Transient Autoreactive PF4 and Antiphospholipid Antibodies in COVID-19 Vaccine Recipients
Abstract
:1. Introduction
2. Materials and Methods
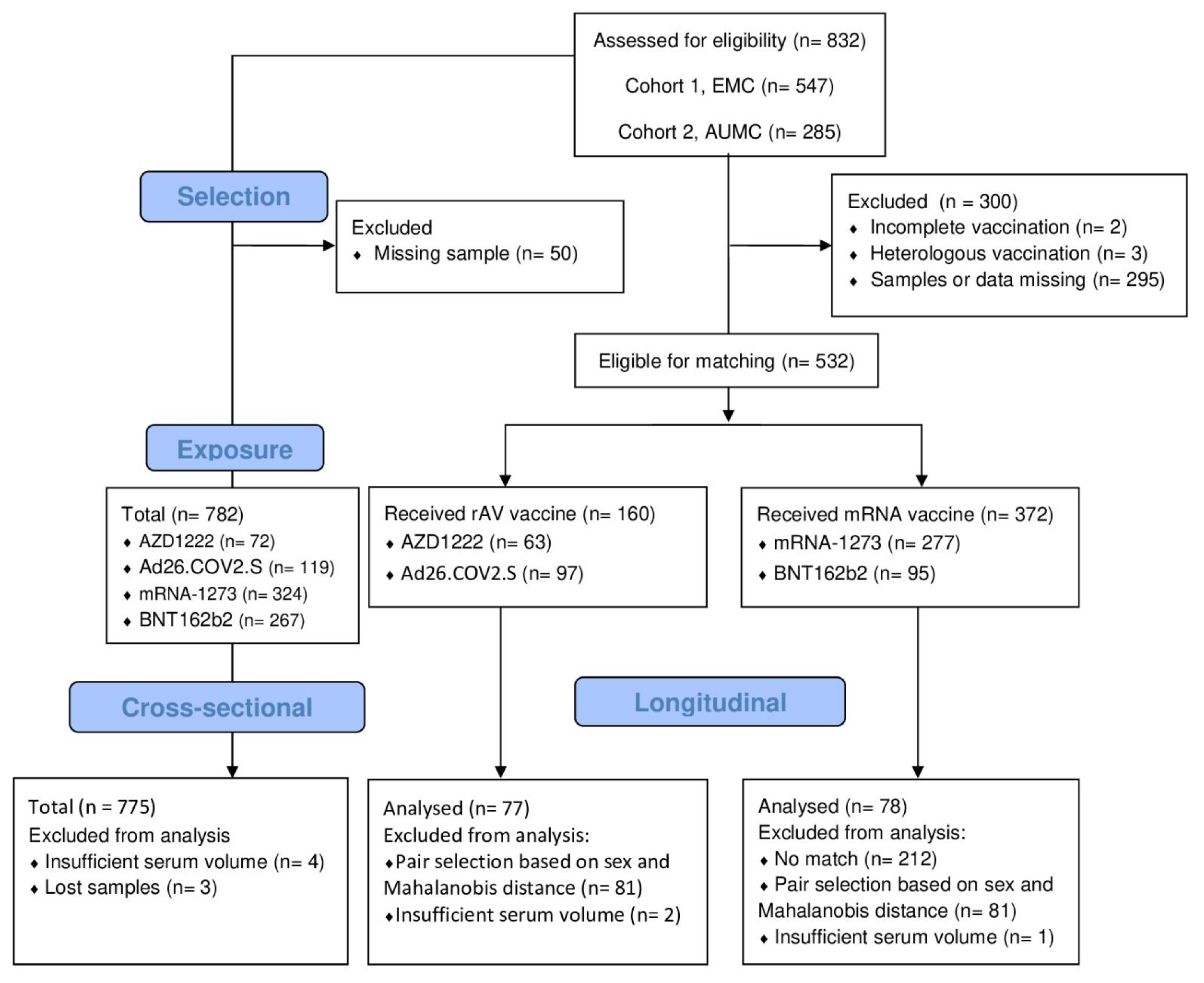
2.1. Study Population
2.2. Datasets
2.2.1. PF4 Antibody ELISA
2.2.2. PF4-Dependent Platelet Activation Assay (PIPAA)
2.2.3. Antiphospholipid Antibody ELISA
2.3. Endpoints
2.4. Sample Size Consideration
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.1.1. aPF4 Levels after COVID-19 Vaccination
3.1.2. aPL Antibodies after COVID-19 Vaccination
3.1.3. Correlations of aPF4 with aPL Antibodies
3.2. Seroconversion
3.3. Cross-Sectional Analysis of Post-Vaccination aPF4 Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aβ2GP | anti-beta2-Glycoprotein |
| ACD | Adenine Citrate Dextrose |
| aCL | anti-CardioLipin |
| aPF4 | Autoantibodies against Platelet Factor 4 |
| aPL | Antiphospholipid |
| AUMC | Amsterdam University Medical Centers |
| 95% CI | 95% Confidence Interval |
| COVID-19 | Coronavirus Disease 2019 |
| CS | Cross-sectional dataset |
| ECM | Erasmus Medical Center |
| EDTA | EthyleneDiamineTetraacetic Acid |
| HCWs | Health-Care Workers |
| FC | Fold Change |
| HIT | Heparin Induced Thrombocytopenia |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IQR | InterQuartile Range |
| L | Longitudinal dataset |
| mRNA | messenger RNA |
| O.D | Optical Density |
| rAV | recombinant AdenoVirus |
| PF4 | Platelet Factor 4 |
| PIPAA | PF4-dependent platelet activation assay |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome-associated CoronaVirus-2 |
| SD | Standard Deviation |
| VITT | Vaccine-Induced Immune Thrombotic Thrombocytopenia |
References
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 2023, CD015477. [Google Scholar] [CrossRef]
- Sadoff, J.; Davis, K.; Douoguih, M. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination—Response from the Manufacturer. N. Engl. J. Med. 2021, 384, 1965–1966. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, K.; Shankar, N.K.; Yadavalli, M.; Ferreira, C.; Foskett, N.; Putsepp, K.; Ferstenberg, L.B.; Nord, M.; Da Silva, H.G.; Bhuyan, P. Geographical distribution of TTS cases following AZD1222 (ChAdOx1 nCoV-19) vaccination. Lancet Glob. Health 2022, 10, e33–e34. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Selleng, K.; Palankar, R.; Wesche, J.; Handtke, S.; Wolff, M.; Aurich, K.; Lalk, M.; Methling, K.; Völker, U.; et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood 2021, 138, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Huynh, A.; Arnold, D.M.; Ivetic, N.; Clare, R.; Hadzi-Tosev, M.; Liu, Y.; Smith, J.W.; Bissola, A.L.; Daka, M.; Kelton, J.G.; et al. Antibodies against platelet factor 4 and the risk of cerebral venous sinus thrombosis in patients with vaccine-induced immune thrombotic thrombocytopenia. J. Thromb. Haemost. 2023, 21, 2833–2843. [Google Scholar] [CrossRef]
- Huynh, A.; Arnold, D.M.; Michael, J.V.; Clare, R.; Smith, J.W.; Daka, M.; Ianosi-Irimie, M.; McKenzie, S.E.; Kelton, J.G.; Nazy, I. Characteristics of VITT antibodies in patients vaccinated with Ad26.COV2.S. Blood Adv. 2023, 7, 246–250. [Google Scholar] [CrossRef]
- Kanack, A.J.; Bayas, A.; George, G.; Abou-Ismail, M.Y.; Singh, B.; Kohlhagen, M.C.; Splinter, N.P.; Christ, M.; Naumann, M.; Moser, K.A.; et al. Monoclonal and oligoclonal anti-platelet factor 4 antibodies mediate VITT. Blood 2022, 140, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yarovoi, S.V.; Zhu, Z.; Rauova, L.; Hayes, V.; Lebedeva, T.; Liu, Q.; Poncz, M.; Arepally, G.; Cines, D.B.; et al. Atomic description of the immune complex involved in heparin-induced thrombocytopenia. Nat. Commun. 2015, 6, 8277. [Google Scholar] [CrossRef]
- Krauel, K.; Pötschke, C.; Weber, C.; Kessler, W.; Fürll, B.; Ittermann, T.; Maier, S.; Hammerschmidt, S.; Bröker, B.M.; Greinacher, A. Platelet factor 4 binds to bacteria, inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood 2011, 117, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Hayward, C.P.; Boshkov, L.K.; Santos, A.V.; Sheppard, J.A.; Bode, A.P.; Kelton, J.G. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: An explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood 1994, 84, 3691–3699. [Google Scholar] [CrossRef] [PubMed]
- Kelton, J.; Sheridan, D.; Santos, A.; Smith, J.; Steeves, K.; Smith, C.; Brown, C.; Murphy, W. Heparin-induced thrombocytopenia: Laboratory studies. Blood 1988, 72, 925–930. [Google Scholar] [CrossRef]
- Arepally, G.M. Heparin-induced thrombocytopenia. Blood 2017, 129, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Basciano, P.A.; Knopman, J.; Bernstein, R.A. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood 2014, 123, 3651–3654. [Google Scholar] [CrossRef]
- Jay, R.M.; Warkentin, T.E. Fatal heparin-induced thrombocytopenia (HIT) during warfarin thromboprophylaxis following orthopedic surgery: Another example of ‘spontaneous’ HIT? J. Thromb. Haemost. JTH 2008, 6, 1598–1600. [Google Scholar] [CrossRef]
- Liu, Q.; Miao, H.; Li, S.; Zhang, P.; Gerber, G.F.; Follmann, D.; Ji, H.; Zeger, S.L.; Chertow, D.S.; Quinn, T.C.; et al. Anti-PF4 antibodies associated with disease severity in COVID-19. Proc. Natl. Acad. Sci. USA 2022, 119, e2213361119. [Google Scholar] [CrossRef]
- Selleng, S.; Malowsky, B.; Strobel, U.; Wessel, A.; Ittermann, T.; Wollert, H.G.; Warkentin, T.E.; Greinacher, A. Early-onset and persisting thrombocytopenia in post-cardiac surgery patients is rarely due to heparin-induced thrombocytopenia, even when antibody tests are positive. J. Thromb. Haemost. 2010, 8, 30–36. [Google Scholar] [CrossRef]
- Bauer, T.L.; Arepally, G.; Konkle, B.A.; Mestichelli, B.; Shapiro, S.S.; Cines, D.B.; Poncz, M.; McNulty, S.; Amiral, J.; Hauck, W.W.; et al. Prevalence of heparin-associated antibodies without thrombosis in patients undergoing cardiopulmonary bypass surgery. Circulation 1997, 95, 1242–1246. [Google Scholar] [CrossRef]
- Trossaërt, M.; Gaillard, A.; Commin, P.L.; Amiral, J.; Vissac, A.M.; Fressinaud, E. High incidence of anti-heparin/platelet factor 4 antibodies after cardiopulmonary bypass surgery. Br. J. Haematol. 1998, 101, 653–655. [Google Scholar] [CrossRef]
- Visentin, G.P.; Malik, M.; Cyganiak, K.A.; Aster, R.H. Patients treated with unfractionated heparin during open heart surgery are at high risk to form antibodies reactive with heparin:platelet factor 4 complexes. J. Lab. Clin. Med. 1996, 128, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Jouni, R.; Hackbarth, C.; Hietkamp, B.; Selleng, S.; Koster, A.; Jensch, I.; Linde, J.V.D.; Schwertz, H.; Bakchoul, T.; Hundt, M.; et al. Further insights into the anti-PF4/heparin IgM immune response. Thromb. Haemost. 2016, 115, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.M.; Levine, D.A.; Blumberg, N.; Fisher, G.G.; Kabeto, M.; Langa, K.M. Antigenic challenge in the etiology of autoimmune disease in women. J. Autoimmun. 2012, 38, J97–J102. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Muri, J.; Cecchinato, V.; Cavalli, A.; Shanbhag, A.A.; Matkovic, M.; Biggiogero, M.; Maida, P.A.; Moritz, J.; Toscano, C.; Ghovehoud, E.; et al. Autoantibodies against chemokines post-SARS-CoV-2 infection correlate with disease course. Nat. Immunol. 2023, 24, 604–611. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Mouquet, H.; Nussenzweig, M.C. Polyreactive antibodies in adaptive immune responses to viruses. Cell. Mol. Life Sci. 2012, 69, 1435–1445. [Google Scholar] [CrossRef]
- McNally, T.; Purdy, G.; Mackie, I.J.; Machin, S.J.; Isenberg, D.A. The use of an anti-β2-glycoprotein-I assay for discrimination between anticardiolipin antibodies associated with infection and increased risk of thrombosis. Br. J. Haematol. 1995, 91, 471–473. [Google Scholar] [CrossRef]
- Krutzke, L.; Rösler, R.; Allmendinger, E.; Engler, T.; Wiese, S.; Kochanek, S. Process- and product-related impurities in the ChAdOx1 nCov-19 vaccine. eLife 2022, 11, e78513. [Google Scholar] [CrossRef]
- Woodruff, M.C.; Ramonell, R.P.; Haddad, N.S.; Anam, F.A.; Rudolph, M.E.; Walker, T.A.; Truong, A.D.; Dixit, A.N.; Han, J.E.; Cabrera-Mora, M.; et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature 2022, 611, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Guthmiller, J.J.; Lan, L.Y.-L.; Fernández-Quintero, M.L.; Han, J.; Utset, H.A.; Bitar, D.J.; Hamel, N.J.; Stovicek, O.; Li, L.; Tepora, M.; et al. Polyreactive Broadly Neutralizing B cells Are Selected to Provide Defense against Pandemic Threat Influenza Viruses. Immunity 2020, 53, 1230–1244.e1235. [Google Scholar] [CrossRef]
- van Gils, M.J.; Lavell, A.; van der Straten, K.; Appelman, B.; Bontjer, I.; Poniman, M.; Burger, J.A.; Oomen, M.; Bouhuijs, J.H.; van Vught, L.A.; et al. Antibody responses against SARS-CoV-2 variants induced by four different SARS-CoV-2 vaccines in health care workers in the Netherlands: A prospective cohort study. PLoS Med. 2022, 19, e1003991. [Google Scholar] [CrossRef] [PubMed]
- Geers, D.; Shamier, M.C.; Bogers, S.; den Hartog, G.; Gommers, L.; Nieuwkoop, N.N.; Schmitz, K.S.; Rijsbergen, L.C.; van Osch, J.A.T.; Dijkhuizen, E.; et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021, 6, eabj1750. [Google Scholar] [CrossRef] [PubMed]
- Gezondheidsraad [Dutch Health Organisation] Aanbieding Advies Inzet AstraZeneca-vaccin [Recommendation on the Deployment of AstraZeneca Vaccine]. Available online: https://www.gezondheidsraad.nl/documenten/adviezen/2021/04/09/inzet-astrazeneca-vaccin (accessed on 5 June 2023).
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Michels, I.; Kiefel, V.; Mueller-Eckhardt, C. A rapid and sensitive test for diagnosing heparin-associated thrombocytopenia. Thromb. Haemost. 1991, 66, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef]
- Koopman, P.A.R. Confidence Intervals for the Ratio of Two Binomial Proportions. Biometrics 1984, 40, 513–517. [Google Scholar] [CrossRef]
- Fagerland, M.W.; Lydersen, S.; Laake, P. Recommended confidence intervals for two independent binomial proportions. Stat. Methods Med. Res. 2015, 24, 224–254. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Sørvoll, I.H.; Horvei, K.D.; Ernstsen, S.L.; Lægreid, I.J.; Lund, S.; Grønli, R.H.; Olsen, M.K.; Jacobsen, H.K.; Eriksson, A.; Halstensen, A.M.; et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J. Thromb. Haemost. 2021, 19, 1813–1818. [Google Scholar] [CrossRef]
- Cohen, T.S.; Kelly, E.J.; Nylander, S.; Bansal, H.; Jepson, B.M.; Bhuyan, P.; Sobieszczyk, M.E.; Falsey, A.R. Serum levels of anti-PF4 IgG after AZD1222 (ChAdOx1 nCoV-19) vaccination. Sci. Rep. 2022, 12, 7961. [Google Scholar] [CrossRef]
- Hantrakun, N.; Sinsakolwat, P.; Tantiworawit, A.; Rattarittamrong, E.; Rattanathammethee, T.; Hantrakool, S.; Piriyakhuntorn, P.; Punnachet, T.; Niprapan, P.; Wongtagan, O.; et al. Longitudinal Profiles of Anti-Platelet Factor 4 Antibodies in Thai People Who Received ChAdOx1 nCoV-19 Vaccination. Vaccines 2023, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Thiele, T.; Ulm, L.; Holtfreter, S.; Schönborn, L.; Kuhn, S.O.; Scheer, C.; Warkentin, T.E.; Bröker, B.M.; Becker, K.; Aurich, K.; et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood 2021, 138, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.O.; Bombaci, M.; Bodio, C.; Lonati, P.A.; Gobbini, A.; Lorenzo, M.; Torresani, E.; Dubini, A.; Bulgarelli, I.; Solari, F.; et al. Anti-Phospholipid Antibodies and Coronavirus Disease 2019: Vaccination Does Not Trigger Early Autoantibody Production in Healthcare Workers. Front. Immunol. 2022, 13, 930074. [Google Scholar] [CrossRef] [PubMed]
- Thurm, C.; Reinhold, A.; Borucki, K.; Kahlfuss, S.; Feist, E.; Schreiber, J.; Reinhold, D.; Schraven, B. Homologous and Heterologous Anti-COVID-19 Vaccination Does Not Induce New-Onset Formation of Autoantibodies Typically Accompanying Lupus Erythematodes, Rheumatoid Arthritis, Celiac Disease and Antiphospholipid Syndrome. Vaccines 2022, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Jaycox, J.R.; Lucas, C.; Yildirim, I.; Dai, Y.; Wang, E.Y.; Monteiro, V.; Lord, S.; Carlin, J.; Kita, M.; Buckner, J.H.; et al. SARS-CoV-2 mRNA vaccines decouple anti-viral immunity from humoral autoimmunity. Nat. Commun. 2023, 14, 1299. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, D.; Caruso, V.; Belardi, R.; Bernardini, S.; Nuccetelli, M. Evaluation of autoantibody profile in healthy subjects after mRNA vaccination against COVID-19. Int. Immunopharmacol. 2023, 122, 110592. [Google Scholar] [CrossRef] [PubMed]
- Gazitt, T.; Eviatar, T.; Shear, J.; Meidan, R.; Furer, V.; Feld, J.; Haddad, A.; Elias, M.; Hijazi, N.; Stein, N.; et al. Development of Autoantibodies Following BNT162b2 mRNA COVID-19 Vaccination and Their Association with Disease Flares in Adult Patients with Autoimmune Inflammatory Rheumatic Diseases (AIIRD) and the General Population: Results of 1-Year Prospective Follow-Up Study. Vaccines 2023, 11, 476. [Google Scholar]
- Ogrič, M.; Žigon, P.; Podovšovnik, E.; Lakota, K.; Sodin-Semrl, S.; Rotar, Ž.; Čučnik, S. Differences in SARS-CoV-2-Specific Antibody Responses After the First, Second, and Third Doses of BNT162b2 in Naïve and Previously Infected Individuals: A 1-Year Observational Study in Healthcare Professionals. Front. Immunol. 2022, 13, 876533. [Google Scholar] [CrossRef]
- Blank, R.B.; Haberman, R.H.; Qian, K.; Samanovic, M.; Castillo, R.; Jimenez Hernandez, A.; Vasudevapillai Girija, P.; Catron, S.; Uddin, Z.; Rackoff, P.; et al. Low incidence and transient elevation of autoantibodies post mRNA COVID-19 vaccination in inflammatory arthritis. Rheumatology 2022, 62, 467–472. [Google Scholar] [CrossRef]
- Noureldine, H.A.; Maamari, J.; El Helou, M.O.; Chedid, G.; Farra, A.; Husni, R.; Mokhbat, J.E. The effect of the BNT162b2 vaccine on antinuclear antibody and antiphospholipid antibody levels. Immunol. Res. 2022, 70, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Świerkot, J.; Madej, M.; Szmyrka, M.; Korman, L.; Sokolik, R.; Andrasiak, I.; Morgiel, E.; Sebastian, A. The Risk of Autoimmunity Development following mRNA COVID-19 Vaccination. Viruses 2022, 14, 2655. [Google Scholar] [CrossRef] [PubMed]
- Shome, M.; Chung, Y.; Chavan, R.; Park, J.G.; Qiu, J.; Labaer, J. Serum autoantibodyome reveals that healthy individuals share common autoantibodies. Cell Rep. 2022, 39, 110873. [Google Scholar] [CrossRef] [PubMed]
- Schönborn, L.; Esteban, O.; Wesche, J.; Dobosz, P.; Broto, M.; Rovira Puig, S.; Fuhrmann, J.; Torres, R.; Serra, J.; Llevadot, R.; et al. Heparin- and Vaccine-Independent Anti-Platelet Factor 4 Immunothrombosis. SSRN [Preprint] 2023. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Baskin-Miller, J.; Raybould, A.L.; Sheppard, J.I.; Daka, M.; Nazy, I.; Moll, S. Adenovirus-Associated Thrombocytopenia, Thrombosis, and VITT-like Antibodies. N. Engl. J. Med. 2023, 389, 574–577. [Google Scholar] [CrossRef] [PubMed]


| Dataset | rAV | mRNA | AZD1222 (rAV) | Ad26.COV2.S (rAV) | mRNA-1273 (mRNA) | BNT162b2 (mRNA) | |
|---|---|---|---|---|---|---|---|
| Subjects (n, %) | LO | 77 | 78 | 39 (25.2) | 38 (25.0) | 52 (33.5) | 26 (16.8) |
| CS | 187 | 588 | 72 (9.3) | 115 (14.8) | 322 (41.5) | 266 (34.3) | |
| Age (mean, SD) | LO | 50.4 (14.1) | 43.0 (11.8) | 61.6 (1.5) | 39.3 (12.1) | 42.8 (11.6) | 43.4 (12.3) |
| CS | 46.4 (15.4) | 46.7 (11.8) | 61.0 (5.6) | 37.8 (12.5) | 39.5 (11.5) | 41.4 (11.8) | |
| Males (n, %) | LO | 29 (37.2) | 29 (37.2) | 16 (41.0) | 13 (34.2) | 15 (28.8) | 14 (53.8) |
| CS | 37 (19.8) | 149 (25.3) | 17 (23.6) | 20 (17.4) | 59 (18.3) | 90 (33.8) | |
| COVID-19 infection prior to vaccination (n, %) | LO | 12 (15.4) | 9 (11.5) | 6 (15.4) | 6 (15.8) | 6 (11.5) | 2 (7.7) |
| CS | 39 (20.9) | 153 (26.0) | 8 (11.1) | 31 (27.0) | 81 (25.2) | 72 (27.1) |
| # | Vaccine | aPF4 (FC) | aβ2GP (FC) | aCL IgG (FC) | aCL IgM (FC) | ||||
|---|---|---|---|---|---|---|---|---|---|
| T2 | T3 | T2 | T3 | T2 | T3 | T2 | T3 | ||
| 1 | Ad26.COV2.S | 2.4 | - | 1.6 | - | 1.0 | - | 0.6 | - |
| 2 | Ad26.COV2.S | 0.1 | - | 3.4 | - | 1.3 | - | 1.0 | - |
| 3 | Ad26.COV2.S | 1.2 | - | 1.6 | - | 1.3 | - | 2.9 | - |
| 4 | AZD1222 | 2.3 | 1.8 | 1.5 | 1.2 | 2.7 | 1.5 | 1.2 | 1.1 |
| 5 | AZD1222 | 0.9 | 0.7 | 0.9 | 2.7 | 0.8 | 1.7 | 0.9 | 4.0 |
| 6 | AZD1222 | - | 1.1 | - | 2.1 | - | 1.1 | - | 1.4 |
| 7 | AZD1222 | 1.3 | 1.3 | 1.3 | 1.9 | 1.1 | 1.3 | 2.7 | 1.7 |
| 8 | AZD1222 | 1.2 | 1.1 | 1.3 | 1.4 | 0.8 | 0.7 | 2.7 | 1.5 |
| 9 | AZD1222 | 0.9 | 0.6 | 0.9 | 2.7 | 0.8 | 1.7 | 0.9 | 4.0 |
| 10 | AZD1222 | 0.7 | 0.8 | 0.9 | 1.4 | 0.8 | 0.7 | 2.5 | 1.2 |
| 11 | BNT162b2 | 0.9 | 1.0 | 1.1 | 2.7 | 1.1 | 1.1 | 1.1 | 1.2 |
| 12 | mRNA-1273 | 1.5 | 2.5 | 0.8 | 0.9 | 0.8 | 0.9 | 1.0 | 0.6 |
| 13 | mRNA-1273 | 0.8 | 1.0 | 1.0 | 0.9 | 1.2 | 0.7 | 2.2 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raadsen, M.P.; Visser, C.; Lavell, A.H.A.; van de Munckhof, A.A.G.A.; Coutinho, J.M.; de Maat, M.P.M.; GeurtsvanKessel, C.H.; Amsterdam UMC COVID-19 S3/HCW Study Group; Bomers, M.K.; Haagmans, B.L.; et al. Transient Autoreactive PF4 and Antiphospholipid Antibodies in COVID-19 Vaccine Recipients. Vaccines 2023, 11, 1851. https://doi.org/10.3390/vaccines11121851
Raadsen MP, Visser C, Lavell AHA, van de Munckhof AAGA, Coutinho JM, de Maat MPM, GeurtsvanKessel CH, Amsterdam UMC COVID-19 S3/HCW Study Group, Bomers MK, Haagmans BL, et al. Transient Autoreactive PF4 and Antiphospholipid Antibodies in COVID-19 Vaccine Recipients. Vaccines. 2023; 11(12):1851. https://doi.org/10.3390/vaccines11121851
Chicago/Turabian StyleRaadsen, Matthijs P., Chantal Visser, A. H. Ayesha Lavell, Anita A. G. A. van de Munckhof, Jonathan M. Coutinho, Moniek P. M. de Maat, Corine H. GeurtsvanKessel, Amsterdam UMC COVID-19 S3/HCW Study Group, Marije K. Bomers, Bart L. Haagmans, and et al. 2023. "Transient Autoreactive PF4 and Antiphospholipid Antibodies in COVID-19 Vaccine Recipients" Vaccines 11, no. 12: 1851. https://doi.org/10.3390/vaccines11121851
APA StyleRaadsen, M. P., Visser, C., Lavell, A. H. A., van de Munckhof, A. A. G. A., Coutinho, J. M., de Maat, M. P. M., GeurtsvanKessel, C. H., Amsterdam UMC COVID-19 S3/HCW Study Group, Bomers, M. K., Haagmans, B. L., van Gorp, E. C. M., Porcelijn, L., & Kruip, M. J. H. A. (2023). Transient Autoreactive PF4 and Antiphospholipid Antibodies in COVID-19 Vaccine Recipients. Vaccines, 11(12), 1851. https://doi.org/10.3390/vaccines11121851






