Low-Grade Cervical Intraepithelial Neoplasia (CIN1) Evolution: Analysis of Opportunistic Preventive Vaccination Role
Abstract
:1. Introduction
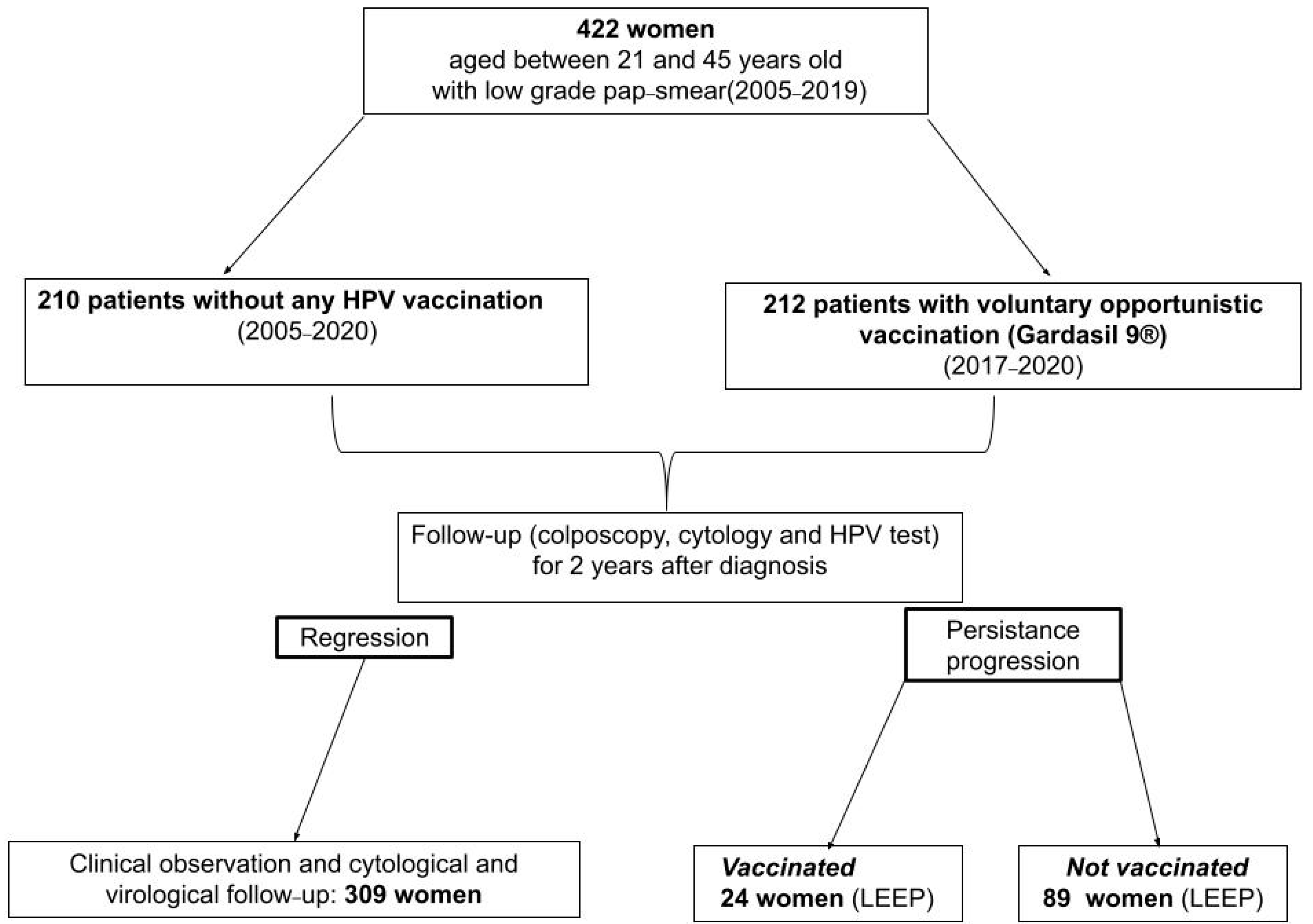
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
Key Points
- -
- HPV vaccine is intended to prevent HPV infection, and protect the recipient from HPV-induced cancers of the cervix, vagina, vulva, anus, penis, and oropharynx.
- -
- The use of the HPV vaccine as a treatment for Cervical Intraepithelial Neoplasia (CIN) is unique.
- -
- It is well known that CIN1 is predominantly a reversible lesion, the vast majority reverting over time to normal epithelium. Only a small percentage progress to CIN2+, which is typically irreversible. In the vast majority, unless treated with ablative or excisional methods, the lesion tends to progress to high-grade cervical lesions.
- -
- The authors investigated the use of the HPV vaccine in women with CIN1. Indeed they successfully demonstrated that a larger percentage of CIN1 lesions heal or regress, with vaccination than in those who remained unvaccinated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203, Erratum in Lancet Glob. Health. 2022, 10, e41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefani, C.; Liverani, C.A.; Bianco, V.; Penna, C.; Guarnieri, T.; Comparetto, C.; Monti, E.; Valente, I.; Pieralli, A.L.; Fiaschi, C.; et al. Spontaneous regression of low-grade cervical intraepithelial lesions is positively improved by topical bovine colostrum preparations (GINEDIE®). A multicentre, observational, italian pilot study. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 728–733. [Google Scholar] [PubMed]
- Abdulrahman, Z.; Kortekaas, K.E.; De Vos Van Steenwijk, P.J.; Van Der Burg, S.H.; Van Poelgeest, M.I. The immune microenvironment in vulvar (pre)cancer: Review of literature and implications for immunotherapy. Expert Opin. Biol. Ther. 2018, 18, 1223–1233. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Bosi, S.; Bassi, C.; Ferracin, M.; Lanza, G.; Gafà, R.; Magri, E.; Selvatici, R.; Torresani, S.; Marci, R.; et al. Gene expression changes in progression of cervical neoplasia revealed by microarray analysis of cervical neoplastic keratinocytes. J. Cell Physiol. 2015, 230, 806–812. [Google Scholar] [CrossRef]
- Halle, M.K.; Munk, A.C.; Engesæter, B.; Akbari, S.; Frafjord, A.; Hoivik, E.A.; Forsse, D.; Fasmer, K.E.; Woie, K.; Haldorsen, I.S.; et al. A Gene Signature Identifying CIN3 Regression and Cervical Cancer Survival. Cancers 2021, 13, 5737. [Google Scholar] [CrossRef]
- Darragh, T.M.; Colgan, T.J.; Cox, J.T.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch. Pathol. Lab. Med. 2012, 136, 1266–1297, Erratum in Arch. Pathol. Lab. Med. 2013, 137, 738. [Google Scholar] [CrossRef]
- Massad, L.S.; Einstein, M.H.; Huh, W.K.; Katki, H.A.; Kinney, W.K.; Schiffman, M.; Solomon, D.; Wentzensen, N.; Lawson, H.W. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet. Gynecol. 2013, 121, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Colposcopia Italia. Available online: https://www.colposcopiaitaliana.com/wp-content/uploads/2020/07/Capitolo_1_Gestione_delle_lesioni_citologiche.pdf (accessed on 26 November 2022).
- Cox, J.T.; Schiffman, M.; Solomon, D.; ASCUS-LSIL Triage Study (ALTS) Group. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am. J. Obstet. Gynecol. 2003, 188, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Katki, H.A.; Schiffman, M.; Castle, P.E.; Fetterman, B.; Poitras, N.E.; Lorey, T.; Cheung, L.; Raine-Bennett, T.R.; Gage, J.C.; Kinney, W.K. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J. Low. Genit. Tract. Dis. 2013, 17 (Suppl. S1), S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Paraskevaidis, E.; Martin-Hirsch, P.; Prendiville, W.; Dillner, J. Clinical utility of HPV-DNA detection: Triage of minor cervical lesions, follow-up of women treated for high-grade CIN: An update of pooled evidence. Gynecol. Oncol. 2005, 99 (Suppl. S1), S7–S11. [Google Scholar] [CrossRef]
- Arbyn, M.; Sasieni, P.; Meijer, C.J.; Clavel, C.; Koliopoulos, G.; Dillner, J. Chapter 9: Clinical applications of HPV testing: A summary of meta-analyses. Vaccine 2006, 24 (Suppl. S3), S3/78–S3/89. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: Management of abnormal cervical cytology and histology. Obstet. Gynecol. 2008, 112, 1419–1444. [Google Scholar] [CrossRef] [PubMed]
- Spinillo, A.; Gardella, B.; Roccio, M.; Alberizzi, P.; Cesari, S.; Patrizia, M.; Silini, E.M. Multiple human papillomavirus infection with or without type 16 and risk of cervical intraepithelial neoplasia among women with cervical cytological abnormalities. Cancer Causes Control 2014, 25, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- van der Marel, J.; Quint, W.G.; Schiffman, M.; Van De Sandt, M.M.; Zuna, R.E.; Dunn, S.T.; Smith, K.; Mathews, C.; Gold, M.A.; Walker, J.; et al. Molecular mapping of high-grade cervical intraepithelial neoplasia shows etiological dominance of HPV16. Int. J. Cancer 2012, 131, E946–E953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senapati, R.; Nayak, B.; Kar, S.K.; Dwibedi, B. HPV genotypes co-infections associated with cervical carcinoma: Special focus on phylogenetically related and non-vaccine targeted genotypes. PLoS ONE 2017, 12, e0187844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaccarella, S.; Franceschi, S.; Snijders, P.J.; Herrero, R.; Meijer, C.J.; Plummer, M.; The IARC HPV Prevalence Surveys Study Group. Concurrent infection with multiple human papillomavirus types: Pooled analysis of the IARC HPV Prevalence Surveys. Cancer Epidemiol. Biomark. Prev. 2010, 19, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Spinillo, A.; Dal Bello, B.; Gardella, B.; Roccio, M.; Dacco’, M.D.; Silini, E.M. Multiple human papillomavirus infection and high grade cervical intraepithelial neoplasia among women with cytological diagnosis of atypical squamous cells of undetermined significance or low grade squamous intraepithelial lesions. Gynecol. Oncol. 2009, 113, 115–119. [Google Scholar] [CrossRef]
- Spinillo, A.; Gardella, B.; Chiesa, A.; Cesari, S.; Alberizzi, P.; Silini, E.M. Diagnostic accuracy of colposcopy in relation to human papillomavirus genotypes and multiple infection. Gynecol. Oncol. 2014, 134, 527–533. [Google Scholar] [CrossRef]
- Biryukov, J.; Meyers, C. Superinfection Exclusion between Two High-Risk Human Papillomavirus Types during a Coinfection. J. Virol. 2018, 92, e01993-17. [Google Scholar] [CrossRef]
- De Vincenzo, R.; Conte, C.; Ricci, C.; Scambia, G.; Capelli, G. Long-term efficacy and safety of human papillomavirus vaccination. Int. J. Womens Health 2014, 6, 999–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5, CD009069. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, A.; Parazzini, F.; Martella, F.; Pieralli, A.; Bay, P.; Tonetti, A.; Svelato, A.; Bertacca, G.; Lombardi, S.; Joura, E.A. SPERANZA project: HPV vaccination after treatment for CIN2. Gynecol. Oncol. 2018, 151, 229–234. [Google Scholar] [CrossRef]
- De Vincenzo, R.; Caporale, N.; Bertoldo, V.; Ricci, C.; Evangelista, M.; Bizzarri, N.; Anchora, L.P.; Scambia, G.; Capelli, G. HPV and Cytology Testing in Women Undergoing 9-Valent HPV Opportunistic Vaccination: A Single-Cohort Follow Up Study. Vaccines 2021, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.hologic.com (accessed on 23 January 2023).
- Available online: https://www.fujirebio.com (accessed on 23 January 2023).
- Muñoz, N.; Castellsagué, X.; Berrington de González, A.; Gissmann, L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006, 24 (Suppl. S3), S3/1–S3/10. [Google Scholar] [CrossRef]
- Bornstein, J.; Bentley, J.; Bösze, P.; Girardi, F.; Haefner, H.; Menton, M.; Perrotta, M.; Prendiville, W.; Russell, P.; Sideri, M.; et al. 2011 colposcopic terminology of the International Federation for Cervical Pathology and Colposcopy. Obstet. Gynecol. 2012, 120, 166–172. [Google Scholar] [CrossRef] [Green Version]
- StataCorp, L.P. Stata Statistical 17.0: Release 13; StataCorp LP: College Station, TX, USA, 2013. [Google Scholar]
- Wheeler, C.M.; Skinner, S.R.; Del Rosario-Raymundo, M.R.; Garland, S.M.; Chatterjee, A.; Lazcano-Ponce, E.; Salmerón, J.; McNeil, S.; Stapleton, J.T.; Bouchard, C.; et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect. Dis. 2016, 16, 1154–1168. [Google Scholar] [CrossRef]
- Apter, D.; Wheeler, C.M.; Paavonen, J.; Castellsagué, X.; Garland, S.M.; Skinner, S.R.; Naud, P.; Salmerón, J.; Chow, S.-N.; Kitchener, H.C.; et al. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: Final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin. Vaccine Immunol. 2015, 22, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Teoh, D.; Nam, G.; Aase, D.A.; Russell, R.; Melton, G.B.; Kulasingam, S.; Vogel, R.I. Test Performance of Cervical Cytology Among Adults with vs without Human Papillomavirus Vaccination. JAMA Netw. Open 2022, 5, e2214020. [Google Scholar] [CrossRef]
- Lee, G.Y.; Inthasorn, P.; Laowahutanont, P.; Lawpoolsri, S.; Kamolratanakul, S.; Lungchukiet, P.; Oh, J.; Termrungruanglert, W.; Taechakraichana, N.; Pitisuttithum, P. Long-term effectiveness of human papillomavirus vaccines among adult women: A real-world scenario. Vaccine 2022, 40, 1968–1976. [Google Scholar] [CrossRef]
- Di Donato, V.; Caruso, G.; Bogani, G.; Cavallari, E.N.; Palaia, G.; Perniola, G.; Ralli, M.; Sorrenti, S.; Romeo, U.; Pernazza, A.; et al. HPV Vaccination after Primary Treatment of HPV-Related Disease across Different Organ Sites: A Multidisciplinary Comprehensive Review and Meta-Analysis. Vaccines 2022, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, K.S.; Kalliala, I.; Bowden, S.J.; Athanasiou, A.; Paraskevaidi, M.; Paraskevaidis, E.; Dillner, J.; Nieminen, P.; Strander, B.; Sasieni, P.; et al. Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: Systematic review and meta-analysis. BMJ 2022, 378, e070135. [Google Scholar] [CrossRef] [PubMed]
- Koutsky, L. Epidemiology of genital human papillomavirus infection. Am. J. Med. 1997, 102, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Baseman, J.G.; Koutsky, L.A. The epidemiology of human papillomavirus infections. J. Clin. Virol. 2005, 32 (Suppl. S1), S16–S24. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Bierman, R.; Beardsley, L.; Chang, C.J.; Burk, R.D. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998, 338, 423–428. [Google Scholar] [CrossRef]
- Muntinga, C.L.P.; de Vos van Steenwijk, P.J.; Bekkers, R.L.M.; van Esch, E.M.G. Importance of the Immune Microenvironment in the Spontaneous Regression of Cervical Squamous Intraepithelial Lesions (cSIL) and Implications for Immunotherapy. J. Clin. Med. 2022, 11, 1432. [Google Scholar] [CrossRef]
- Woo, Y.L.; Sterling, J.; Damay, I.; Coleman, N.; Crawford, R.; Van Der Burg, S.; Stanley, M. Characterising the local immune responses in cervical intraepithelial neoplasia: A cross-sectional and longitudinal analysis. BJOG 2008, 115, 1616–1622. [Google Scholar] [CrossRef]
- Monnier-Benoit, S.; Mauny, F.; Riethmuller, D.; Guerrini, J.-S.; Căpîlna, M.; Félix, S.; Seillès, E.; Mougin, C.; Prétet, J.-L. Immunohistochemical analysis of CD4+ and CD8+ T-cell subsets in high risk human papillomavirus-associated pre-malignant and malignant lesions of the uterine cervix. Gynecol. Oncol. 2006, 102, 22–31. [Google Scholar] [CrossRef]
- Litwin, T.R.; Irvin, S.R.; Chornock, R.L.; Sahasrabuddhe, V.V.; Stanley, M.; Wentzensen, N. Infiltrating T-cell markers in cervical carcinogenesis: A systematic review and meta-analysis. Br. J. Cancer 2021, 124, 831–841. [Google Scholar] [CrossRef]
- Jayshree, R.S. The Immune Microenvironment in Human Papilloma Virus-Induced Cervical Lesions-Evidence for Estrogen as an Immunomodulator. Front. Cell. Infect. Microbiol. 2021, 11, 649815. [Google Scholar] [CrossRef]
- Pruski, D.; Łagiedo-Żelazowska, M.; Millert-Kalińska, S.; Sikora, J.; Jach, R.; Przybylski, M. Immunity after HPV Vaccination in Patients after Sexual Initiation. Vaccines 2022, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J.; Jenkins, D.; Bosch, F.X.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.-N.; Apter, D.L.; Kitchener, H.C.; Castellsague, X.; et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial [published correction appears in Lancet 2007, 370, 1414]. Lancet 2007, 369, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Olsson, S.E.; Block, S.; Castellsague, X.; Gray, G.E.; Herrera, T.; Huang, L.-M.; Kim, D.S.; Pitisuttithum, P.; Chen, J.; et al. Immunogenicity and Safety of a 9-Valent HPV Vaccine. Pediatrics 2015, 136, e28–e39. [Google Scholar] [CrossRef] [Green Version]
- Villa, L.L.; Costa, R.L.; Petta, C.A.; Andrade, R.P.; Paavonen, J.; Iversen, O.-E.; Olsson, S.-E.; Høye, J.; Steinwall, M.; Riis-Johannessen, G.; et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br. J. Cancer 2006, 95, 1459–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viscidi, R.P.; Schiffman, M.; Hildesheim, A.; Herrero, R.; Castle, P.E.; Bratti, M.C.; Rodriguez, A.C.; Sherman, M.E.; Wang, S.; Clayman, B.; et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: Results from a population-based study in Costa Rica. Cancer Epidemiol. Biomark. Prev. 2004, 13, 324–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, J.J.; Koutsky, L.A.; Hughes, J.P.; Lee, S.K.; Kuypers, J.; Kiviat, N.; Galloway, D.A. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 2000, 181, 1911–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelstein, Z.R.; Carter, J.J.; Garg, R.; Winer, R.L.; Feng, Q.; Galloway, D.A.; Koutsky, L.A. Serum antibody response following genital {alpha}9 human papillomavirus infection in young men. J. Infect. Dis. 2011, 204, 209–216. [Google Scholar] [CrossRef] [PubMed]

| Patients Characteristics | Vaccinated Group n = 212 (%) | Non-Vaccinated Group n = 210 (%) | p-Value |
|---|---|---|---|
| Europe Extra Europe | 202 (95.38) | 205 (97.61) | 0.613 |
| 10 (4.72) | 5 (2.38) | ||
| Italian | 197 (92.92) | 200 (95.24) | 0.432 |
| HIV positivity | 12 (5.66) | 24 (11.43) | 0.025 |
| Parous | 74 (34.91) | 104 (49.52) | 0.002 |
| Smoking | 163 (76.87) | 156 (74.29) | |
| <10 cigarettes/day | 21 (9.91) | 30 (14.29) | 0.030 |
| ≥10 cigarettes/day | 21 (9.91) | 24 (11.43) | |
| Previous smoke | 7 (3.30) | 0 (0) | |
| Contraception | |||
| No | 104 (49.06) | 120 (57.14) | |
| Condoms | 14 (6.6) | 10 (4.76) | 0.090 |
| Oral therapy | 94 (44.34) | 77 (36.67) | |
| Intra Uterine Device | 0 (0) | 3 (1.43) |
| Lesion Characteristics | Vaccinated Group n = 212 (%) | Non-Vaccinated Group n = 210 (%) | p-Value |
|---|---|---|---|
| Colposcopy | |||
| No lesion | 54 (25.47) | 43 (20.48) | 0.021 |
| G1 lesion | 137 (64.62) | 126 (60.00) | |
| G2 lesion | 21 (9.91) | 41 (19.52) | |
| Transformation zone | |||
| Type 1 | 160 (75.47) | 128 (60.95) | 0.005 |
| Type 2 | 35 (16.51) | 24 (11.43) | |
| Type 3 | 17 (8.02) | 0 (0) | |
| Lesions extension | |||
| No lesion | 52 (24.53) | 43 (20.48) | |
| <50% of cervix | 107 (50.47) | 91 (43.33) | 0.031 |
| >50% of cervix | 41 (19.33) | 50 (23.80) | |
| Endocervical lesion | 12 (5.66) | 26 (12.38) | |
| HPV status negative | 8 (3.77) | 6 (2.86) | 0.528 |
| HPV genotype negative/untypable | 40 (18.87) | 24 (11.43) | 0.252 |
| Single-genotype infection | 89 (41.99) | 103 (49.05) | |
| Multiple-genotypes infection | 83 (39.15) | 83 (39.52) | |
| Class of risk | |||
| HPV genotype negative/untypable | 41 (19.34) | 23 (19.95) | |
| Low Risk-HPV single | 15 (7.08) | 20 (9.52) | 0.120 |
| Low Risk-HPV multiple | 0 (0) | 2 (0.95) | |
| Low Risk-High Risk HPV | 33 (15.57) | 31 (14.76) | |
| High Risk-HPV single | 77 (36.32) | 79 (37.62) | |
| High Risk-HPV multiple | 43 (20.28) | 54 (25.71) | |
| Positivity for one of HPV genotypes included in anti-HPV nonavalent vaccine | 121 (57.08) | 141 (67.14) | 0.021 |
| Positivity for multiple HPV genotypes included in anti-HPV nonavalent vaccine | 63 (29.72) | 75 (35.71) | 0.083 |
| Histological Characteristics at Follow-Up | Vaccinated Group n = 212 (%) | Non-Vaccinated Group n = 210 (%) | p-Value |
|---|---|---|---|
| Loop Electrosurgical Excision Procedure | 24 (11.32) | 89 (42.38) | <0.001 |
| Specimen with CIN1 on the margin | 7 (3.3) | 10 (4.76) | 0.521 |
| Endocervical Glandular involvement for CIN1 | 6 (2.83) | 4 (1.9) | 0.495 |
| Spontaneous regression of LSIL/CIN1 | 162 (76.42) | 143 (68.10) | |
| Persistence of LSIL/CIN1 | 46 (21.70) | 52 (24.76) | 0.019 |
| Progression to HSIL/CIN2 | 4 (1.89) | 15 (7.14) |
| Vaccinated Group n = 212 (%) | Non-Vaccinated Group n = 210 (%) | p-Value | |||
|---|---|---|---|---|---|
| HPV 16 Status | HPV 16 Positive at Entry | HPV 16 Negative at Entry | HPV 16 Positive at Entry | HPV 16 Negative at Entry | |
| HPV 16 positive at follow up | 10 (4.72) | 40 (18.87) | 10 (4.76) | 47 (22.38) | 0.807 |
| HPV 16 negative at follow up | 9 (4.25) | 153 (72.17) | 19 (9.05) | 134 (63.81) | 0.046 |
| Outcome | Variables | Relative Risk | 95% CI [RR] |
|---|---|---|---|
| CIN1 persistence | Age Not vaccine administration HIV status negative LSIL cytology | 0.994 1.266 0.518 0.782 | 0.970–1.018 0.774–2.068 0.242–1.109 0.481–1.271 |
| progression to CIN2+ | Age Not vaccine administration HIV status negative LSIL cytology | 1.005 3.472 0.299 0.343 | 0.961–1.051 1.066–11.320 0.088–1.018 0.131–0.896 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardella, B.; Dominoni, M.; Pasquali, M.F.; Melito, C.; Fiandrino, G.; Cesari, S.; La Verde, M.; Spinillo, A. Low-Grade Cervical Intraepithelial Neoplasia (CIN1) Evolution: Analysis of Opportunistic Preventive Vaccination Role. Vaccines 2023, 11, 284. https://doi.org/10.3390/vaccines11020284
Gardella B, Dominoni M, Pasquali MF, Melito C, Fiandrino G, Cesari S, La Verde M, Spinillo A. Low-Grade Cervical Intraepithelial Neoplasia (CIN1) Evolution: Analysis of Opportunistic Preventive Vaccination Role. Vaccines. 2023; 11(2):284. https://doi.org/10.3390/vaccines11020284
Chicago/Turabian StyleGardella, Barbara, Mattia Dominoni, Marianna Francesca Pasquali, Chiara Melito, Giacomo Fiandrino, Stefania Cesari, Marco La Verde, and Arsenio Spinillo. 2023. "Low-Grade Cervical Intraepithelial Neoplasia (CIN1) Evolution: Analysis of Opportunistic Preventive Vaccination Role" Vaccines 11, no. 2: 284. https://doi.org/10.3390/vaccines11020284





