Ex Vivo Blockade of the PD-1 Pathway Improves Recall IFNγ Responses of HIV-Infected Persons on Antiretroviral Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Blood Samples
2.3. Anti-Tetanus Antibody Levels
2.4. Measurement of PD-1 Expression Levels on T Cells
2.5. Measurement of Recall IFNγ Responses to Measles Virus and Tetanus Toxin
2.6. Statistical Analysis
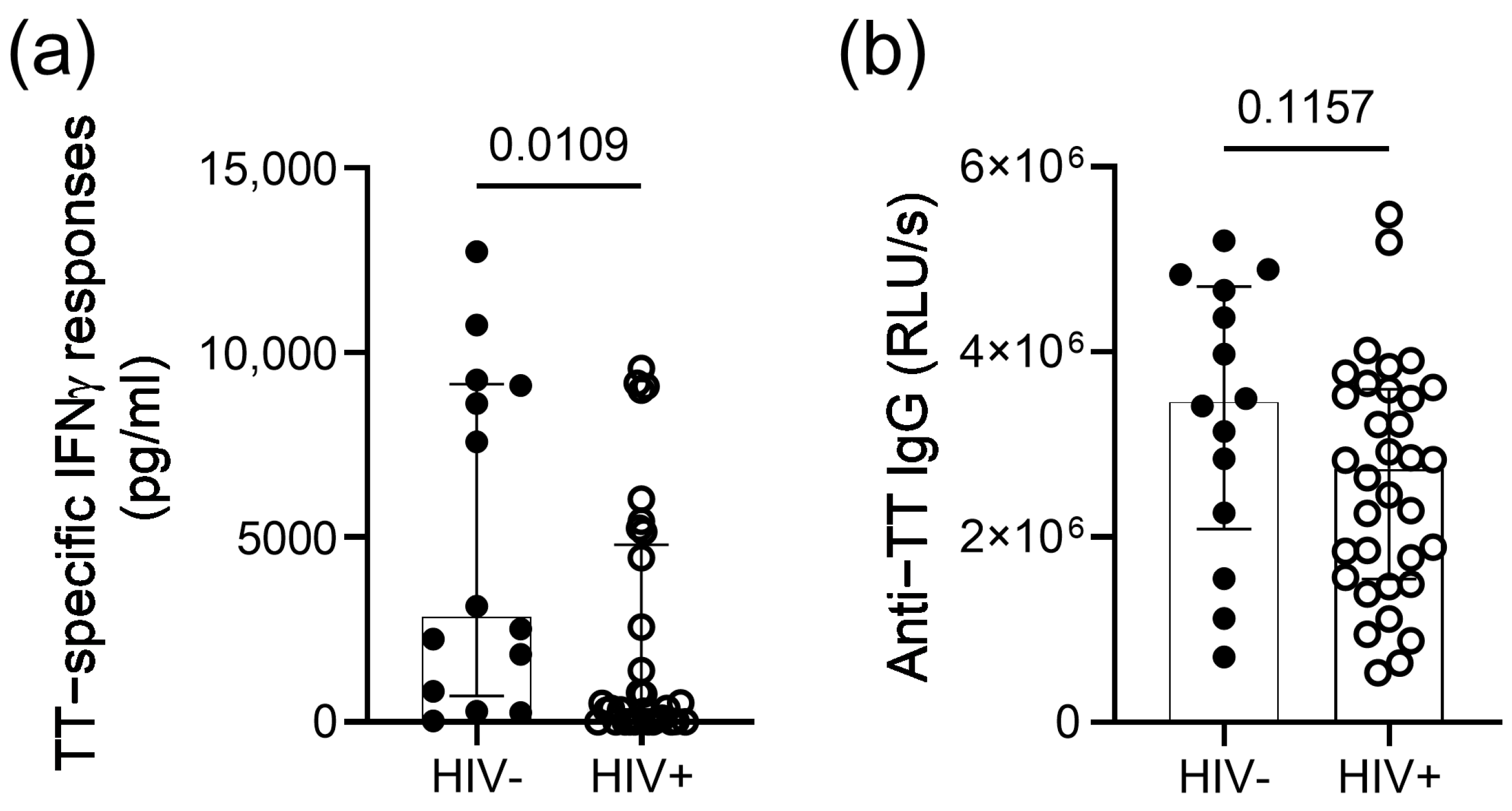
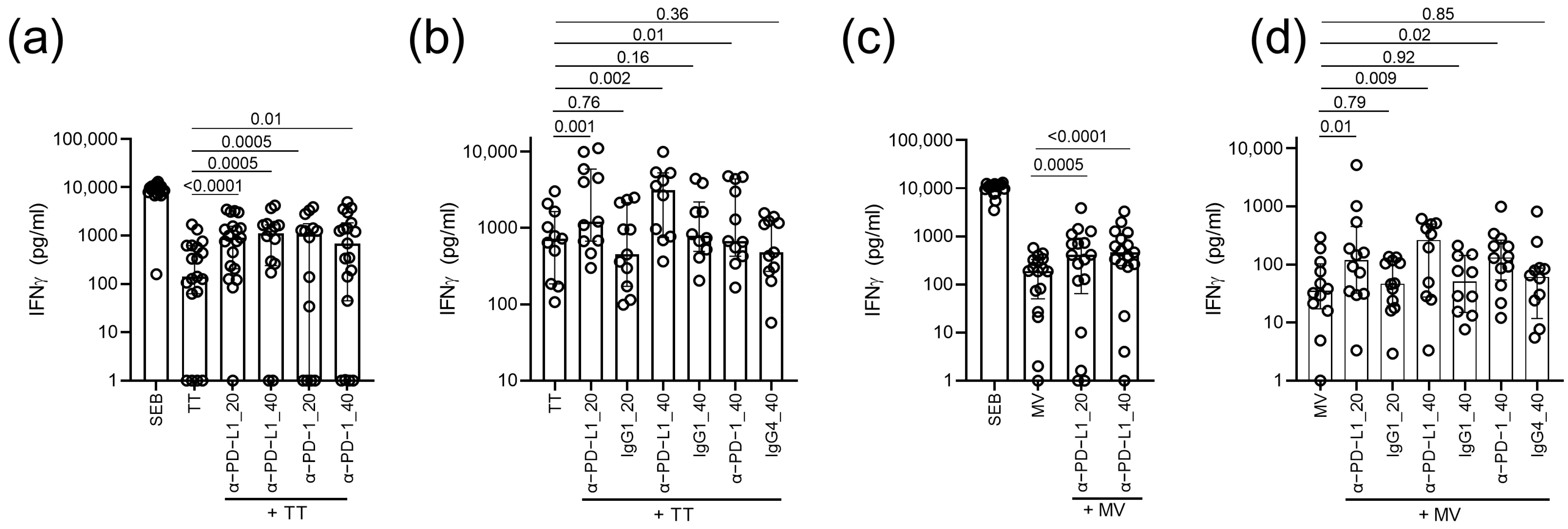
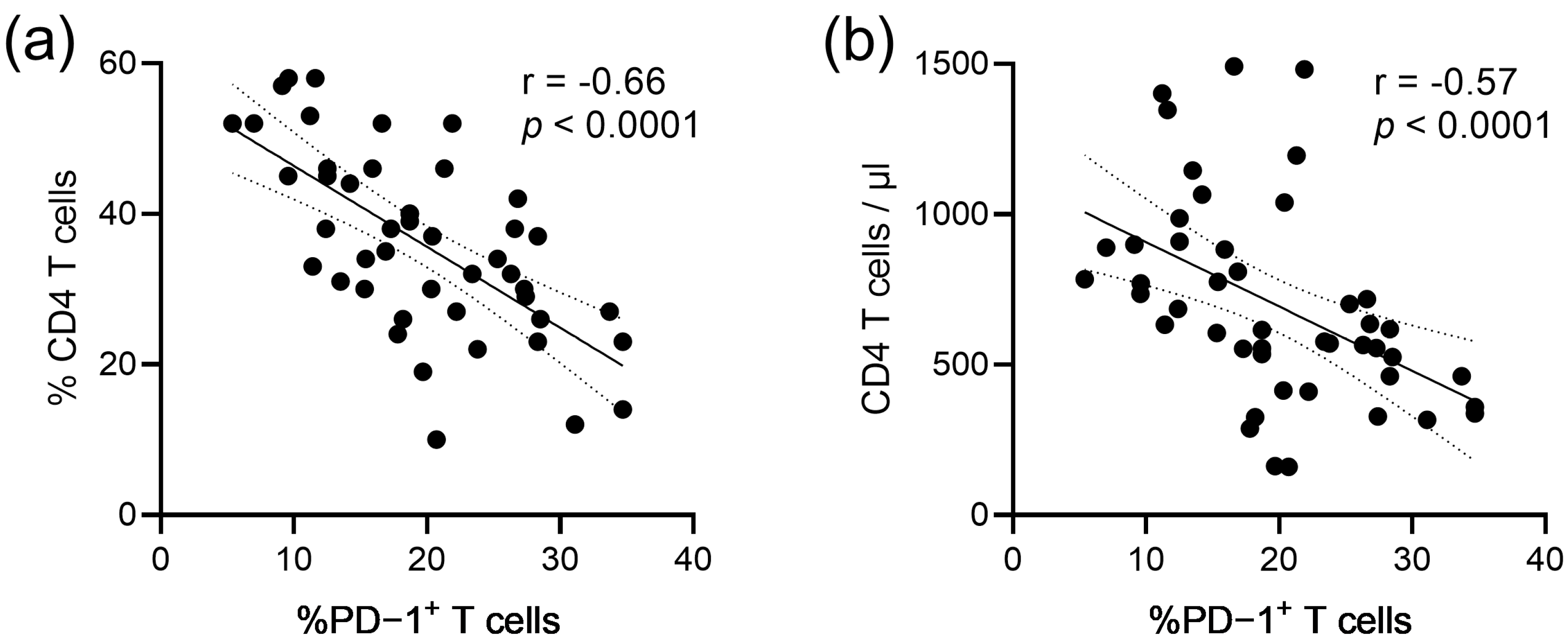
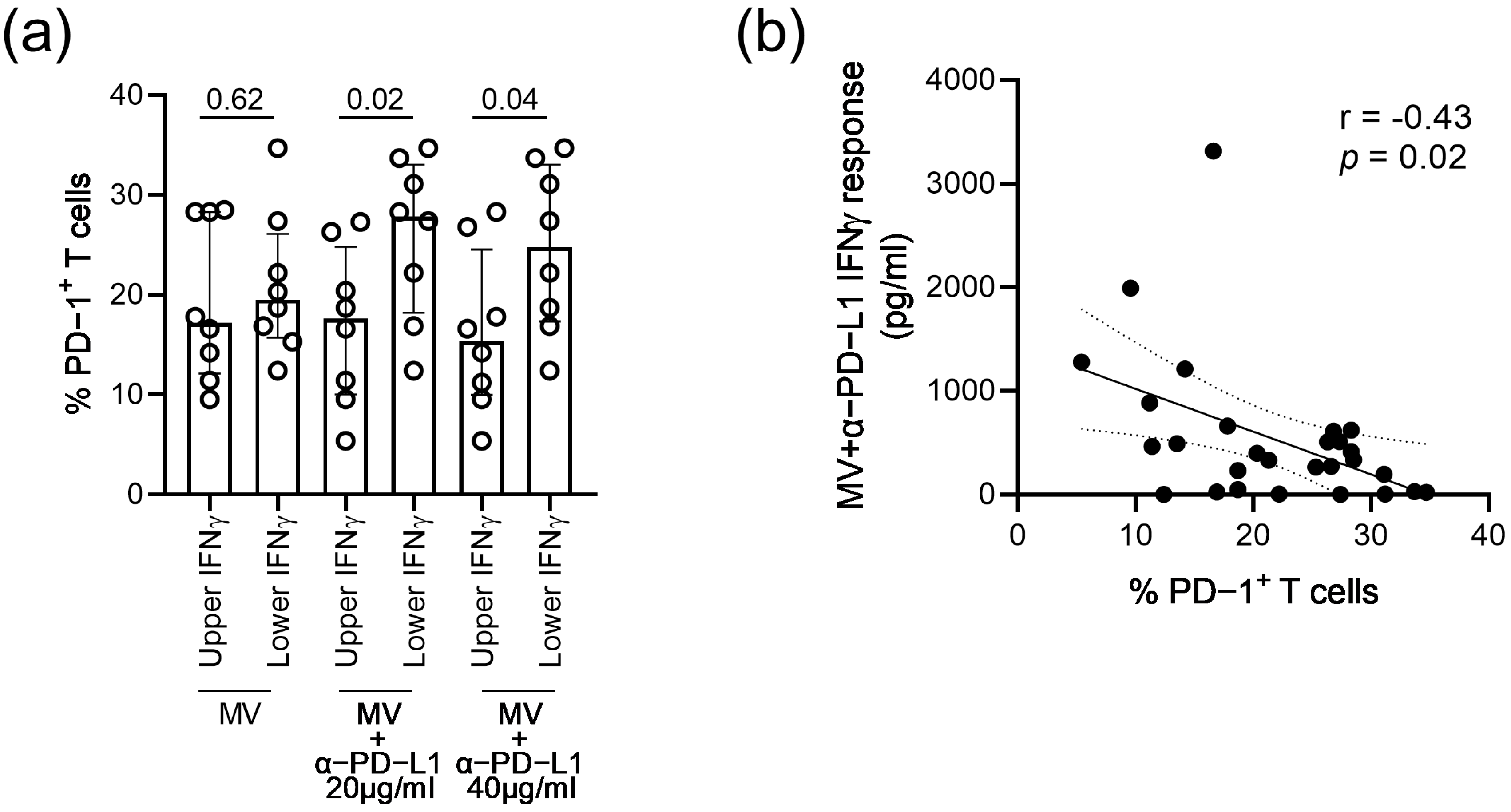
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wherry, E.J. T Cell Exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Crawford, A.; Angelosanto, J.M.; Kao, C.; Doering, T.A.; Odorizzi, P.M.; Barnett, B.E.; Wherry, E.J. Molecular and Transcriptional Basis of Cd4(+) T Cell Dysfunction During Chronic Infection. Immunity 2014, 40, 289–302. [Google Scholar] [CrossRef]
- D’Souza, M.; Fontenot, A.P.; Mack, D.G.; Lozupone, C.; Dillon, S.; Meditz, A.; Wilson, C.C.; Connick, E.; Palmer, B.E. Programmed Death 1 Expression on Hiv-Specific Cd4+ T Cells Is Driven by Viral Replication and Associated with T Cell Dysfunction. J. Immunol. 2007, 179, 1979–1987. [Google Scholar] [CrossRef]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. Pd-1 Expression on Hiv-Specific T Cells Is Associated with T-Cell Exhaustion and Disease Progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef]
- Haase, A.T.; Henry, K.; Zupancic, M.; Sedgewick, G.; Faust, R.A.; Melroe, H.; Cavert, W.; Gebhard, K.; Staskus, K.; Zhang, Z.Q.; et al. Quantitative Image Analysis of Hiv-1 Infection in Lymphoid Tissue. Science 1996, 274, 985–989. [Google Scholar] [CrossRef]
- Jones, L.E.; Perelson, A.S. Transient Viremia, Plasma Viral Load, and Reservoir Replenishment in Hiv-Infected Patients on Antiretroviral Therapy. J. Acquir. Immune. Defic. Syndr. 2007, 45, 483–493. [Google Scholar] [CrossRef]
- Nakanjako, D.; Ssewanyana, I.; Mayanja-Kizza, H.; Kiragga, A.; Colebunders, R.; Manabe, Y.C.; Nabatanzi, R.; Kamya, M.R.; Cao, H. High T-Cell Immune Activation and Immune Exhaustion among Individuals with Suboptimal Cd4 Recovery after 4 Years of Antiretroviral Therapy in an African Cohort. BMC Infect. Dis 2001, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Pallikkuth, S.; Fischl, M.A.; Pahwa, S. Combination Antiretroviral Therapy with Raltegravir Leads to Rapid Immunologic Reconstitution in Treatment-Naive Patients with Chronic Hiv Infection. J. Infect. Dis. 2013, 208, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y.; et al. Hiv-Infected Individuals with Low Cd4/Cd8 Ratio Despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened Cd8+ T Cell Activation, and Increased Risk of Non-Aids Morbidity and Mortality. PLoS Pathog. 2014, 10, e1004078. [Google Scholar] [CrossRef] [PubMed]
- Grabmeier-Pfistershammer, K.; Steinberger, P.; Rieger, A.; Leitner, J.; Kohrgruber, N. Identification of Pd-1 as a Unique Marker for Failing Immune Reconstitution in Hiv-1-Infected Patients on Treatment. J. Acquir. Immune. Defic. Syndr. 2011, 56, 118–124. [Google Scholar] [CrossRef]
- Hurst, J.; Hoffmann, M.; Pace, M.; Williams, J.P.; Thornhill, J.; Hamlyn, E.; Meyerowitz, J.; Willberg, C.; Koelsch, K.K.; Robinson, N.; et al. Immunological Biomarkers Predict Hiv-1 Viral Rebound after Treatment Interruption. Nat. Commun. 2015, 6, 8495. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Hammarlund, E.; Gao, L.; Holman, S.; Michel, K.G.; Glesby, M.; Villacres, M.C.; Golub, E.T.; Roan, N.R.; French, A.L.; et al. Loss of Preexisting Immunological Memory among Human Immunodeficiency Virus-Infected Women Despite Immune Reconstitution with Antiretroviral Therapy. J. Infect. Dis. 2020, 222, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Elrefaei, M.; McElroy, M.D.; Preas, C.P.; Hoh, R.; Deeks, S.; Martin, J.; Cao, H. Central Memory Cd4+ T Cell Responses in Chronic Hiv Infection Are Not Restored by Antiretroviral Therapy. J. Immunol. 2004, 173, 2184–2189. [Google Scholar] [CrossRef] [PubMed]
- Puissant-Lubrano, B.; Combadiere, B.; Duffy, D.; Wincker, N.; Frachette, M.J.; Ait-Mohand, H.; Verrier, B.; Katlama, C.; Autran, B. Influence of Antigen Exposure on the Loss of Long-Term Memory to Childhood Vaccines in Hiv-Infected Patients. Vaccine 2009, 27, 3576–3583. [Google Scholar] [CrossRef]
- Burton, C.T.; Goodall, R.L.; Samri, A.; Autran, B.; Kelleher, A.D.; Poli, G.; Pantaleo, G.; Gotch, F.M.; Imami, N.; Initio Trial International Co-ordinating Committee. Restoration of Anti-Tetanus Toxoid Responses in Patients Initiating Highly Active Antiretroviral Therapy with or without a Boost Immunization: An Initio Substudy. Clin. Exp. Immunol. 2008, 152, 252–257. [Google Scholar]
- Hardy, G.A.; Imami, N.; Sullivan, A.K.; Pires, A.; Burton, C.T.; Nelson, M.R.; Gazzard, B.G.; Gotch, F.M. Reconstitution of Cd4+ T Cell Responses in Hiv-1 Infected Individuals Initiating Highly Active Antiretroviral Therapy (Haart) Is Associated with Renewed Interleukin-2 Production and Responsiveness. Clin. Exp. Immunol. 2003, 134, 98–106. [Google Scholar] [CrossRef]
- Wendland, T.; Furrer, H.; Vernazza, P.L.; Frutig, K.; Christen, A.; Matter, L.; Malinverni, R.; Pichler, W.J. Haart in Hiv-Infected Patients: Restoration of Antigen-Specific Cd4 T-Cell Responses in Vitro Is Correlated with Cd4 Memory T-Cell Reconstitution, Whereas Improvement in Delayed Type Hypersensitivity Is Related to a Decrease in Viraemia. AIDS 1999, 13, 1857–1862. [Google Scholar] [CrossRef]
- Gay, C.L.; Bosch, R.J.; Ritz, J.; Hataye, J.M.; Aga, E.; Tressler, R.L.; Mason, S.W.; Hwang, C.K.; Grasela, D.M.; Ray, N.; et al. Clinical Trial of the Anti-Pd-L1 Antibody Bms-936559 in Hiv-1 Infected Participants on Suppressive Antiretroviral Therapy. J. Infect. Dis. 2017, 215, 1725–1733. [Google Scholar] [CrossRef]
- Geretti, A.M.; Doyle, T. Immunization for Hiv-Positive Individuals. Curr. Opin. Infect. Dis. 2010, 23, 32–38. [Google Scholar] [CrossRef]
- Kerneis, S.; Launay, O.; Turbelin, C.; Batteux, F.; Hanslik, T.; Boelle, P.Y. Long-Term Immune Responses to Vaccination in Hiv-Infected Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2014, 58, 1130–1139. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring Function in Exhausted Cd8 T Cells During Chronic Viral Infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.J.; Callendret, B.; Zhu, B.; Freeman, G.J.; Hasselschwert, D.L.; Satterfield, W.; Sharpe, A.H.; Dustin, L.B.; Rice, C.M.; Grakoui, A.; et al. Immunotherapy of Chronic Hepatitis C Virus Infection with Antibodies against Programmed Cell Death-1 (Pd-1). Proc. Natl. Acad. Sci. USA 2013, 110, 15001–15006. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.B.; Yoder, C.; Metcalf, J.A.; DerSimonian, R.; Orenstein, J.M.; Stevens, R.A.; Falloon, J.; Polis, M.A.; Lane, H.C.; Sereti, I. Incomplete Cd4 T Cell Recovery in Hiv-1 Infection after 12 Months of Highly Active Antiretroviral Therapy Is Associated with Ongoing Increased Cd4 T Cell Activation and Turnover. J. Acquir. Immune. Defic. Syndr. 2003, 33, 125–133. [Google Scholar] [CrossRef]
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed Cell Death 1 Forms Negative Costimulatory Microclusters That Directly Inhibit T Cell Receptor Signaling by Recruiting Phosphatase Shp2. J. Exp. Med. 2012, 209, 1201–1217. [Google Scholar] [CrossRef]
- Ghoneim, H.E.; Fan, Y.; Moustaki, A.; Abdelsamed, H.A.; Dash, P.; Dogra, P.; Carter, R.; Awad, W.; Neale, G.; Thomas, P.G.; et al. De Novo Epigenetic Programs Inhibit Pd-1 Blockade-Mediated T Cell Rejuvenation. Cell 2017, 170, 142–157. [Google Scholar] [CrossRef]
- Pauken, K.E.; Sammons, M.A.; Odorizzi, P.M.; Manne, S.; Godec, J.; Khan, O.; Drake, A.M.; Chen, Z.; Sen, D.R.; Kurachi, M.; et al. Epigenetic Stability of Exhausted T Cells Limits Durability of Reinvigoration by Pd-1 Blockade. Science 2016, 354, 1160–1165. [Google Scholar] [CrossRef]
- Wang, T.W.; Johmura, Y.; Suzuki, N.; Omori, S.; Migita, T.; Yamaguchi, K.; Hatakeyama, S.; Yamazaki, S.; Shimizu, E.; Imoto, S.; et al. Blocking Pd-L1-Pd-1 Improves Senescence Surveillance and Ageing Phenotypes. Nature 2022, 611, 358–364. [Google Scholar] [CrossRef]
- De Sousa Linhares, A.; Battin, C.; Jutz, S.; Leitner, J.; Hafner, C.; Tobias, J.; Wiedermann, U.; Kundi, M.; Zlabinger, G.J.; Grabmeier-Pfistershammer, K.; et al. Therapeutic Pd-L1 Antibodies Are More Effective Than Pd-1 Antibodies in Blocking Pd-1/Pd-L1 Signaling. Sci. Rep. 2019, 9, 11472. [Google Scholar] [CrossRef] [PubMed]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed Death-1 Ligand 1 Interacts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.T.; Cheong, H.C.; Tang, T.F.; Rajasuriar, R.; Cheng, K.K.; Looi, C.Y.; Wong, W.F.; Kamarulzaman, A. Immune Checkpoint Molecules and Glucose Metabolism in Hiv-Induced T Cell Exhaustion. Biomedicines 2022, 10, 2809. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.; Wherry, E.J. Coregulation of Cd8+ T Cell Exhaustion by Multiple Inhibitory Receptors During Chronic Viral Infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Chew, G.M.; Fujita, T.; Webb, G.M.; Burwitz, B.J.; Wu, H.L.; Reed, J.S.; Hammond, K.B.; Clayton, K.L.; Ishii, N.; Abdel-Mohsen, M.; et al. Tigit Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in Hiv and Siv Infection. PLoS Pathog. 2016, 12, e1005349. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, R.; Zietse, M.; Dumoulin, D.W.; Hendrikx, J.; Aerts, J.; van der Veldt, A.A.M.; Koch, B.C.P.; Sleijfer, S.; van Leeuwen, R.W.F. Alternative Dosing Strategies for Immune Checkpoint Inhibitors to Improve Cost-Effectiveness: A Special Focus on Nivolumab and Pembrolizumab. Lancet. Oncol. 2022, 23, e552–e561. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Osuch, S.; Metzner, K.J.; Cortes, K.C. Reversal of T Cell Exhaustion in Chronic Hcv Infection. Viruses 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]

| TT Seropositive (n = 30) | MV Seropositive (n = 29) | |
|---|---|---|
| Age, median years (IQR) | 50 (40–56) | 49 (41–55) |
| Male-to-female ratio | 19:11 | 17:12 |
| CD4 T cell count, median cells/µL (IQR) | 676 (423–897) | 672 (462–772) |
| CD4:CD8 T cell ratio, median (IQR) | 1.9 (1.2–2.6) | 1.3 (1.1–1.4) |
| Plasma virus load, median copies/mL (IQR) | <20 (<20–<20) | <20 (<20–<20) |
| IFNγ Responses | CD4 T Cell Count Rho; p | % CD4 T Cells Rho; p | CD4:CD8 Ratio Rho; p |
|---|---|---|---|
| TT only | 0.43; 0.01 | 0.41; 0.02 | −0.06; 0.76 |
| TT + αPD-L1 20 µg/mL | 0.50; 0.005 | 0.49; 0.005 | −0.06; 0.76 |
| TT + αPD-1 40 µg/mL | 0.54; 0.001 | 0.55; 0.001 | −0.15; 0.42 |
| MV only | 0.24; 0.21 | 0.26; 0.18 | 0.42; 0.02 |
| MV + αPD-L1 20 µg/mL | 0.49; 0.006 | 0.41; 0.03 | 0.41; 0.02 |
| MV + αPD-L1 40 µg/mL | 0.46; 0.02 | 0.48; 0.01 | 0.45; 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischhaber, N.; Schmiedeberg, M.; Kübel, S.; Harrer, E.G.; Harrer, T.; Nganou-Makamdop, K. Ex Vivo Blockade of the PD-1 Pathway Improves Recall IFNγ Responses of HIV-Infected Persons on Antiretroviral Therapy. Vaccines 2023, 11, 211. https://doi.org/10.3390/vaccines11020211
Fischhaber N, Schmiedeberg M, Kübel S, Harrer EG, Harrer T, Nganou-Makamdop K. Ex Vivo Blockade of the PD-1 Pathway Improves Recall IFNγ Responses of HIV-Infected Persons on Antiretroviral Therapy. Vaccines. 2023; 11(2):211. https://doi.org/10.3390/vaccines11020211
Chicago/Turabian StyleFischhaber, Natalie, Moritz Schmiedeberg, Sabrina Kübel, Ellen G. Harrer, Thomas Harrer, and Krystelle Nganou-Makamdop. 2023. "Ex Vivo Blockade of the PD-1 Pathway Improves Recall IFNγ Responses of HIV-Infected Persons on Antiretroviral Therapy" Vaccines 11, no. 2: 211. https://doi.org/10.3390/vaccines11020211
APA StyleFischhaber, N., Schmiedeberg, M., Kübel, S., Harrer, E. G., Harrer, T., & Nganou-Makamdop, K. (2023). Ex Vivo Blockade of the PD-1 Pathway Improves Recall IFNγ Responses of HIV-Infected Persons on Antiretroviral Therapy. Vaccines, 11(2), 211. https://doi.org/10.3390/vaccines11020211






