Double-Blind, Placebo-Controlled, Dose-Escalating Study Evaluating the Safety and Immunogenicity of an Epitope-Specific Chemically Defined Nanoparticle RSV Vaccine
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
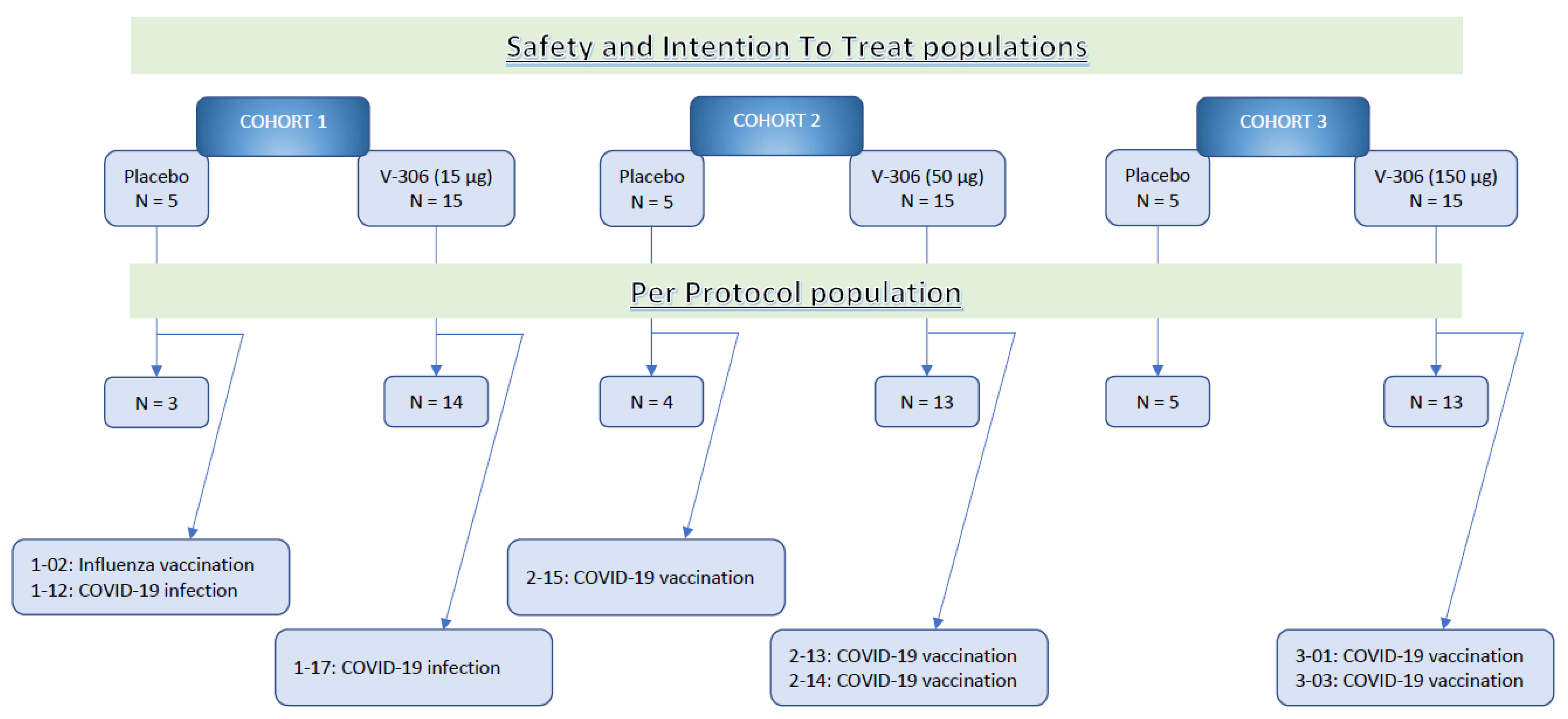
Study Population Demographics and Baseline Characteristics
4. Reactogenicity and Safety
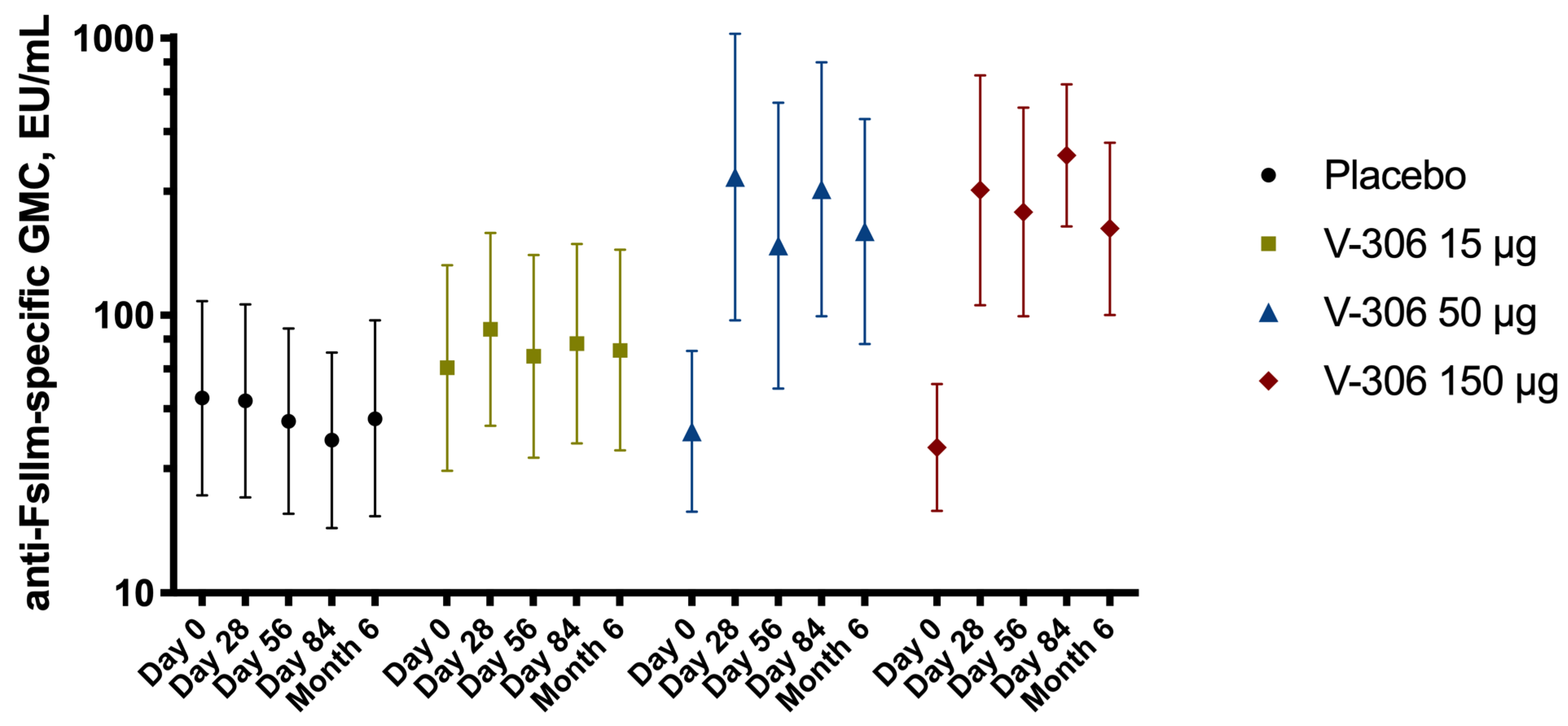
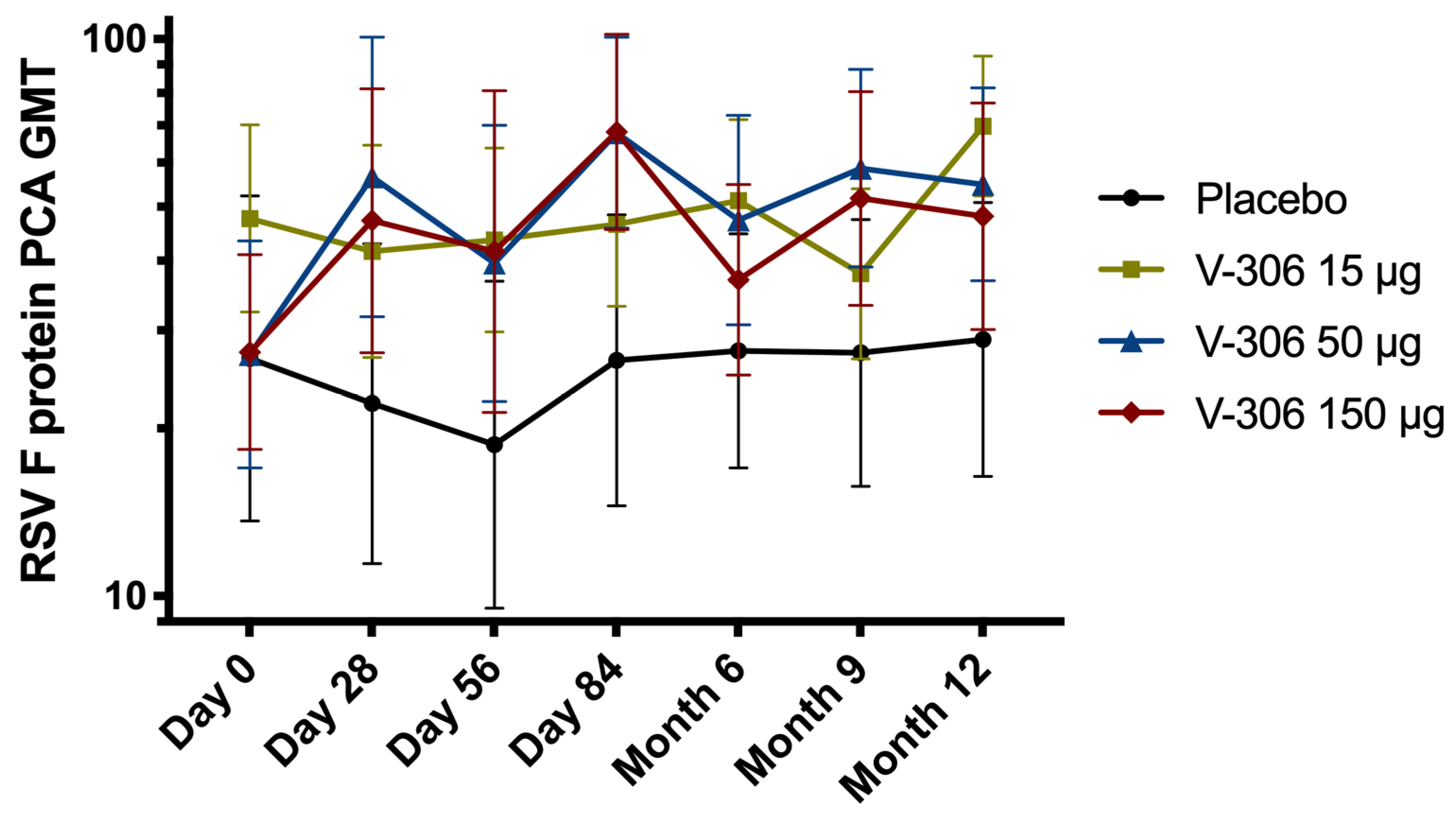
5. Humoral Immune Response
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shan, J.; Britton, P.N.; King, C.L.; Booy, R. The immunogenicity and safety of respiratory syncytial virus vaccines in development: A systematic review. Influenza Other Respir. Viruses 2021, 15, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Cantú-Flores, K.; Rivera-Alfaro, G.; Muñoz-Escalante, J.C.; Noyola, D.E. Global distribution of respiratory syncytial virus A and B infections: A systematic review. Pathog. Glob. Health 2022, 116, 398–409. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Tripp, R.A. Immunopathology of RSV: An Updated Review. Viruses 2021, 13, 2478. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.M.; van Wijhe, M.; Lehtonen, T.; Stona, L.; Teirlinck, A.C.; Fernandez, L.V.; Li, Y.; Osei-Yeboah, R.; Fischer, T.K.; Heikkinen, T.; et al. A Systematic Review of European Clinical Practice Guidelines for Respiratory Syncytial Virus Prophylaxis. J. Infect. Dis. 2022, 226, S110–S116. [Google Scholar] [CrossRef] [PubMed]
- Glowinski, R.; Mejias, A.; Ramilo, O. New preventive strategies for respiratory syncytial virus infection in children. Curr. Opin. Virol. 2021, 51, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Navarro Alonso, J.A.; Bont, L.J.; Bozzola, E.; Herting, E.; Lega, F.; Mader, S.; Nunes, M.C.; Ramilo, O.; Valiotis, G.; Olivier, C.W.; et al. RSV: Perspectives to strengthen the need for protection in all infants. Emerg. Themes Epidemiol. 2021, 18, 15. [Google Scholar] [CrossRef]
- Eichinger, K.M.; Kosanovich, J.L.; Lipp, M.; Empey, K.M.; Petrovsky, N. Strategies for active and passive pediatric RSV immunization. Ther. Adv. Vaccines Immunother. 2021, 9. [Google Scholar] [CrossRef]
- Zuniga, A.; Rassek, O.; Vrohlings, M.; Marrero-Nodarse, A.; Moehle, K.; Robinson, J.A.; Ghasparian, A. An epitope-specific chemically defined nanoparticle vaccine for respiratory syncytial virus. NPJ Vaccines 2021, 6, 85. [Google Scholar] [CrossRef]
- Boato, F.; Thomas, R.M.; Ghasparian, A.; Freund-Renard, A.; Moehle, K.; Robinson, J.A. Synthetic Virus-Like Particles from Self-Assembling Coiled-Coil Lipopeptides and Their Use in Antigen Display to the Immune System. Angew. Chem. Int. Ed. 2007, 46, 9015–9018. [Google Scholar] [CrossRef]
- Ghasparian, A.; Riedel, T.; Koomullil, J.; Moehle, K.; Gorba, C.; Svergun, D.I.; Perriman, A.W.; Mann, S.; Tamborrini, M.; Pluschke, G.; et al. Engineered Synthetic Virus-Like Particles and Their Use in Vaccine Delivery. Eur. J. Chem. Biol. 2011, 12, 100–109. [Google Scholar] [CrossRef]
- Sharma, R.; Ghasparian, A.; Robinson, J.A.; McCullough, K.C. Synthetic Virus-Like Particles Target Dendritic Cell Lipid Rafts for Rapid Endocytosis Primarily but Not Exclusively by Macropinocytosis. PLoS ONE 2012, 7, e43248. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ghasparian, A.; Robinson, J.A.; McCullough, K.C. Dendritic Cell Sensing of Hydrophobic Di- and Triacylated Lipopeptides Self-Assembled within Synthetic Virus-like Particles. J. Immunol. 2017, 199, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Hervé, P.-L.; Dhelft, V.; Zuniga, A.; Ghasparian, A.; Rassek, O.; Yim, K.C.; Donne, N.; Lambert, P.-H.; Benhamou, P.-H.; Sampson, H.A.; et al. Epicutaneous immunization using synthetic virus-like particles efficiently boosts protective immunity to respiratory syncytial virus. Vaccine 2021, 39, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Acosta, P.L.; Caballero, M.T.; Polack, F.P. Brief History and Characterization of Enhanced Respiratory Syncytial Virus Disease. Clin. Vaccine Immunol. 2015, 23, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Falloon, J.; Yu, J.; Esser, M.T.; Villafana, T.; Yu, L.; Dubovsky, F.; Takas, T.; Levin, M.J.; Falsey, A.R. An Adjuvanted, Postfusion F Protein-Based Vaccine Did Not Prevent Respiratory Syncytial Virus Illness in Older Adults. J. Infect. Dis. 2017, 216, 1362–1370. [Google Scholar] [CrossRef]
- Blais, N.; Gagné, M.; Hamuro, Y.; Rheault, P.; Boyer, M.; Steff, A.-M.; Baudoux, G.; Dewar, V.; Demers, J.; Ruelle, J.-L.; et al. Characterization of Pre-F-GCN4t, a Modified Human Respiratory Syncytial Virus Fusion Protein Stabilized in a Noncleaved Prefusion Conformation. J. Virol. 2017, 91, e02437-16. [Google Scholar] [CrossRef]
- Patel, N.; Tian, J.-H.; Flores, R.; Jacobson, K.; Walker, M.; Portnoff, A.; Gueber-Xabier, M.; Massare, M.J.; Glenn, G.; Ellingsworth, L.; et al. Flexible RSV Prefusogenic Fusion Glycoprotein Exposes Multiple Neutralizing Epitopes that May Collectively Contribute to Protective Immunity. Vaccines 2020, 8, 607. [Google Scholar] [CrossRef]
- Chen, G.L.; Mithani, R.; Kapoor, A.; Lu, S.; El Asmar, L.; Panozzo, C.A.; Shaw, C.A.; Stoszek, S.K.; August, A. Safety and Immunogenicity of mRNA-1345, an mRNA-Based RSV Vaccine in Younger and Older Adult Cohorts: Results from a Phase 1, Randomized Clinical Trial. Open Forum Infect. Dis. 2022, 9 (Suppl. 2). [Google Scholar] [CrossRef]
- Aliprantis, A.O.; Wolford, D.; Caro, L.; Haas, B.M.; Ma, H.; Montgomery, D.L.; Sterling, L.M.; Hunt, A.; Cox, K.S.; Vora, K.A.; et al. A Phase 1 Randomized, Double-Blind, Placebo-Controlled Trial to Assess the Safety, Tolerability, and Pharmacokinetics of a Respiratory Syncytial Virus Neutralizing Monoclonal Antibody MK-1654 in Healthy Adults. Clin. Pharmacol. Drug Dev. 2021, 10, 556–566. [Google Scholar] [CrossRef]
- Ward, A.B.; Wilson, I.A. Innovations in structure-based antigen design and immune monitoring for next generation vaccines. Curr. Opin. Immunol. 2020, 65, 50–56. [Google Scholar] [CrossRef]
- Cayatte, C.; Bennett, A.S.; Rajani, G.M.; Hostetler, L.; Maynard, S.K.; Lazzaro, M.; McTamney, P.; Ren, K.; O’Day, T.; McCarthy, M.P.; et al. Inferior immunogenicity and efficacy of respiratory syncytial virus fusion protein-based subunit vaccine candidates in aged versus young mice. PLoS ONE 2017, 12, e0188708. [Google Scholar] [CrossRef] [PubMed]
- Fries, L.; Shinde, V.; Stoddard, J.J.; Thomas, D.N.; Kpamegan, E.; Lu, H.; Smith, G.; Hickman, S.P.; Piedra, P.; Glenn, G.M. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun. Ageing 2017, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Falloon, J.; Talbot, H.K.; Curtis, C.; Ervin, J.; Krieger, D.; Dubovsky, F.; Takas, T.; Yu, J.; Yu, L.; Lambert, S.L.; et al. Dose Selection for an Adjuvanted Respiratory Syncytial Virus F Protein Vaccine for Older Adults Based on Humoral and Cellular Immune Responses. Clin. Vaccine Immunol. 2017, 24, e00157-17. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, I.; Wicht, O.; Luytjes, W.; Leenhouts, K.; Rottier, P.J.M.; van Kuppeveld, F.J.M.; Haijema, B.J.; de Haan, C.A.M. Characterization of Epitope-Specific Anti-Respiratory Syncytial Virus (Anti-RSV) Antibody Responses after Natural Infection and after Vaccination with Formalin-Inactivated RSV. J. Virol. 2016, 90, 5965–5977. [Google Scholar] [CrossRef]
- Glenn, G.M.; Fries, L.F.; Smith, G.; Kpamegan, E.; Lu, H.; Guebre-Xabier, M.; Hickman, S.P.; Flyer, D. Modeling maternal fetal RSV F vaccine induced antibody transfer in guinea pigs. Vaccine 2015, 33, 6488–6492. [Google Scholar] [CrossRef]
- Raghunandan, R.; Lu, H.; Zhou, B.; Xabier, M.G.; Massare, M.J.; Flyer, D.C.; Fries, L.F.; Smith, G.E.; Glenn, G.M. An insect cell derived respiratory syncytial virus (RSV) F nanoparticle vaccine induces antigenic site II antibodies and protects against RSV challenge in cotton rats by active and passive immunization. Vaccine 2014, 32, 6485–6492. [Google Scholar] [CrossRef]
- Phung, E.; Chang, L.A.; Morabito, K.M.; Kanekiyo, M.; Chen, M.; Nair, D.; Kumar, A.; Chen, G.L.; Ledgerwood, J.E.; Graham, B.S.; et al. Epitope-Specific Serological Assays for RSV: Conformation Matters. Vaccines 2019, 7, 23. [Google Scholar] [CrossRef]
- Crank, M.C.; Ruckwardt, T.J.; Chen, M.; Morabito, K.M.; Phung, E.; Costner, P.J.; Holman, L.A.; Hickman, S.P.; Berkowitz, N.M.; Gordon, I.J.; et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019, 365, 505–509. [Google Scholar] [CrossRef]
- Andreano, E.; Paciello, I.; Bardelli, M.; Tavarini, S.; Sammicheli, C.; Frigimelica, E.; Guidotti, S.; Torricelli, G.; Biancucci, M.; D’Oro, U.; et al. The respiratory syncytial virus (RSV) prefusion F-protein functional antibody repertoire in adult healthy donors. EMBO Mol. Med. 2021, 13, e14035. [Google Scholar] [CrossRef]
- Mousa, J.J.; Sauer, M.F.; Sevy, A.M.; Finn, J.A.; Bates, J.T.; Alvarado, G.; King, H.G.; Loerinc, L.B.; Fong, R.H.; Doranz, B.J.; et al. Structural basis for nonneutralizing antibody competition at antigenic site II of the respiratory syncytial virus fusion protein. Proc. Natl. Acad. Sci. USA 2016, 113, E6849–E6858. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; McLellan, J.S.; Kallewaard, N.L.; Ulbrandt, N.D.; Palaszynski, S.; Zhang, J.; Moldt, B.; Khan, A.; Svabek, C.; McAuliffe, J.M.; et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci. Transl. Med. 2017, 9, eaaj1928. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Cots, M.B.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Chen, Z.; Cox, K.S.; Su, H.-P.; Callahan, C.; Fridman, A.; Zhang, L.; Patel, S.B.; Cejas, P.J.; Swoyer, R.; et al. A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat. Commun. 2019, 10, 4153. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Raghunandan, R.; Wu, Y.; Liu, Y.; Massare, M.; Nathan, M.; Zhou, B.; Lu, H.; Boddapati, S.; Li, J.; et al. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS ONE 2012, 7, e50852. [Google Scholar] [CrossRef] [Green Version]

| Placebo N = 15 | V-306 15µg N = 15 | V-306 50µg N = 15 | V-306 150µg N = 15 | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| At least one local symptom | 1 | 6.7 | 13 | 86.7 | 13 | 86.7 | 15 | 100.0 |
| At least one local severe symptom | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Pain | 1 | 6.7 | 13 | 86.7 | 13 | 86.7 | 15 | 100.0 |
| Induration | 0 | 0.0 | 1 | 6.7 | 2 | 13.3 | 5 | 33.3 |
| Erythema | 0 | 0.0 | 1 | 6.7 | 0 | 0.0 | 0 | 0.0 |
| Swelling | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 6.7 |
| At least one systemic symptom | 7 | 46.7 | 9 | 60.0 | 13 | 86.7 | 12 | 80.0 |
| At least one systemic severe symptom | 0 | 0.0 | 1 | 6.7 | 0 | 0.0 | 0 | 0.0 |
| Headache | 5 | 33.3 | 8 | 53.3 | 9 | 60.0 | 7 | 46.7 |
| Fatigue | 5 | 33.3 | 7 | 46.7 | 9 | 60.0 | 9 | 60.0 |
| Fatigue (severe) | 0 | 0.0 | 1 | 6.7 | 0 | 0.0 | 0 | 0.0 |
| Generalized myalgia | 2 | 13.3 | 3 | 20.0 | 0 | 0.0 | 1 | 6.7 |
| Fever | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leroux-Roels, I.; Bruhwyler, J.; Stergiou, L.; Sumeray, M.; Joye, J.; Maes, C.; Lambert, P.-H.; Leroux-Roels, G. Double-Blind, Placebo-Controlled, Dose-Escalating Study Evaluating the Safety and Immunogenicity of an Epitope-Specific Chemically Defined Nanoparticle RSV Vaccine. Vaccines 2023, 11, 367. https://doi.org/10.3390/vaccines11020367
Leroux-Roels I, Bruhwyler J, Stergiou L, Sumeray M, Joye J, Maes C, Lambert P-H, Leroux-Roels G. Double-Blind, Placebo-Controlled, Dose-Escalating Study Evaluating the Safety and Immunogenicity of an Epitope-Specific Chemically Defined Nanoparticle RSV Vaccine. Vaccines. 2023; 11(2):367. https://doi.org/10.3390/vaccines11020367
Chicago/Turabian StyleLeroux-Roels, Isabel, Jacques Bruhwyler, Lilli Stergiou, Mark Sumeray, Jasper Joye, Cathy Maes, Paul-Henri Lambert, and Geert Leroux-Roels. 2023. "Double-Blind, Placebo-Controlled, Dose-Escalating Study Evaluating the Safety and Immunogenicity of an Epitope-Specific Chemically Defined Nanoparticle RSV Vaccine" Vaccines 11, no. 2: 367. https://doi.org/10.3390/vaccines11020367





