42,150 | 800,850 | 52,500 | 997,500 | 94,650 | 1,798,350 |
| Cost of molecular test for screening (in pooling) | 140,500 | 281,000 | 175,000 | 350,000 | 315,500 | 631,000 |
| Cost of rapid test | 28,100 | 196,700 | 35,000 | 245,000 | 63,100 | 441,700 |
| Total | 210,750 | 1,278,550 | 262,500 | 1,592,500 | 473,250 | 2,871,050 |
| ESTIMATES | FIRST PERIOD | SECOND PERIOD |
|---|---|---|
| Productivity loss (EUR) | 2,120,195.72 | 2,348,841.18 |
| Hospital admission (EUR) | 463,440.00 | 151,770.00 |
| At-home treatment (EUR) | 617.76 | 305.73 |
| Contact tracing and screening (EUR) | 1,278,550.00 | 1,592,500.00 |
| Total (EUR) | 3,862,803.48 | 4,093,416.91 |
| Confirmed cases | 654.00 | 2,621 |
| Total per person (EUR) | 5906.43 | 1561.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fabio, S.; La Torre, G. Analysis of the Economic Burden of COVID-19 on the Workers of a Teaching Hospital in the Centre of Italy: Changes in Productivity Loss and Healthcare Costs Pre and Post Vaccination Campaign. Vaccines 2023, 11, 1791. https://doi.org/10.3390/vaccines11121791
Di Fabio S, La Torre G. Analysis of the Economic Burden of COVID-19 on the Workers of a Teaching Hospital in the Centre of Italy: Changes in Productivity Loss and Healthcare Costs Pre and Post Vaccination Campaign. Vaccines. 2023; 11(12):1791. https://doi.org/10.3390/vaccines11121791
Chicago/Turabian StyleDi Fabio, Sara, and Giuseppe La Torre. 2023. "Analysis of the Economic Burden of COVID-19 on the Workers of a Teaching Hospital in the Centre of Italy: Changes in Productivity Loss and Healthcare Costs Pre and Post Vaccination Campaign" Vaccines 11, no. 12: 1791. https://doi.org/10.3390/vaccines11121791
APA StyleDi Fabio, S., & La Torre, G. (2023). Analysis of the Economic Burden of COVID-19 on the Workers of a Teaching Hospital in the Centre of Italy: Changes in Productivity Loss and Healthcare Costs Pre and Post Vaccination Campaign. Vaccines, 11(12), 1791. https://doi.org/10.3390/vaccines11121791







