Assessment of Immune Responses to a Trivalent Pichinde Virus-Vectored Vaccine Expressing Hemagglutinin Genes from Three Co-Circulating Influenza A Virus Subtypes in Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Reagents, and Viruses
2.2. Vaccines Used in the Study
2.3. Vaccination and Challenge Experiment
2.4. Immunological Assay
2.5. Quantification of Viral Load
2.6. Pathological Analysis of Lungs
2.7. Statistical Analysis
3. Results
3.1. Antibody Responses after Vaccination
3.2. Viral Genomic RNA in Nasal Secretions and Lungs
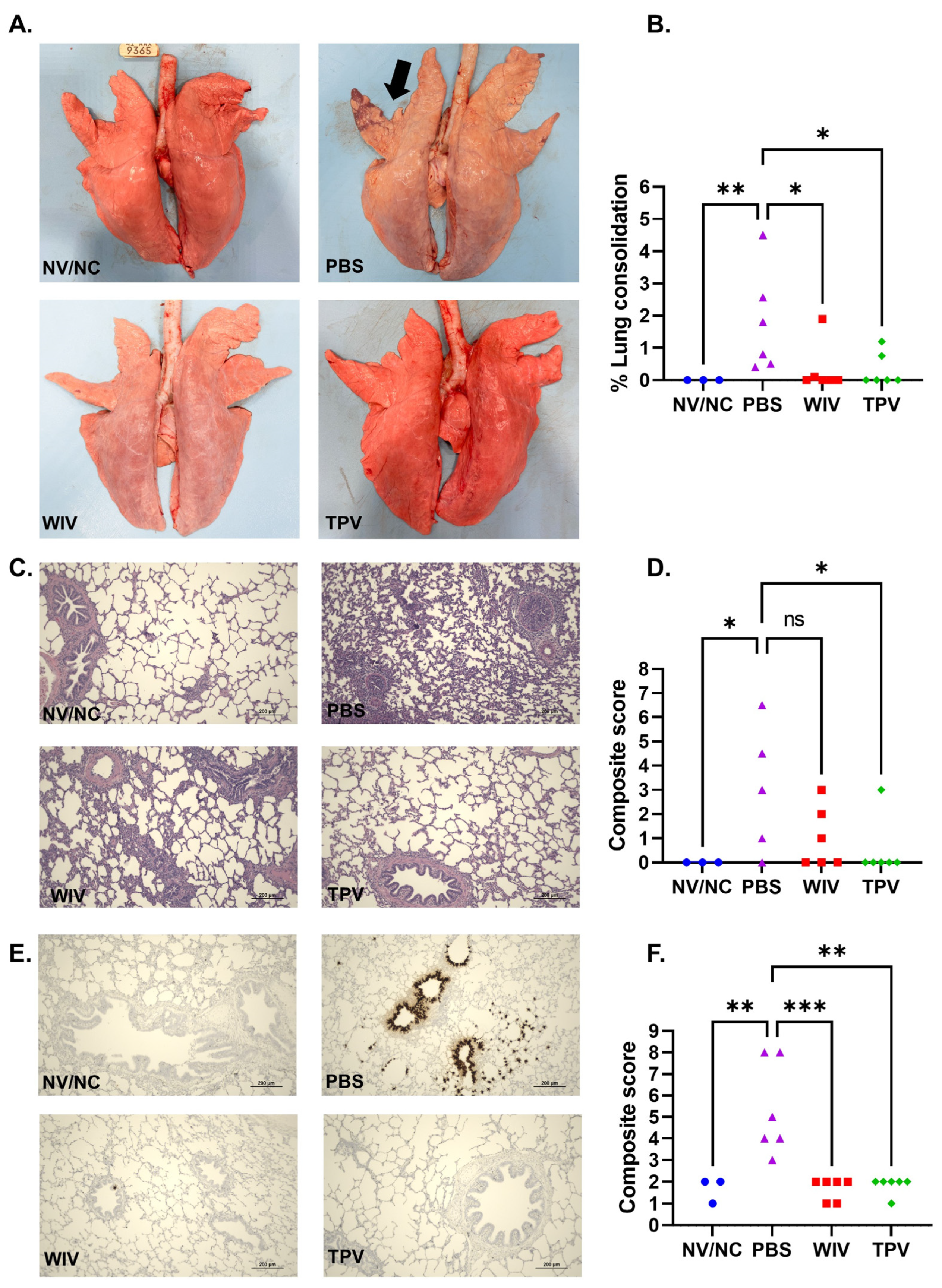
3.3. Macroscopic and Microscopic Lung Lesions
3.4. Antibody Responses against the PICV Vector (Anti-Vectored Immunity)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, A.; Awada, L.; Brown, I.; Chen, H.; Claes, F.; Dauphin, G.; Donis, R.; Culhane, M.; Hamilton, K.; Lewis, N.; et al. Review of Influenza A Virus in Swine Worldwide: A Call for Increased Surveillance and Research. Zoonoses Public Health 2014, 61, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Janke, B.H. Influenza A virus infections in swine: Pathogenesis and diagnosis. Vet. Pathol. 2014, 51, 410–426. [Google Scholar] [CrossRef] [PubMed]
- Calderon Diaz, J.A.; Fitzgerald, R.M.; Shalloo, L.; Rodrigues da Costa, M.; Niemi, J.; Leonard, F.C.; Kyriazakis, I.; Garcia Manzanilla, E. Financial Analysis of Herd Status and Vaccination Practices for Porcine Reproductive and Respiratory Syndrome Virus, Swine Influenza Virus, and Mycoplasma hyopneumoniae in Farrow-to-Finish Pig Farms Using a Bio-Economic Simulation Model. Front. Vet. Sci. 2020, 7, 556674. [Google Scholar] [CrossRef] [PubMed]
- Haden, C.; Painter, T.; Fangman, T.; Holtkamp, D.J. Assessing Production Parameters and Economic Impact of Swine Influenza, PRRS and Mycoplasma Hyopneumoniae on Finishing Pigs in a Large Production System. In Proceedings of the American Association of Swine Veterinarians Annual Meeting, Denver, CO, USA, 10–13 March 2012; Available online: https://vetmed.iastate.edu/sites/default/files/vdpam/Cara%20Haden%20AASV%20Abstract.pdf (accessed on 28 November 2023).
- Ma, W.; Kahn, R.E.; Richt, J.A. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. J. Mol. Genet. Med. Int. J. Biomed. Res. 2008, 3, 158–166. [Google Scholar] [CrossRef]
- Walia, R.R.; Anderson, T.K.; Vincent, A.L. Regional patterns of genetic diversity in swine influenza A viruses in the United States from 2010 to 2016. Influenza Other Respir. Viruses 2018, 13, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.K.; Campbell, B.A.; Nelson, M.I.; Lewis, N.S.; Janas-Martindale, A.; Killian, M.L.; Vincent, A.L. Characterization of co-circulating swine influenza A viruses in North America and the identification of a novel H1 genetic clade with antigenic significance. Virus Res. 2015, 201, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.K.; Macken, C.A.; Lewis, N.S.; Scheuermann, R.H.; Van Reeth, K.; Brown, I.H.; Swenson, S.L.; Simon, G.; Saito, T.; Berhane, Y.; et al. A Phylogeny-Based Global Nomenclature System and Automated Annotation Tool for H1 Hemagglutinin Genes from Swine Influenza A Viruses. mSphere 2016, 1, e00275-16. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.K.; Chang, J.; Arendsee, Z.W.; Venkatesh, D.; Souza, C.K.; Kimble, J.B.; Lewis, N.S.; Davis, C.T.; Vincent, A.L. Swine Influenza A Viruses and the Tangled Relationship with Humans. Cold Spring Harb. Perspect. Med. 2021, 11, a038737. [Google Scholar] [CrossRef]
- Anderson, T.K.; Nelson, M.I.; Kitikoon, P.; Swenson, S.L.; Korslund, J.A.; Vincent, A.L. Population dynamics of cocirculating swine influenza A viruses in the United States from 2009 to 2012. Influenza Other Respir. Viruses 2013, 7, 42–51. [Google Scholar] [CrossRef]
- Salvesen, H.A.; Whitelaw, C.B.A. Current and prospective control strategies of influenza A virus in swine. Porc. Health Manag. 2021, 7, 23. [Google Scholar] [CrossRef]
- Mancera Gracia, J.C.; Pearce, D.S.; Masic, A.; Balasch, M. Influenza A Virus in Swine: Epidemiology, Challenges and Vaccination Strategies. Front. Vet. Sci. 2020, 7, 647. [Google Scholar] [CrossRef] [PubMed]
- Sandbulte, M.R.; Spickler, A.R.; Zaabel, P.K.; Roth, J.A. Optimal Use of Vaccines for Control of Influenza A Virus in Swine. Vaccines 2015, 3, 22–73. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.L.; Lager, K.M.; Janke, B.H.; Gramer, M.R.; Richt, J.A. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Vet. Microbiol. 2008, 126, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Gramer, M.R.; Joo, H.S. Efficacy of swine influenza A virus vaccines against an H3N2 virus variant. Can. J. Vet. Res. 2007, 71, 207–212. [Google Scholar] [PubMed]
- Vincent, A.L.; Ma, W.J.; Lager, K.M.; Richt, J.A.; Janke, B.H.; Sandbulte, M.R.; Gauger, P.C.; Loving, C.L.; Webby, R.J.; Garcia-Sastre, A. Live Attenuated Influenza Vaccine Provides Superior Protection from Heterologous Infection in Pigs with Maternal Antibodies without Inducing Vaccine-Associated Enhanced Respiratory Disease. J. Virol. 2012, 86, 10597–10605. [Google Scholar] [CrossRef] [PubMed]
- Gauger, P.C.; Loving, C.L.; Khurana, S.; Lorusso, A.; Perez, D.R.; Kehrli, M.E., Jr.; Roth, J.A.; Golding, H.; Vincent, A.L. Live attenuated influenza A virus vaccine protects against A(H1N1)pdm09 heterologous challenge without vaccine associated enhanced respiratory disease. Virology 2014, 471–473, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Masic, A.; Lu, X.; Li, J.; Mutwiri, G.K.; Babiuk, L.A.; Brown, E.G.; Zhou, Y. Immunogenicity and protective efficacy of an elastase-dependent live attenuated swine influenza virus vaccine administered intranasally in pigs. Vaccine 2010, 28, 7098–7108. [Google Scholar] [CrossRef]
- Masic, A.; Booth, J.S.; Mutwiri, G.K.; Babiuk, L.A.; Zhou, Y. Elastase-dependent live attenuated swine influenza A viruses are immunogenic and confer protection against swine influenza A virus infection in pigs. J. Virol. 2009, 83, 10198–10210. [Google Scholar] [CrossRef]
- Richt, J.A.; Lekcharoensuk, P.; Lager, K.M.; Vincent, A.L.; Loiacono, C.M.; Janke, B.H.; Wu, W.H.; Yoon, K.J.; Webby, R.J.; Solorzano, A.; et al. Vaccination of pigs against swine influenza viruses by using an NS1-truncated modified live-virus vaccine. J. Virol. 2006, 80, 11009–11018. [Google Scholar] [CrossRef]
- Sharma, A.; Zeller, M.A.; Li, G.; Harmon, K.M.; Zhang, J.; Hoang, H.; Anderson, T.K.; Vincent, A.L.; Gauger, P.C. Detection of live attenuated influenza vaccine virus and evidence of reassortment in the U.S. swine population. J. Vet. Diagn. Investig. 2020, 32, 301–311. [Google Scholar] [CrossRef]
- Loeffen, W.L.; de Vries, R.P.; Stockhofe, N.; van Zoelen-Bos, D.; Maas, R.; Koch, G.; Moormann, R.J.; Rottier, P.J.; de Haan, C.A. Vaccination with a soluble recombinant hemagglutinin trimer protects pigs against a challenge with pandemic (H1N1) 2009 influenza virus. Vaccine 2011, 29, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Rajao, D.S.; Loving, C.L.; Gauger, P.C.; Kitikoon, P.; Vincent, A.L. Influenza A virus hemagglutinin protein subunit vaccine elicits vaccine-associated enhanced respiratory disease in pigs. Vaccine 2014, 32, 5170–5176. [Google Scholar] [CrossRef] [PubMed]
- Bragstad, K.; Vinner, L.; Hansen, M.S.; Nielsen, J.; Fomsgaard, A. A polyvalent influenza A DNA vaccine induces heterologous immunity and protects pigs against pandemic A(H1N1)pdm09 virus infection. Vaccine 2013, 31, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Gorres, J.P.; Lager, K.M.; Kong, W.P.; Royals, M.; Todd, J.P.; Vincent, A.L.; Wei, C.J.; Loving, C.L.; Zanella, E.L.; Janke, B.; et al. DNA Vaccination Elicits Protective Immune Responses against Pandemic and Classic Swine Influenza Viruses in Pigs. Clin. Vaccine Immunol. 2011, 18, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Borggren, M.; Nielsen, J.; Karlsson, I.; Dalgaard, T.S.; Trebbien, R.; Williams, J.A.; Fomsgaard, A. A polyvalent influenza DNA vaccine applied by needle-free intradermal delivery induces cross-reactive humoral and cellular immune responses in pigs. Vaccine 2016, 34, 3634–3640. [Google Scholar] [CrossRef]
- Karlsson, I.; Borggren, M.; Rosenstierne, M.W.; Trebbien, R.; Williams, J.A.; Vidal, E.; Vergara-Alert, J.; Foz, D.S.; Darji, A.; Sistere-Oro, M.; et al. Protective effect of a polyvalent influenza DNA vaccine in pigs. Vet. Immunol. Immunopathol. 2018, 195, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Macklin, M.D.; McCabe, D.; McGregor, M.W.; Neumann, V.; Meyer, T.; Callan, R.; Hinshaw, V.S.; Swain, W.F. Immunization of pigs with a particle-mediated DNA vaccine to influenza A virus protects against challenge with homologous virus. J. Virol. 1998, 72, 1491–1496. [Google Scholar] [CrossRef]
- Larsen, D.L.; Olsen, C.W. Effects of DNA dose, route of vaccination, and coadministration of porcine interleukin-6 DNA on results of DNA vaccination against influenza virus infection in pigs. Am. J. Vet. Res. 2002, 63, 653–659. [Google Scholar] [CrossRef]
- Erdman, M.M.; Kamrud, K.I.; Harris, D.L.; Smith, J. Alphavirus replicon particle vaccines developed for use in humans induce high levels of antibodies to influenza virus hemagglutinin in swine: Proof of concept. Vaccine 2010, 28, 594–596. [Google Scholar] [CrossRef]
- Demoulins, T.; Ruggli, N.; Gerber, M.; Thomann-Harwood, L.J.; Ebensen, T.; Schulze, K.; Guzman, C.A.; McCullough, K.C. Self-Amplifying Pestivirus Replicon RNA Encoding Influenza Virus Nucleoprotein and Hemagglutinin Promote Humoral and Cellular Immune Responses in Pigs. Front. Immunol. 2020, 11, 622385. [Google Scholar] [CrossRef]
- Vander Veen, R.L.; Loynachan, A.T.; Mogler, M.A.; Russell, B.J.; Harris, D.L.; Kamrud, K.I. Safety, immunogenicity, and efficacy of an alphavirus replicon-based swine influenza virus hemagglutinin vaccine. Vaccine 2012, 30, 1944–1950. [Google Scholar] [CrossRef]
- do Nascimento, G.M.; Bugybayeva, D.; Patil, V.; Schrock, J.; Yadagiri, G.; Renukaradhya, G.J.; Diel, D.G. An Orf-Virus (ORFV)-Based Vector Expressing a Consensus H1 Hemagglutinin Provides Protection against Diverse Swine Influenza Viruses. Viruses 2023, 15, 994. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.R.; Knudsen, D.; Pineyro, P.; Dhakal, S.; Renukaradhya, G.J.; Diel, D.G. Protective Efficacy of an Orf Virus-Vector Encoding the Hemagglutinin and the Nucleoprotein of Influenza A Virus in Swine. Front. Immunol. 2021, 12, 747574. [Google Scholar] [CrossRef] [PubMed]
- Braucher, D.R.; Henningson, J.N.; Loving, C.L.; Vincent, A.L.; Kim, E.; Steitz, J.; Gambotto, A.A.; Kehrli, M.E., Jr. Intranasal vaccination with replication-defective adenovirus type 5 encoding influenza virus hemagglutinin elicits protective immunity to homologous challenge and partial protection to heterologous challenge in pigs. Clin. Vaccine Immunol. 2012, 19, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Wesley, R.D.; Tang, M.; Lager, K.M. Protection of weaned pigs by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of H3N2 swine influenza virus. Vaccine 2004, 22, 3427–3434. [Google Scholar] [CrossRef]
- Wesley, R.D.; Lager, K.M. Overcoming maternal antibody interference by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of swine influenza virus. Vet. Microbiol. 2006, 118, 67–75. [Google Scholar] [CrossRef]
- Wesley, R.D.; Lager, K.M. Evaluation of a recombinant human adenovirus-5 vaccine administered via needle-free device and intramuscular injection for vaccination of pigs against swine influenza virus. Am. J. Vet. Res. 2005, 66, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Klingbeil, K.; Lange, E.; Blohm, U.; Teifke, J.P.; Mettenleiter, T.C.; Fuchs, W. Protection of pigs against pandemic swine origin H1N1 influenza A virus infection by hemagglutinin- or neuraminidase-expressing attenuated pseudorabies virus recombinants. Virus Res. 2015, 199, 20–30. [Google Scholar] [CrossRef]
- Klingbeil, K.; Lange, E.; Teifke, J.P.; Mettenleiter, T.C.; Fuchs, W. Immunization of pigs with an attenuated pseudorabies virus recombinant expressing the haemagglutinin of pandemic swine origin H1N1 influenza A virus. J. Gen. Virol. 2014, 95, 948–959. [Google Scholar] [CrossRef]
- Dhanwani, R.; Zhou, Y.; Huang, Q.; Verma, V.; Dileepan, M.; Ly, H.; Liang, Y. A Novel Live Pichinde Virus-Based Vaccine Vector Induces Enhanced Humoral and Cellular Immunity after a Booster Dose. J. Virol. 2015, 90, 2551–2560. [Google Scholar] [CrossRef]
- Kumar, P.; Sharafeldin, T.A.; Kumar, R.; Huang, Q.; Liang, Y.; Goyal, S.M.; Porter, R.E.; Ly, H.; Mor, S.K. Development of a Recombinant Pichinde Virus-Vectored Vaccine against Turkey Arthritis Reovirus and Its Immunological Response Characterization in Vaccinated Animals. Pathogens 2021, 10, 197. [Google Scholar] [CrossRef]
- Kumari, S.; Chaudhari, J.; Huang, Q.; Gauger, P.; De Almeida, M.N.; Liang, Y.; Ly, H.; Vu, H.L.X. Immunogenicity and Protective Efficacy of a Recombinant Pichinde Viral-Vectored Vaccine Expressing Influenza Virus Hemagglutinin Antigen in Pigs. Vaccines 2022, 10, 1400. [Google Scholar] [CrossRef] [PubMed]
- WHO. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; World Health Organization: Geneva, Switzerland, 2011; Chapter 2.E; pp. 43–57.
- Halbur, P.G.; Paul, P.S.; Frey, M.L.; Landgraf, J.; Eernisse, K.; Meng, X.J.; Lum, M.A.; Andrews, J.J.; Rathje, J.A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1995, 32, 648–660. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, J.T. Diva vaccines that reduce virus transmission. J. Biotechnol. 1999, 73, 195–205. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, J.T.; Kaashoek, M.J.; Rijsewijk, F.A.; Stegeman, J.A. The use of marker vaccines in eradication of herpesviruses. J. Biotechnol. 1996, 44, 75–81. [Google Scholar] [CrossRef]
- Capua, I.; Schmitz, A.; Jestin, V.; Koch, G.; Marangon, S. Vaccination as a tool to combat introductions of notifiable avian influenza viruses in Europe, 2000 to 2006. Rev. Sci. Tech. 2009, 28, 245–259. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Chaudhari, J.; Huang, Q.; Gauger, P.; De Almeida, M.N.; Ly, H.; Liang, Y.; Vu, H.L.X. Assessment of Immune Responses to a Trivalent Pichinde Virus-Vectored Vaccine Expressing Hemagglutinin Genes from Three Co-Circulating Influenza A Virus Subtypes in Pigs. Vaccines 2023, 11, 1806. https://doi.org/10.3390/vaccines11121806
Kumari S, Chaudhari J, Huang Q, Gauger P, De Almeida MN, Ly H, Liang Y, Vu HLX. Assessment of Immune Responses to a Trivalent Pichinde Virus-Vectored Vaccine Expressing Hemagglutinin Genes from Three Co-Circulating Influenza A Virus Subtypes in Pigs. Vaccines. 2023; 11(12):1806. https://doi.org/10.3390/vaccines11121806
Chicago/Turabian StyleKumari, Sushmita, Jayeshbhai Chaudhari, Qinfeng Huang, Phillip Gauger, Marcelo Nunes De Almeida, Hinh Ly, Yuying Liang, and Hiep L. X. Vu. 2023. "Assessment of Immune Responses to a Trivalent Pichinde Virus-Vectored Vaccine Expressing Hemagglutinin Genes from Three Co-Circulating Influenza A Virus Subtypes in Pigs" Vaccines 11, no. 12: 1806. https://doi.org/10.3390/vaccines11121806





