Homologous and Heterologous Prime-Boost Vaccination: Impact on Clinical Severity of SARS-CoV-2 Omicron Infection among Hospitalized COVID-19 Patients in Belgium
Abstract
:1. Introduction
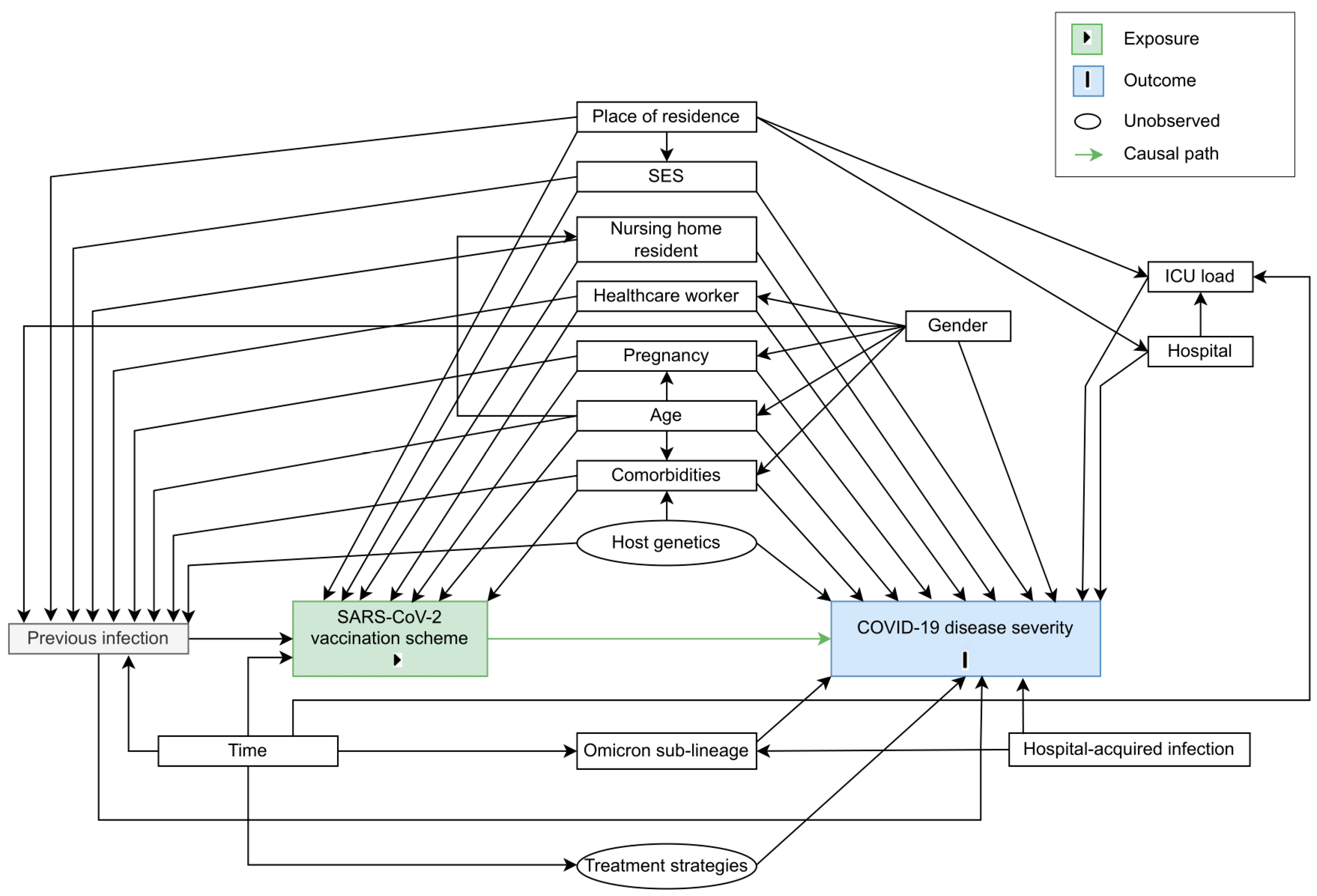
2. Materials and Methods
2.1. Target Trial Specification
2.2. Target Trial Emulation
2.2.1. Data Sources
2.2.2. Eligibility Criteria
2.2.3. Intervention Strategies and Assignment
2.2.4. Follow-up Period and Outcomes of Interest
2.3. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
3.1.1. Main Analysis
3.1.2. Sensitivity Analysis
3.2. Effect Estimates
3.2.1. Target Trial I: Primary Vaccination Plus mRNA Booster Vaccination versus Primary Vaccination without Booster Vaccination
Main Analysis
Sensitivity Analysis
3.2.2. Target Trial II: Heterologous versus Homologous Prime-Boost Vaccination
Main Analysis
Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 Pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and Effectiveness of MRNA BNT162b2 Vaccine against SARS-CoV-2 Infections and COVID-19 Cases, Hospitalisations, and Deaths Following a Nationwide Vaccination Campaign in Israel: An Observational Study Using National Surveillance Data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Mazagatos, C.; Monge, S.; Olmedo, C.; Vega, L.; Gallego, P.; Martín-Merino, E.; Sierra, M.J.; Limia, A.; Larrauri, A.; Spain, W.G.; et al. Effectiveness of MRNA COVID-19 Vaccines in Preventing SARS-CoV-2 Infections and COVID-19 Hospitalisations and Deaths in Elderly Long-Term Care Facility Residents, Spain, Weeks 53 2020 to 13 2021. Eurosurveillance 2021, 26, 2100452. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Baz, I.; Miqueleiz, A.; Casado, I.; Navascués, A.; Trobajo-Sanmartín, C.; Burgui, C.; Guevara, M.; Ezpeleta, C.; Castilla, J.; Working Group for the Study of COVID-19 in Navarra. Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection and Hospitalisation, Navarre, Spain, January to April 2021. Eurosurveillance 2021, 26, 2100438. [Google Scholar] [CrossRef]
- Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Pannaraj, P.S.; Irby, K.; Walker, T.C.; Schwartz, S.P.; et al. Effectiveness of BNT162b2 Vaccine against Critical Covid-19 in Adolescents. N. Engl. J. Med. 2022, 386, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, C.; Lenehan, P.; Puranik, A.; Agarwal, V.; Venkatakrishnan, A.J.; Niesen, M.J.M.; O’Horo, J.C.; Virk, A.; Swift, M.D.; Badley, A.D.; et al. FDA-Authorized MRNA COVID-19 Vaccines Are Effective per Real-World Evidence Synthesized across a Multi-State Health System. Med 2021, 2, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The Impact of Vaccination on Coronavirus Disease 2019 (COVID-19) Outbreaks in the United States. Clin. Infect. Dis. 2021, 73, 2257–2264. [Google Scholar] [CrossRef]
- Federal Public Service (FPS) Health, Food Chain Safety and Environment. Vaccination Coronavirus COVID-19. Available online: https://www.info-coronavirus.be/en/vaccination/ (accessed on 15 March 2022).
- Sciensano. COVID-19—Epidemiologisch Bulletin Van 31 MEI 2022; COVID-19 Epidemiologisch Bulletin; Sciensano: Brussels, Belgium, 2022; Available online: https://covid-19.sciensano.be/sites/default/files/Covid19/Meest%20recente%20update.pdf (accessed on 1 June 2022).
- Sciensano. COVID-19 Wekelijks Epidemiologisch Bulletin (1 December 2022); Wekelijks Epidemiologisch Bulletin; Sciensano: Brussels, Belgium, 2022; Available online: https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_Weekly_report_NL.pdf (accessed on 6 December 2022).
- Bellamkonda, N.; Lambe, U.P.; Sawant, S.; Nandi, S.S.; Chakraborty, C.; Shukla, D. Immune Response to SARS-CoV-2 Vaccines. Biomedicines 2022, 10, 1464. [Google Scholar] [CrossRef]
- Kashte, S.; Gulbake, A.; El-Amin III, S.F.; Gupta, A. COVID-19 Vaccines: Rapid Development, Implications, Challenges and Future Prospects. Hum. Cell. 2021, 34, 711–733. [Google Scholar] [CrossRef]
- Ndwandwe, D.; Wiysonge, C.S. COVID-19 Vaccines. Curr. Opin. Immunol. 2021, 71, 111–116. [Google Scholar] [CrossRef]
- Vanaparthy, R.; Mohan, G.; Vasireddy, D.; Atluri, P. Review of COVID-19 Viral Vector-Based Vaccines and COVID-19 Variants. Infez. Med. 2021, 29, 328–338. [Google Scholar] [CrossRef]
- Huang, Q.; Zeng, J.; Yan, J. COVID-19 MRNA Vaccines. J. Genet. Genom. 2021, 48, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Upreti, S.; Samant, M. A Review on Immunological Responses to SARS-CoV-2 and Various COVID-19 Vaccine Regimens. Pharm. Res. 2022, 39, 2119–2134. [Google Scholar] [CrossRef] [PubMed]
- Lu, S. Heterologous Prime–Boost Vaccination. Curr. Opin. Immunol. 2009, 21, 346–351. [Google Scholar] [CrossRef]
- Levine, M.Z.; Holiday, C.; Jefferson, S.; Gross, F.L.; Liu, F.; Li, S.; Friel, D.; Boutet, P.; Innis, B.L.; Mallett, C.P.; et al. Heterologous Prime-Boost with A(H5N1) Pandemic Influenza Vaccines Induces Broader Cross-Clade Antibody Responses than Homologous Prime-Boost. Npj Vaccines 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Sapkota, B.; Saud, B.; Shrestha, R.; Al-Fahad, D.; Sah, R.; Shrestha, S.; Rodriguez-Morales, A.J. Heterologous Prime–Boost Strategies for COVID-19 Vaccines. J. Travel Med. 2022, 29, taab191. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Jungsomsri, P.; Sangwongwanich, J.; Tawinprai, K.; Siripongboonsitti, T.; Porntharukchareon, T.; Wittayasak, K.; Thonwirak, N.; Soonklang, K.; Sornsamdang, G.; et al. Immunogenicity and Reactogenicity after Heterologous Prime-Boost Vaccination with CoronaVac and ChAdox1 NCov-19 (AZD1222) Vaccines. Hum. Vaccines Immunother. 2022, 18, 2052525. [Google Scholar] [CrossRef] [PubMed]
- Khoo, N.K.H.; Lim, J.M.E.; Gill, U.S.; de Alwis, R.; Tan, N.; Toh, J.Z.N.; Abbott, J.E.; Usai, C.; Ooi, E.E.; Low, J.G.H.; et al. Differential Immunogenicity of Homologous versus Heterologous Boost in Ad26.COV2.S Vaccine Recipients. Med 2022, 3, 104–118.e4. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Hirabara, S.M.; Serdan, T.D.A.; Gorjao, R.; Masi, L.N.; Pithon-Curi, T.C.; Covas, D.T.; Curi, R.; Durigon, E.L. SARS-COV-2 Variants: Differences and Potential of Immune Evasion. Front. Cell. Infect. Microbiol. 2022, 11, 1401. [Google Scholar] [CrossRef]
- Li, X. Omicron: Call for Updated Vaccines. J. Med. Virol. 2022, 94, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; St. Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. MRNA-Based COVID-19 Vaccine Boosters Induce Neutralizing Immunity against SARS-CoV-2 Omicron Variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.-S.; Winkler, M.S.; et al. The Omicron Variant Is Highly Resistant against Antibody-Mediated Neutralization: Implications for Control of the COVID-19 Pandemic. Cell 2022, 185, 447–456.e11. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Ackerson, B.K.; Takhar, H.; Ogun, O.A.; Simmons, S.; Zamparo, J.M.; et al. BNT162b2 Vaccine Effectiveness against SARS-CoV-2 Omicron BA.4 and BA.5. Lancet Infect. Dis. 2022, 22, 1663–1665. [Google Scholar] [CrossRef]
- Jacobsen, H.; Katzmarzyk, M.; Higdon, M.M.; Jiménez, V.C.; Sitaras, I.; Bar-Zeev, N.; Knoll, M.D. Post-Vaccination Neutralization Responses to Omicron Sub-Variants. Vaccines 2022, 10, 1757. [Google Scholar] [CrossRef]
- Björk, J.; Bonander, C.; Moghaddassi, M.; Rasmussen, M.; Malmqvist, U.; Inghammar, M.; Kahn, F. COVID-19 Vaccine Effectiveness against Severe Disease from SARS-CoV-2 Omicron BA.1 and BA.2 Subvariants—Surveillance Results from Southern Sweden, December 2021 to March 2022. Eurosurveillance 2022, 27, 2200322. [Google Scholar] [CrossRef]
- Vanmechelen, B.; Logist, A.-S.; Wawina-Bokalanga, T.; Verlinden, J.; Martí-Carreras, J.; Geenen, C.; Slechten, B.; Cuypers, L.; André, E.; Baele, G.; et al. Identification of the First SARS-CoV-2 Lineage B.1.1.529 Virus Detected in Europe. Microbiol. Resour. Announc. 2022, 11, e01161-21. [Google Scholar] [CrossRef]
- Leuven, U.Z.; Leuven, K.U. Genomic Surveillance of SARS-CoV-2 in Belgium—Report of the National Reference Laboratory; U.Z. Leuven: Leuven, Belgium, 2022; Available online: https://www.uzleuven.be/nl/laboratoriumgeneeskunde/genomic-surveillance-sars-cov-2-belgium/genomic-surveillance-sars-cov-2-belgium-reports (accessed on 12 August 2022).
- Leuven, U.Z. Genomic Surveillance of SARS-CoV-2 in Belgium. Available online: https://www.uzleuven.be/nl/laboratoriumgeneeskunde/genomic-surveillance-sars-cov-2-belgium (accessed on 19 December 2022).
- Saito, A.; Irie, T.; Suzuki, R.; Maemura, T.; Nasser, H.; Uriu, K.; Kosugi, Y.; Shirakawa, K.; Sadamasu, K.; Kimura, I.; et al. Enhanced Fusogenicity and Pathogenicity of SARS-CoV-2 Delta P681R Mutation. Nature 2022, 602, 300–306. [Google Scholar] [CrossRef]
- Dang, S.; Ren, L.; Wang, J. Functional Mutations of SARS-CoV-2: Implications to Viral Transmission, Pathogenicity and Immune Escape. Chin. Med. J. 2022, 135, 1213–1222. [Google Scholar] [CrossRef]
- Van Goethem, N.; Vilain, A.; Wyndham-Thomas, C.; Deblonde, J.; Bossuyt, N.; Lernout, T.; Rebolledo Gonzalez, J.; Quoilin, S.; Melis, V.; Van Beckhoven, D. Rapid Establishment of a National Surveillance of COVID-19 Hospitalizations in Belgium. Arch. Public Health 2020, 78, 121. [Google Scholar] [CrossRef] [PubMed]
- healthdata.be (Sciensano). DATABASE COVID-19 TESTRESULTS—LaboratoryTestResult. Available online: https://covid19lab.healthdata.be/data-collection/laboratorytestresult (accessed on 20 June 2022).
- healthdata.be (Sciensano). DATABASE COVID-19 TESTRESULTS—LaboratoryTestResultVariants. Available online: https://covid19lab.healthdata.be/laboratorytestresultvariants (accessed on 20 June 2022).
- Meurisse, M.; Lajot, A.; Dupont, Y.; Lesenfants, M.; Klamer, S.; Rebolledo, J.; Lernout, T.; Leroy, M.; Capron, A.; Van Bussel, J.; et al. One Year of Laboratory-Based COVID-19 Surveillance System in Belgium: Main Indicators and Performance of the Laboratories (March 2020–2021). Arch. Public Health 2021, 79, 188. [Google Scholar] [CrossRef]
- Zorg en Gezondheid (Vlaamse overheid). Vaccinatie Systeem (Vaccinnet). Available online: https://www.vaccinnet.be/Vaccinnet/welkom.do (accessed on 20 June 2022).
- eHealth. CoBRHA—Common Base Registry for HealthCare Actor. Available online: https://www.ehealth.fgov.be/ehealthplatform/nl/service-cobrha-common-base-registry-for-healthcare-actor (accessed on 20 June 2022).
- Van Goethem, N.; Serrien, B.; Vandromme, M.; Wyndham-Thomas, C.; Catteau, L.; Brondeel, R.; Klamer, S.; Meurisse, M.; Cuypers, L.; André, E.; et al. Conceptual Causal Framework to Assess the Effect of SARS-CoV-2 Variants on COVID-19 Disease Severity among Hospitalized Patients. Arch. Public Health 2021, 79, 185. [Google Scholar] [CrossRef] [PubMed]
- Meurisse, M.; Van Oyen, H.; Blot, K.; Catteau, L.; Serrien, B.; Klamer, S.; Cauët, E.; Robert, A.; Van Goethem, N. Evaluating Methodological Approaches to Assess the Severity of Infection with SARS-CoV-2 Variants: Scoping Review and Applications on Belgian COVID-19 Data. BMC Infect. Dis. 2022, 22, 839. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust Causal Inference Using Directed Acyclic Graphs: The R Package “Dagitty”. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Reinfection with SARS-CoV-2: Implementation of a Surveillance Case Definition within the EU/EEA; European Centre for Disease Prevention and Control: Solna, Sweden, 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/reinfection-sars-cov-2-implementation-surveillance-case-definition-within-eueea (accessed on 6 September 2022).
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Lee, S.; Lee, W. Application of Standardization for Causal Inference in Observational Studies: A Step-by-Step Tutorial for Analysis Using R Software. J. Prev. Med. Public Health 2022, 55, 116–124. [Google Scholar] [CrossRef]
- Ripley, B. Package ‘Boot’. 2022. Available online: https://cran.r-project.org/web/packages/boot/boot.pdf (accessed on 5 December 2022).
- Bours, M.J.L. Tutorial: A Nontechnical Explanation of the Counterfactual Definition of Effect Modification and Interaction. J. Clin. Epidemiol. 2021, 134, 113–124. [Google Scholar] [CrossRef]
- Buchan, S.A.; Chung, H.; Brown, K.A.; Austin, P.C.; Fell, D.B.; Gubbay, J.B.; Nasreen, S.; Schwartz, K.L.; Sundaram, M.E.; Tadrous, M.; et al. Estimated Effectiveness of COVID-19 Vaccines Against Omicron or Delta Symptomatic Infection and Severe Outcomes. JAMA Netw. Open 2022, 5, e2232760. [Google Scholar] [CrossRef]
- Liu, B.; Gidding, H.; Stepien, S.; Cretikos, M.; Macartney, K. Relative Effectiveness of COVID-19 Vaccination with 3 Compared to 2 Doses against SARS-CoV-2 B.1.1.529 (Omicron) among an Australian Population with Low Prior Rates of SARS-CoV-2 Infection. Vaccine 2022, 40, 6288–6294. [Google Scholar] [CrossRef] [PubMed]
- Kissling, E.; Hooiveld, M.; Martínez-Baz, I.; Mazagatos, C.; William, N.; Vilcu, A.-M.; Kooijman, M.N.; Ilić, M.; Domegan, L.; Machado, A.; et al. Effectiveness of Complete Primary Vaccination against COVID-19 at Primary Care and Community Level during Predominant Delta Circulation in Europe: Multicentre Analysis, I-MOVE-COVID-19 and ECDC Networks, July to August 2021. Eurosurveillance 2022, 27, 2101104. [Google Scholar] [CrossRef] [PubMed]
- Button, K.S.; Ioannidis, J.P.A.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.J.; Munafò, M.R. Power Failure: Why Small Sample Size Undermines the Reliability of Neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Yelin, D.; Eckerle, I.; Eberhardt, C.S.; Wang, J.; Cao, B.; Kaiser, L. Definitions for Coronavirus Disease 2019 Reinfection, Relapse and PCR Re-Positivity. Clin. Microbiol. Infect. 2021, 27, 315–318. [Google Scholar] [CrossRef] [PubMed]


| Primary Vaccination (No Booster) (n = 107) | Primary Vaccination, Plus mRNA Booster Vaccination | ||
|---|---|---|---|
| Homologous Prime-Boost Vaccination (n = 504) | Heterologous Prime-Boost Vaccination (n = 257) | ||
| Number/Total Number (Percent) | |||
| Demographics | |||
| Age (years), median (IQR) | 68 (51–80) | 79 (72–85) | 84 (74–89) |
| Male gender, n/N (%) | 60/107 (56.1) | 300/504 (59.5) | 153/257 (59.5) |
| Nursing home resident, n/N (%) | 5/106 (4.7) | 68/497 (13.7) | 26/256 (10.2) |
| Healthcare worker, n/N (%) | 1/105 (1.0) | 4/500 (0.8) | 3/256 (1.2) |
| Comorbidities | |||
| Cardiovascular Disease, n/N (%) | 36/107 (33.6) | 277/504 (55.0) | 151/257 (58.8) |
| History of Arterial Hypertension, n/N (%) | 39/107 (36.4) | 218/504 (43.3) | 104/257 (40.5) |
| Diabetes mellitus, n/N (%) | 27/107 (25.2) | 142/504 (28.2) | 70/257 (27.2) |
| Obesity, n/N (%) | 8/107 (7.5) | 39/504 (7.7) | 23/257 (8.9) |
| Chronic Pulmonary Disease, n/N (%) | 28/107 (26.2) | 119/504 (23.6) | 74/257 (28.8) |
| Chronic Neurological Disease, n/N (%) | 21/107 (19.6) | 111/504 (22.0) | 40/257 (15.6) |
| Chronic Cognitive Deficit, n/N (%) | 15/107 (14.0) | 65/504 (12.9) | 28/257 (10.9) |
| Chronic Renal Disease, n/N (%) | 22/107 (20.6) | 136/504 (27.0) | 85/257 (33.1) |
| Chronic Liver Disease, n/N (%) | 4/107 (3.7) | 18/504 (3.6) | 5/257 (1.9) |
| Solid Cancer, n/N (%) | 20/107 (18.7) | 111/504 (22.0) | 48/257 (18.7) |
| Hematological Cancer, n/N (%) | 6/107 (5.6) | 18/504 (3.6) | 10/257 (3.9) |
| Solid organ transplantation, n/N (%) | 2/107 (1.9) | 7/504 (1.4) | 6/257 (2.3) |
| Chronic Immunosuppression, n/N (%) | 8/107 (7.5) | 18/504 (3.6) | 14/257 (5.4) |
| Pregnancy, n/N (%) | 5/107 (4.7) | 0/504 (0.0) | 1/257 (0.4) |
| Socio-economic status | |||
| Education level, n/N (%) | |||
| Low | 41/72 (56.9) | 300/445 (67.4) | 155/235 (66.0) |
| Middle | 24/72 (33.3) | 87/445 (19.6) | 58/235 (24.7) |
| High | 7/72 (9.7) | 58/445 (13.0) | 22/235 (9.4) |
| Income category, n/N (%) | |||
| Low income | 55/94 (58.5) | 269/479 (56.2) | 146/251 (58.2) |
| Middle income | 32/94 (34.0) | 147/479 (30.7) | 75/251 (29.9) |
| High income | 7/94 (7.4) | 63/479 (13.2) | 30/251 (12.0) |
| Population density a, median (IQR) | 2591 (484–2591) | 798 (390–2591) | 941 (357–2591) |
| Median taxable income per capita b, median (IQR) | 23,807 (23,807–27,514) | 27,075 (23,807–28,985) | 26,892 (23,807–28,855) |
| Exposure | |||
| Hospital-acquired infection, n/N (%) | 7/106 (6.6) | 33/498 (6.6) | 15/256 (5.9) |
| Documented previous infections c, n/N (%) | 8/107 (7.5) | 12/504 (2.4) | 9/257 (3.5) |
| Hospital characteristics | |||
| Mean ICU occupancy during hospital stay, median (IQR) | 13 (7–20) | 8 (4–14) | 9 (5–16) |
| Clinical outcomes | |||
| Severe d COVID-19, n/N (%) | 29/106 (27.4) | 98/501 (19.6) | 40/256 (15.6) |
| ICU admission, n/N (%) | 18/107 (16.8) | 54/504 (10.7) | 19/257 (7.4) |
| In-hospital mortality, n/N (%) | 17/105 (16.2) | 57/500 (11.4) | 30/256 (11.7) |
| ARDS, n/N (%) | 4/107 (3.7) | 18/504 (3.6) | 6/257 (2.3) |
| Hospital length of stay (days), median (IQR) | 9 (4–14) | 9 (5–16) | 9 (5–17) |
| Primary Vaccination, without Booster Vaccination (Control Group) | Primary Vaccination, Plus mRNA Booster Vaccination (Intervention Group) | Intervention Effect | ||
|---|---|---|---|---|
| R [95% CI] | R [95% CI] | RD [95% CI] | RR [95% CI] | |
| Severe COVID-19 | 0.31 [0.19; 0.42] | 0.18 [0.15; 0.21] | −0.13 [−0.24; −0.01] | 0.59 [0.33; 0.85] |
| ICU admission | 0.14 [0.06; 0.21] | 0.10 [0.08; 0.12] | −0.04 [−0.11; 0.04] | 0.74 [0.22; 1.26] |
| In-hospital mortality | 0.24 [0.12; 0.35] | 0.11 [0.09; 0.13] | −0.13 [−0.25; 0.00] | 0.47 [0.15; 0.79] |
| Outcome: Severe COVID-19 | ||||
| Homologous Prime-Boost Scheme (Control Group) | Heterologous Prime-Boost Scheme (Intervention Group) | Intervention Effect | ||
| R [95% CI] | R [95% CI] | RD [95% CI] | RR [95% CI] | |
| Overall | 0.20 [0.16; 0.23] | 0.16 [0.11; 0.20] | −0.04 [−0.10; 0.02] | 0.80 [0.53; 1.07] |
| Omicron BA.1 | 0.19 [0.12; 0.27] | 0.13 [0.06; 0.20] | −0.07 [−0.16; 0.03] | 0.65 [0.22; 1.07] |
| Omicron BA.2 | 0.19 [0.13; 0.24] | 0.14 [0.08; 0.21] | −0.04 [−0.13; 0.04] | 0.77 [0.32; 1.23] |
| Omicron BA.4/5 | 0.21 [0.13; 0.29] | 0.21 [0.10; 0.33] | 0.00 [−0.12; 0.12] | 1.01 [0.41; 1.61] |
| Outcome: ICU admission | ||||
| Homologous prime-boost scheme (control group) | Heterologous prime-boost scheme (intervention group) | Intervention effect | ||
| R [95% CI] | R [95% CI] | RD [95% CI] | RR [95% CI] | |
| Overall | 0.11 [0.08; 0.13] | 0.08 [0.04; 0.11] | −0.03 [−0.07; 0.02] | 0.73 [0.34; 1.12] |
| Omicron BA.1 | 0.08 [0.03; 0.13] | 0.04 [0.00; 0.08] | −0.04 [−0.10; 0.02] | 0.51 [0.00; 1.15] |
| Omicron BA.2 | 0.10 [0.06; 0.15] | 0.06 [0.01; 0.11] | −0.04 [−0.11; 0.02] | 0.59 [0.00; 1.19] |
| Omicron BA.4/5 | 0.15 [0.08; 0.23] | 0.17 [0.06; 0.28] | 0.02 [−0.10; 0.13] | 1.12 [0.27; 1.97] |
| Outcome: In-hospital mortality | ||||
| Homologous prime-boost scheme (control group) | Heterologous prime-boost scheme (intervention group) | Intervention effect | ||
| R [95% CI] | R [95% CI] | RD [95% CI] | RR [95% CI] | |
| Overall | 0.12 [0.09; 0.15] | 0.11 [0.07; 0.15] | 0.00 [−0.05; 0.04] | 0.96 [0.55; 1.38] |
| Omicron BA.1 | 0.14 [0.07; 0.20] | 0.11 [0.04; 0.18] | −0.03 [−0.11; 0.06] | 0.81 [0.18; 1.45] |
| Omicron BA.2 | 0.12 [0.07; 0.16] | 0.11 [0.05; 0.16] | −0.01 [−0.09; 0.06] | 0.89 [0.23; 1.56] |
| Omicron BA.4/5 | 0.09 [0.03; 0.15] | 0.13 [0.04; 0.23] | 0.04 [−0.06; 0.14] | 1.42 [0.00; 2.53 × 105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meurisse, M.; Catteau, L.; van Loenhout, J.A.F.; Braeye, T.; De Mot, L.; Serrien, B.; Blot, K.; Cauët, E.; Van Oyen, H.; Cuypers, L.; et al. Homologous and Heterologous Prime-Boost Vaccination: Impact on Clinical Severity of SARS-CoV-2 Omicron Infection among Hospitalized COVID-19 Patients in Belgium. Vaccines 2023, 11, 378. https://doi.org/10.3390/vaccines11020378
Meurisse M, Catteau L, van Loenhout JAF, Braeye T, De Mot L, Serrien B, Blot K, Cauët E, Van Oyen H, Cuypers L, et al. Homologous and Heterologous Prime-Boost Vaccination: Impact on Clinical Severity of SARS-CoV-2 Omicron Infection among Hospitalized COVID-19 Patients in Belgium. Vaccines. 2023; 11(2):378. https://doi.org/10.3390/vaccines11020378
Chicago/Turabian StyleMeurisse, Marjan, Lucy Catteau, Joris A. F. van Loenhout, Toon Braeye, Laurane De Mot, Ben Serrien, Koen Blot, Emilie Cauët, Herman Van Oyen, Lize Cuypers, and et al. 2023. "Homologous and Heterologous Prime-Boost Vaccination: Impact on Clinical Severity of SARS-CoV-2 Omicron Infection among Hospitalized COVID-19 Patients in Belgium" Vaccines 11, no. 2: 378. https://doi.org/10.3390/vaccines11020378
APA StyleMeurisse, M., Catteau, L., van Loenhout, J. A. F., Braeye, T., De Mot, L., Serrien, B., Blot, K., Cauët, E., Van Oyen, H., Cuypers, L., Belgian Collaborative Group on COVID-19 Hospital Surveillance, COVID-19 Genomics Belgium Consortium, Robert, A., & Van Goethem, N. (2023). Homologous and Heterologous Prime-Boost Vaccination: Impact on Clinical Severity of SARS-CoV-2 Omicron Infection among Hospitalized COVID-19 Patients in Belgium. Vaccines, 11(2), 378. https://doi.org/10.3390/vaccines11020378






