Preliminary Study on the Efficacy of a Recombinant, Subunit SARS-CoV-2 Animal Vaccine against Virulent SARS-CoV-2 Challenge in Cats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cells, Virus, and Virus Challenge Procedure
2.3. Clinical Observations and Sample Collection
2.4. Virus-Neutralizing Antibodies Test
2.5. ELISA Antibody Test
2.6. RT-qPCR for Detection of SARS-CoV-2 in Tissues and Nasal, Oral, and Rectal Swabs
2.7. Necropsy Procedures and Microscopic Examinations (Histopathology)
2.8. Statistical Analyses
3. Results
3.1. Clinical Observations: Vaccination Phase
3.2. Clinical Observations: SARS-CoV-2 Challenge Phase
3.3. Determination of Serological Responses to Vaccination and Challenge
3.3.1. ELISA Antibody Test
3.3.2. Virus-Neutralizing Antibodies Test
3.4. RT-qPCR Detection of SARS-CoV-2 in Tissues, and Nasal, Oral, and Rectal Swabs
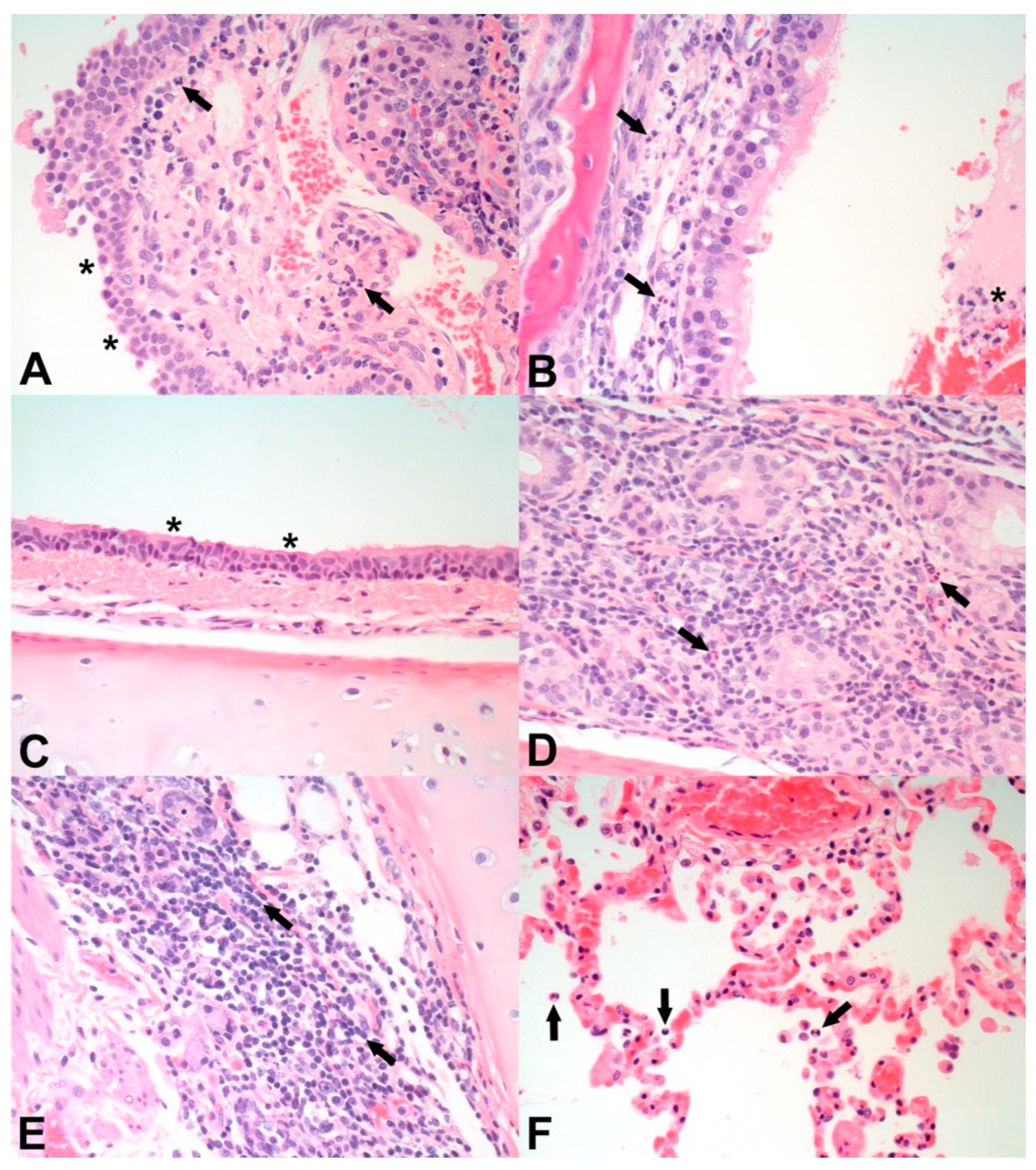
3.5. Macroscopic and Microscopic Pathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michelitsch, A.; Wernike, K.; Ulrich, L.; Mettenleiter, T.C.; Beer, M. SARS-CoV-2 in animals: From potential hosts to animal models. Adv. Virus Res. 2021, 110, 59–102. [Google Scholar] [CrossRef]
- Meekins, D.A.; Gaudreault, N.N.; Richt, J.A. Natural and Experimental SARS-CoV-2 Infection in Domestic and Wild Animals. Viruses 2021, 13, 1993. [Google Scholar] [CrossRef]
- Cleary, S.J.; Pitchford, S.C.; Amison, R.T.; Carrington, R.; Robaina Cabrera, C.L.; Magnen, M.; Looney, M.R.; Gray, E.; Page, C.P. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br. J. Pharmacol. 2020, 177, 4851–4865. [Google Scholar] [CrossRef] [PubMed]
- Meekins, D.A.; Morozov, I.; Trujillo, J.D.; Gaudreault, N.N.; Bold, D.; Carossino, M.; Artiaga, B.L.; Indran, S.V.; Kwon, T.; Balaraman, V.; et al. Susceptibility of swine cells and domestic pigs to SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Cool, K.; Trujillo, J.D.; Morozov, I.; Meekins, D.A.; McDowell, C.; Bold, D.; Carossino, M.; Balaraman, V.; Mitzel, D.; et al. Susceptibility of sheep to experimental co-infection with the ancestral lineage of SARS-CoV-2 and its alpha variant. Emerg. Microbes Infect. 2022, 11, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Cool, K.; Gaudreault, N.N.; Morozov, I.; Trujillo, J.D.; Meekins, D.A.; McDowell, C.; Carossino, M.; Bold, D.; Mitzel, D.; Kwon, T.; et al. Infection and transmission of ancestral SARS-CoV-2 and its alpha variant in pregnant white-tailed deer. Emerg. Microbes Infect. 2022, 11, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.V.; Vaccari, M.; Doyle-Meyers, L.A.; Roy, C.J.; Russell-Lodrigue, K.; Fahlberg, M.; Monjure, C.J.; Beddingfield, B.; Plante, K.S.; Plante, J.A.; et al. Acute Respiratory Distress in Aged, SARS-CoV-2-Infected African Green Monkeys but Not Rhesus Macaques. Am. J. Pathol. 2021, 191, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.L.; Nambulli, S.; McMillen, C.M.; White, A.G.; Tilston-Lunel, N.L.; Albe, J.R.; Cottle, E.; Dunn, M.D.; Frye, L.J.; Gilliland, T.H.; et al. SARS-CoV-2 infection of African green monkeys results in mild respiratory disease discernible by PET/CT imaging and shedding of infectious virus from both respiratory and gastrointestinal tracts. PLoS Pathog. 2020, 16, e1008903. [Google Scholar] [CrossRef]
- Oreshkova, N.; Molenaar, R.J.; Vreman, S.; Harders, F.; Oude Munnink, B.B.; Hakze-van der Honing, R.W.; Gerhards, N.; Tolsma, P.; Bouwstra, R.; Sikkema, R.S.; et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance 2020, 25, 2001005, Erratum in: Eurosurveillance 2021, 26, 210325c. [Google Scholar] [CrossRef]
- Shuai, L.; Zhong, G.; Yuan, Q.; Wen, Z.; Wang, C.; He, X.; Liu, R.; Wang, J.; Zhao, Q.; Liu, Y.; et al. Replication, pathogenicity, and transmission of SARS-CoV-2 in minks. Natl. Sci. Rev. 2020, 8, nwaa291. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.M.; Root, J.J.; Porter, S.M.; Walker, A.E.; Guilbert, L.; Hawvermale, D.; Pepper, A.; Maison, R.M.; Hartwig, A.E.; Gordy, P.; et al. Peridomestic Mammal Susceptibility to Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Emerg. Infect Dis. 2021, 27, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Rosenke, K.; Meade-White, K.; Letko, M.; Clancy, C.; Hansen, F.; Liu, Y.; Okumura, A.; Tang-Huau, T.L.; Li, R.; Saturday, G.; et al. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg. Microbes Infect. 2020, 9, 2673–2684. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, S.G.; Kim, S.M.; Kim, E.H.; Park, S.J.; Yu, K.M.; Chang, J.H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020, 27, 704–709.e2. [Google Scholar] [CrossRef] [PubMed]
- Schlottau, K.; Rissmann, M.; Graaf, A.; Schön, J.; Sehl, J.; Wylezich, C.; Höper, D.; Mettenleiter, T.C.; Balkema-Buschmann, A.; Harder, T.; et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: An experimental transmission study. Lancet Microbe 2020, 1, e218–e225. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.; Chan, J.F.; Yuen, T.T.; Yoon, C.; Hu, J.C.; Wen, L.; Hu, B.; Yang, D.; Wang, Y.; Hou, Y.; et al. Emerging SARS-CoV-2 variants expand species tropism to murines. EBioMedicine 2021, 73, 103643. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Ramirez, S.; Rendon-Marin, S.; Jaimes, J.A.; Martinez-Gutierrez, M.; Ruiz-Saenz, J. SARS-CoV-2 Clinical Outcome in Domestic and Wild Cats: A Systematic Review. Animals 2021, 11, 2056. [Google Scholar] [CrossRef] [PubMed]
- van der Leij, W.J.R.; Broens, E.M.; Hesselink, J.W.; Schuurman, N.; Vernooij, J.C.M.; Egberink, H.F. Serological Screening for Antibodies against SARS-CoV-2 in Dutch Shelter Cats. Viruses 2021, 13, 1634. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Saz, S.; Giner, J.; Tobajas, A.P.; Perez, M.D.; Gonzalez-Ramirez, A.M.; Macias-Leon, J.; Gonzalez, A.; Verde, M.; Yzuel, A.; Hurtado-Guerrero, R.; et al. Serological evidence of SARS-CoV-2 and co-infections in stray cats in Spain. Transbound. Emerg. Dis. 2022, 69, 1056–1064. [Google Scholar] [CrossRef]
- Barroso-Arevalo, S.; Barneto, A.; Ramos, A.M.; Rivera, B.; Sanchez, R.; Sanchez-Morales, L.; Perez-Sancho, M.; Buendia, A.; Ferreras, E.; Ortiz-Menendez, J.C.; et al. Large-scale study on virological and serological prevalence of SARS-CoV-2 in cats and dogs in Spain. Transbound. Emerg. Dis. 2022, 69, e759–e774. [Google Scholar] [CrossRef]
- Barua, S.; Hoque, M.; Adekanmbi, F.; Kelly, P.; Jenkins-Moore, M.; Torchetti, M.K.; Chenoweth, K.; Wood, T.; Wang, C. Antibodies to SARS-CoV-2 in dogs and cats, USA. Emerg. Microbes Infect. 2021, 10, 1669–1674. [Google Scholar] [CrossRef]
- Curukoglu, A.; Ergoren, M.C.; Ozgencil, F.E.; Sayiner, S.; Ince, M.E.; Sanlidag, T. First direct human-to-cat transmission of the SARS-CoV-2 B.1.1.7 variant. Aust. Vet. J. 2021, 99, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Jarrah, S.A.; Kmetiuk, L.B.; Valleriani, F.; Bonfini, B.; Lorusso, A.; Vasinioti, V.; Decaro, N.; Dos Santos, M.T.; Spohr, K.A.H.; Pratelli, A.; et al. SARS-CoV-2 antibodies in dogs and cats in a highly infected area of Brazil during the pandemic. Front. Vet. Sci. 2023, 10, 1111728. [Google Scholar] [CrossRef] [PubMed]
- Hamer, S.A.; Pauvolid-Corrêa, A.; Zecca, I.B.; Davila, E.; Auckland, L.D.; Roundy, C.M.; Tang, W.; Torchetti, M.K.; Killian, M.L.; Jenkins-Moore, M.; et al. SARS-CoV-2 Infections and Viral Isolations among Serially Tested Cats and Dogs in Households with Infected Owners in Texas, USA. Viruses 2021, 13, 938. [Google Scholar] [CrossRef] [PubMed]
- Calvet, G.A.; Pereira, S.A.; Ogrzewalska, M.; Pauvolid-Corrêa, A.; Resende, P.C.; Tassinari, W.S.; Costa, A.P.; Keidel, L.O.; da Rocha, A.S.B.; da Silva, M.F.B.; et al. Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with COVID-19 in Rio de Janeiro, Brazil. PLoS ONE 2021, 16, e0250853. [Google Scholar] [CrossRef] [PubMed]
- Sila, T.; Sunghan, J.; Laochareonsuk, W.; Surasombatpattana, S.; Kongkamol, C.; Ingviya, T.; Siripaitoon, P.; Kositpantawong, N.; Kanchanasuwan, S.; Hortiwakul, T.; et al. Suspected Cat-to-Human Transmission of SARS-CoV-2, Thailand, July-September 2021. Emerg. Infect Dis. 2022, 28, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bellon, H.; Rodon, J.; Fernandez-Bastit, L.; Almagro, V.; Padilla-Sole, P.; Lorca-Oro, C.; Valle, R.; Roca, N.; Grazioli, S.; Trogu, T.; et al. Monitoring Natural SARS-CoV-2 Infection in Lions (Panthera leo) at the Barcelona Zoo: Viral Dynamics and Host Responses. Viruses 2021, 13, 1683. [Google Scholar] [CrossRef] [PubMed]
- McAloose, D.; Laverack, M.; Wang, L.; Killian, M.L.; Caserta, L.C.; Yuan, F.; Mitchell, P.K.; Queen, K.; Mauldin, M.R.; Cronk, B.D.; et al. From People to Panthera: Natural SARS-CoV-2 Infection in Tigers and Lions at the Bronx Zoo. mBio 2020, 11, e02220-20. [Google Scholar] [CrossRef]
- Wang, L.; Gyimesi, Z.S.; Killian, M.L.; Torchetti, M.; Olmstead, C.; Fredrickson, R.; Terio, K.A. Detection of SARS-CoV-2 clade B.1.2 in three snow leopards. Transbound. Emerg. Dis. 2022, 69, e3346–e3351. [Google Scholar] [CrossRef]
- Koeppel, K.N.; Mendes, A.; Strydom, A.; Rotherham, L.; Mulumba, M.; Venter, M. SARS-CoV-2 Reverse Zoonoses to Pumas and Lions, South Africa. Viruses 2022, 14, 120. [Google Scholar] [CrossRef]
- Siegrist, A.A.; Richardson, K.L.; Ghai, R.R.; Pope, B.; Yeadon, J.; Culp, B.; Behravesh, C.B.; Liu, L.; Brown, J.A.; Boyer, L.V. Probable Transmission of SARS-CoV-2 from African Lion to Zoo Employees, Indiana, USA, 2021. Emerg. Infect Dis. 2023, 29, 1102–1108. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Gordy, P.W.; Nehring, M.; Byas, A.D.; VandeWoude, S.; Ragan, I.K.; Maison, R.M.; Bowen, R.A. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc. Natl. Acad. Sci. USA 2020, 117, 26382–26388. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Trujillo, J.D.; Carossino, M.; Meekins, D.A.; Morozov, I.; Madden, D.W.; Indran, S.V.; Bold, D.; Balaraman, V.; Kwon, T.; et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg. Microbes Infect. 2020, 9, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Hatta, M.; Chiba, S.; Maemura, T.; Fan, S.; Takeda, M.; Kinoshita, N.; Hattori, S.I.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; et al. Transmission of SARS-CoV-2 in Domestic Cats. N. Engl. J. Med. 2020, 383, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Bienzle, D.; Rousseau, J.; Marom, D.; MacNicol, J.; Jacobson, L.; Sparling, S.; Prystajecky, N.; Fraser, E.; Weese, J.S. Risk Factors for SARS-CoV-2 Infection and Illness in Cats and Dogs1. Emerg. Infect. Dis. 2022, 28, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Bashor, L.; Gagne, R.B.; Bosco-Lauth, A.; Stenglein, M.; VandeWoude, S. Rapid evolution of SARS-CoV-2 in domestic cats. Virus Evol. 2022, 8, veac092. [Google Scholar] [CrossRef]
- Ura, T.; Yamashita, A.; Mizuki, N.; Okuda, K.; Shimada, M. New vaccine production platforms used in developing SARS-CoV-2 vaccine candidates. Vaccine 2021, 39, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, P.; Zhang, X.; Yu, Z.; Zhang, W.; Sun, H. SARS-CoV-2 vaccine candidates in rapid development. Hum. Vaccines Immunother. 2021, 17, 644–653. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schafer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA Vaccine Development Enabled by Prototype Pathogen Preparedness. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Carossino, M.; Morozov, I.; Trujillo, J.D.; Meekins, D.A.; Madden, D.W.; Cool, K.; Artiaga, B.L.; McDowell, C.; Bold, D.; et al. Experimental re-infected cats do not transmit SARS-CoV-2. Emerg. Microbes Infect. 2021, 10, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Song, Z.; Xue, J.; Gao, H.; Liu, J.; Wang, J.; Guo, Q.; Zhao, B.; Qu, Y.; Qi, F.; et al. Susceptibility and Attenuated Transmissibility of SARS-CoV-2 in Domestic Cats. J. Infect. Dis. 2021, 223, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Hoyte, A.; Webster, M.; Ameiss, K.; Conlee, D.A.; Hainer, N.; Hutchinson, K.; Burakova, Y.; Dominowski, P.J.; Baima, E.T.; King, V.L.; et al. Experimental veterinary SARS-CoV-2 vaccine cross neutralization of the Delta (B.1.617.2) variant virus in cats. Vet. Microbiol. 2022, 268, 109395. [Google Scholar] [CrossRef] [PubMed]
| Group | DPC | BT Least Squares Mean | BT Standard Error | BT 90% Confidence Limits | Minimum | Maximum |
|---|---|---|---|---|---|---|
| T01 | 0 | 212 | 62 | 127–354 | 150 | 300 |
| 14 | 3553 | 1035 | 2128–5932 | 2700 | 8100 | |
| T02 | 0 | 42,089 * | 12,260 | 25,211–70,266 | 24,300 | 72,900 |
| 14 | 95,865 * | 27,924 | 57,423–160,043 | 72,900 | 218,000 |
| Group | DPC | BT Least Squares Mean | BT Standard Error | BT 90% Confidence Limits | Minimum | Maximum |
|---|---|---|---|---|---|---|
| T01 | 0 | 10.0 | 2.49 | 6.5 to 15.5 | 10 | 10 |
| 14 | 160.0 | 39.81 | 103.4 to 247.6 | 160 | 160 | |
| T02 | 0 | 320.0 * | 79.61 | 206.8 to 495.3 | 160 | 1280 |
| 14 | 2560.0 * | 636.90 | 1654.1 to 3962.1 | 2560 # | 2560 # |
| Swab | Day Post-Challenge | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | ||
| Nasal | T01 | 0.00 × 10+00 | 2.42 × 10+09 | 2.04 × 10+10 | 1.55 × 10+09 | 5.54 × 10+07 | 4.02 × 10+05 | 2.65 × 10+05 | 1.26 × 10+05 |
| T02 | 0.00 × 10+00 | 1.64 × 10+06 * | 2.37 × 10+05 * | 1.20 × 10+06 * | 8.36 × 10+03 * | 0.00 × 10+00 * | 1.41 × 10+01 * | 0.00 × 10+00 * | |
| Oral | T01 | 0.00 × 10+00 | 9.71 × 10+08 | 9.64 × 10+08 | 2.12 × 10+08 | 1.84 × 10+04 | 1.49 × 10+01 | 1.15 × 10+03 | 2.60 × 10+02 |
| T02 | 0.00 × 10+00 | 6.00 × 10+04 * | 3.66 × 10+07 | 5.18 × 10+06 | 1.95 × 10+01 * | 1.55 × 10+01 | 2.95 × 10+02 | 0.00 × 10+00 * | |
| Rectal | T01 | 0.00 × 10+00 | 5.81 × 10+08 | 1.08 × 10+08 | 1.48 × 10+10 | 1.90 × 10+07 | 1.16 × 10+06 | 1.26 × 10+05 | 4.83 × 10+03 |
| T02 | 0.00 × 10+00 | 0.00 × 10+00 * | 5.52 × 10+05 | 3.74 × 10+09 | 1.72 × 10+08 | 2.92 × 10+04 | 2.50 × 10+02 | 1.57 × 10+01 | |
| Nasal Turbinate | Trachea/Bronchi | Lungs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Cat ID | Epithelial Changes | Gland/Duct Changes | Inflammation | Epithelial Changes | Gland/Duct Changes | Inflammation | Alveolar space, Septal Changes | Bronchi/Intraluminal Exudate | Perivascular Cuffing | Congestion | Edema | Bronchi/Bronchiole Epithelial | Focal Alveolar Thrombi |
| T01 | M194474 | 2 | 1 | 3 | 1 | 1 | 0 | 0 | 2 | 0 | 3.5 | 2 | 0 | 0 |
| T01 | M192790 | 2 | 1 | 4 | 2 | 2 | 0 | 1 | 2.5 | 2 | 3.5 | 2 | 0 | 0 |
| T01 | M192144 | 2 | 1 | 1.5 | 1 | 2 | 0 | 1.5 | 1.5 | 1.5 | 4 | 2 | 0 | 0 |
| T01 | M191539 | 0 | 1.5 | 2 | 0 | 2 | 0 | 0 | 1 | 0 | 3 | 2 | 0 | 0 |
| AVG (SD) | 1.5 (0.93) | 1.1 (0.35) | 2.6 (1.06) | 1.0 (0.76) | 1.75 (0.46) | 0 (0) | 0.63 (0.74) | 1.75 (0.7) | 0.88 (0.99) | 3.5 (0.53) | 2.0 (0) | 0 (0) | 0 (0) | |
| T02 | M194229 | 0 | 3 | 2.5 | 1 | 1 | 0 | 1 | 1 | 0 | 3.5 | 2 | 0 | 0 |
| T02 | M192812 | 2 | 1 | 1.5 | 1 | 3 | 1 | 2 | 1 | 2 | 3.5 | 2 | 0 | 1 |
| T02 | M192128 | 0 | 1.5 | 2.5 | 0 | 1 | 0 | 1 | 1.5 | 1 | 3.5 | 2 | 0 | 0 |
| T02 | M191806 | 2 | 1 | 3.5 | 0 | 1 | 0 | 1.5 | 1.5 | 1 | 3 | 2 | 0 | 0 |
| AVG (SD) | 1.0 (1.07) | 1.2 (0.92) | 2.5 (0.93) | 0.5 (0.86) | 1.5 (0.93) | 0.25 (0.46) | 1.4 (0.52) | 1.13 (0.46) | 1 (0.75) | 3.38 (0.52) | 2.0 (0) | 0 (0) | 0.25 (0.46) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozov, I.; Gaudreault, N.N.; Trujillo, J.D.; Indran, S.V.; Cool, K.; Kwon, T.; Meekins, D.A.; Balaraman, V.; Artiaga, B.L.; Madden, D.W.; et al. Preliminary Study on the Efficacy of a Recombinant, Subunit SARS-CoV-2 Animal Vaccine against Virulent SARS-CoV-2 Challenge in Cats. Vaccines 2023, 11, 1831. https://doi.org/10.3390/vaccines11121831
Morozov I, Gaudreault NN, Trujillo JD, Indran SV, Cool K, Kwon T, Meekins DA, Balaraman V, Artiaga BL, Madden DW, et al. Preliminary Study on the Efficacy of a Recombinant, Subunit SARS-CoV-2 Animal Vaccine against Virulent SARS-CoV-2 Challenge in Cats. Vaccines. 2023; 11(12):1831. https://doi.org/10.3390/vaccines11121831
Chicago/Turabian StyleMorozov, Igor, Natasha N. Gaudreault, Jessie D. Trujillo, Sabarish V. Indran, Konner Cool, Taeyong Kwon, David A. Meekins, Velmurugan Balaraman, Bianca Libanori Artiaga, Daniel W. Madden, and et al. 2023. "Preliminary Study on the Efficacy of a Recombinant, Subunit SARS-CoV-2 Animal Vaccine against Virulent SARS-CoV-2 Challenge in Cats" Vaccines 11, no. 12: 1831. https://doi.org/10.3390/vaccines11121831
APA StyleMorozov, I., Gaudreault, N. N., Trujillo, J. D., Indran, S. V., Cool, K., Kwon, T., Meekins, D. A., Balaraman, V., Artiaga, B. L., Madden, D. W., McDowell, C., Njaa, B., Retallick, J., Hainer, N., Millership, J., Wilson, W. C., Tkalcevic, G., Vander Horst, H., Burakova, Y., ... Richt, J. A. (2023). Preliminary Study on the Efficacy of a Recombinant, Subunit SARS-CoV-2 Animal Vaccine against Virulent SARS-CoV-2 Challenge in Cats. Vaccines, 11(12), 1831. https://doi.org/10.3390/vaccines11121831











