Children’s SARS-CoV-2 Infection and Their Vaccination
Abstract
:1. Introduction
2. Vaccine Hesitancy in Parents to Have Their Children Vaccinated
3. Multi-Inflammatory Syndrome in Children
4. Status of Vaccination in Children
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acuti Martellucci, C.; Flacco, M.E.; Cappadona, R.; Bravi, F.; Mantovani, L.; Manzoli, L. SARS-CoV-2 pandemic: An overview. Adv. Biol. Regul. 2020, 77, 100736. [Google Scholar] [CrossRef]
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiao, Z.; Ye, K.; He, X.; Sun, B.; Qin, Z.; Yu, J.; Yao, J.; Wu, Q.; Bao, Z.; et al. SARS-CoV-2: Characteristics and current advances in research. Virol. J. 2020, 17, 117. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Islam, S.; Islam, T.; Islam, M.R. New Coronavirus Variants are Creating More Challenges to Global Healthcare System: A Brief Report on the Current Knowledge. Clin. Pathol. 2022, 15, 2632010X221075584. [Google Scholar] [CrossRef]
- Otto, S.P.; Day, T.; Arino, J.; Colijn, C.; Dushoff, J.; Li, M.; Mechai, S.; Van Domselaar, G.; Wu, J.; Earn, D.J.D.; et al. The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Curr. Biol. 2021, 31, R918–R929. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Bucholc, M.; Bradley, D.; Bennett, D.; Patterson, L.; Spiers, R.; Gibson, D.; Van Woerden, H.; Bjourson, A.J. Identifying pre-existing conditions and multimorbidity patterns associated with in-hospital mortality in patients with COVID-19. Sci. Rep. 2022, 12, 17313. [Google Scholar] [CrossRef] [PubMed]
- Dadras, O.; SeyedAlinaghi, S.; Karimi, A.; Shamsabadi, A.; Qaderi, K.; Ramezani, M.; Mirghaderi, S.P.; Mahdiabadi, S.; Vahedi, F.; Saeidi, S.; et al. COVID-19 mortality and its predictors in the elderly: A systematic review. Health Sci. Rep. 2022, 5, e657. [Google Scholar] [CrossRef]
- Brodin, P. SARS-CoV-2 infections in children: Understanding diverse outcomes. Immunity 2022, 55, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.A.; Herold, K.C.; Herold, B.C.; Chou, J.; Randolph, A.; Kane, B.; McFarland, S.; Gurdasani, D.; Pagel, C.; Hotez, P.; et al. COVID-19 and children. Science 2022, 377, 1144–1149. [Google Scholar] [CrossRef]
- Viner, R.M.; Mytton, O.T.; Bonell, C.; Melendez-Torres, G.J.; Ward, J.; Hudson, L.; Waddington, C.; Thomas, J.; Russell, S.; van der Klis, F.; et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared with Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2021, 175, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2021, 106, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Ortega Pacheco, Y.J.; Barrero Toncel, V.I. The impact of school closure on children’s well-being during the COVID-19 pandemic. Asian J. Psychiatr. 2022, 67, 102957. [Google Scholar] [CrossRef] [PubMed]
- Khemiri, H.; Ayouni, K.; Triki, H.; Haddad-Boubaker, S. SARS-CoV-2 infection in pediatric population before and during the Delta (B.1.617.2) and Omicron (B.1.1.529) variants era. Virol. J. 2022, 19, 144. [Google Scholar] [CrossRef]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, Cd015477. [Google Scholar] [CrossRef]
- Leech, G.; Rogers-Smith, C.; Monrad, J.T.; Sandbrink, J.B.; Snodin, B.; Zinkov, R.; Rader, B.; Brownstein, J.S.; Gal, Y.; Bhatt, S.; et al. Mask wearing in community settings reduces SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. USA 2022, 119, e2119266119. [Google Scholar] [CrossRef] [PubMed]
- Talic, S.; Shah, S.; Wild, H.; Gasevic, D.; Maharaj, A.; Ademi, Z.; Li, X.; Xu, W.; Mesa-Eguiagaray, I.; Rostron, J.; et al. Effectiveness of public health measures in reducing the incidence of COVID-19, SARS-CoV-2 transmission, and COVID-19 mortality: Systematic review and meta-analysis. BMJ 2021, 375, e068302. [Google Scholar] [CrossRef]
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef]
- Gupta, S.L.; Jaiswal, R.K. Neutralizing antibody: A savior in the COVID-19 disease. Mol. Biol. Rep. 2022, 49, 2465–2474. [Google Scholar] [CrossRef]
- Gupta, S.L.; Jaiswal, R.K. Relevant of neutralizing antibody during SARS-CoV-2 infection and their therapeutic usage. Mol. Biol. Rep. 2022, 49, 10137–10140. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Ma, X.; Yu, H.; Zhang, Z.; Bian, P.; Han, Y.; Sun, J.; Liu, Y.; Yang, C.; Geng, J.; et al. The different clinical characteristics of corona virus disease cases between children and their families in China—The character of children with COVID-19. Emerg. Microbes Infect. 2020, 9, 707–713. [Google Scholar] [CrossRef]
- Chen, F.; Tian, Y.; Zhang, L.; Shi, Y. The role of children in household transmission of COVID-19: A systematic review and meta-analysis. Int. J. Infect. Dis. 2022, 122, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, B.D.; Goerne, T.; Geweniger, A.; Henneke, P.; Elling, R. Intra-Household and Close-Contact SARS-CoV-2 Transmission Among Children—A Systematic Review. Front. Pediatr. 2021, 9, 613292. [Google Scholar] [CrossRef] [PubMed]
- Yonker, L.M.; Boucau, J.; Regan, J.; Choudhary, M.C.; Burns, M.D.; Young, N.; Farkas, E.J.; Davis, J.P.; Moschovis, P.P.; Bernard Kinane, T.; et al. Virologic Features of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children. J. Infect. Dis. 2021, 224, 1821–1829. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Qian, H.; Cao, S.; Dong, B.; Yan, X.; Luo, S.; Zhou, M.; Zhou, S.; Ning, B.; Zhao, L. Is there possibility of vertical transmission of COVID-19: A systematic review. Transl. Pediatr. 2021, 10, 423–434. [Google Scholar] [CrossRef] [PubMed]
- AbdelMassih, A.; Fouda, R.; Essam, R.; Negm, A.; Khalil, D.; Habib, D.; Afdal, G.; Ismail, H.-A.; Aly, H.; Genedy, I.; et al. COVID-19 during pregnancy should we really worry from vertical transmission or rather from fetal hypoxia and placental insufficiency? A systematic review. Egypt. Pediatr. Assoc. Gaz. 2021, 69, 12. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Wake, A.D. Intrauterine Vertical Transmission of SARS-CoV-2 Infection Among Confirmed Cases of Pregnant Women: “A Double Burden for the Pregnant Women”—A Systematic Review. Glob. Pediatr. Health 2022, 9, 2333794X221089765. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, J.; Shi, Y.; Yi, M. Research progress in vertical transmission of SARS-CoV-2 among infants born to mothers with COVID-19. Future Virol. 2022, 17, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, J.C.; Carrascosa Moreno, N.P.; Tovar-Gálvez, M.I.; Cortés-Martín, J.; Liñán-González, A.; Alvarado Olmedo, L.; Rodríguez-Blanque, R. COVID-19 in Pregnant Women, Maternal-Fetal Involvement, and Vertical Mother-to-Child Transmission: A Systematic Review. Biomedicines 2022, 10, 2554. [Google Scholar] [CrossRef]
- Shook, L.L.; Atyeo, C.G.; Yonker, L.M.; Fasano, A.; Gray, K.J.; Alter, G.; Edlow, A.G. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA 2022, 327, 1087–1089. [Google Scholar] [CrossRef]
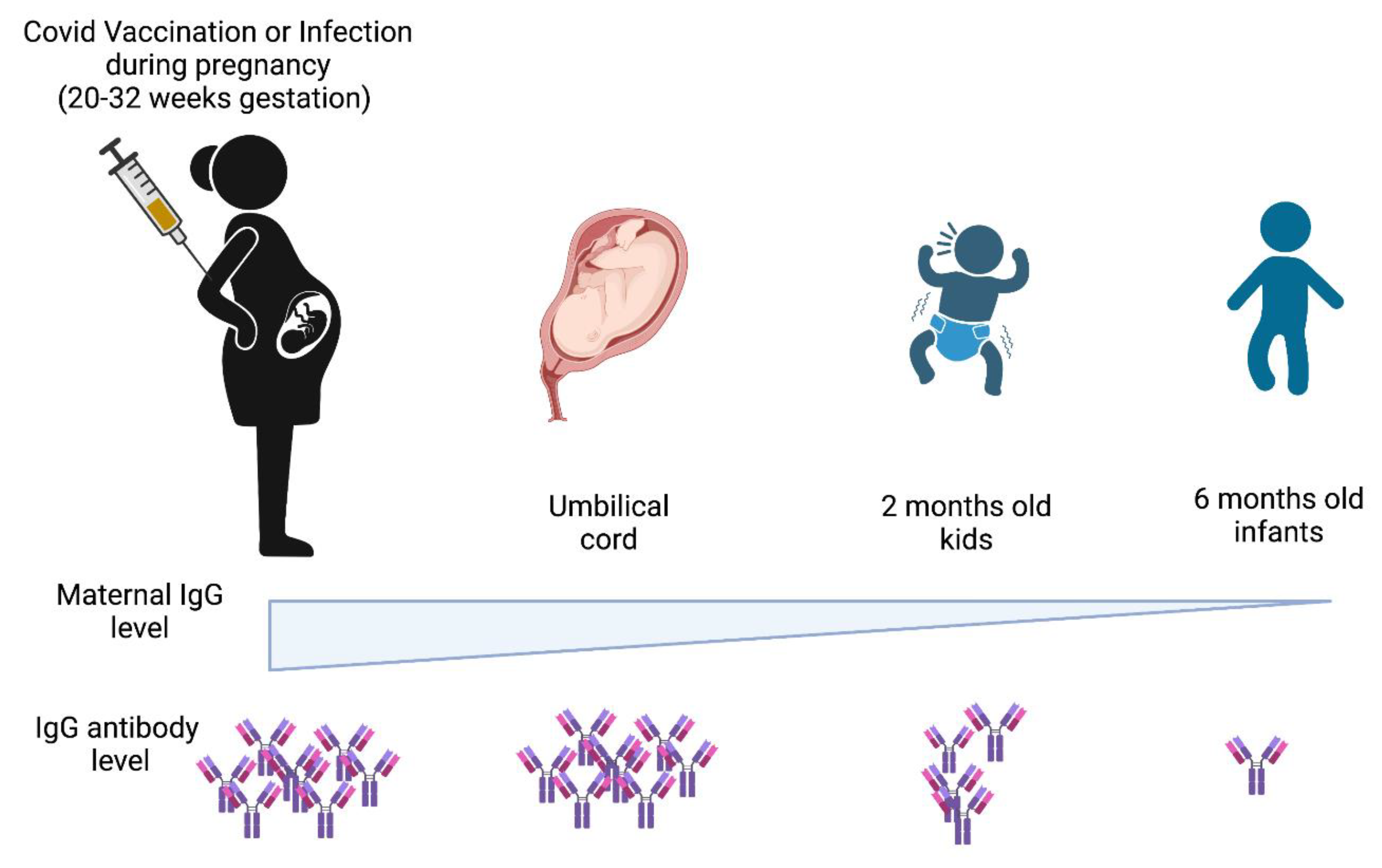
- Liu, S.; Zhong, J.; Zhang, D. Transplacental Transfer of Maternal Antibody against SARS-CoV-2 and Its Influencing Factors: A Review. Vaccines 2022, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.D.; Muir, C.; Atyeo, C.; Davis, J.P.; Demidkin, S.; Akinwunmi, B.; Fasano, A.; Gray, K.J.; Alter, G.; Shook, L.L.; et al. Relationship between Anti-Spike Antibodies and Risk of SARS-CoV-2 Infection in Infants Born to COVID-19 Vaccinated Mothers. Vaccines 2022, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, P.; Zheng, J.; Liu, P.; Wei, C.; Guo, J.; Zhang, Y.; Zhao, D. Dynamic changes of acquired maternal SARS-CoV-2 IgG in infants. Sci. Rep. 2021, 11, 8021. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG Placental Transfer in Healthy and Pathological Pregnancies. Clin. Dev. Immunol. 2012, 2012, 985646. [Google Scholar] [CrossRef]
- Atyeo, C.G.; Shook, L.L.; Brigida, S.; De Guzman, R.M.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.M.; Sheehan, M.L.; McSweeney, E.; et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat. Commun. 2022, 13, 3571. [Google Scholar] [CrossRef]
- Laguila Altoé, A.; Marques Mambriz, A.P.; Cardozo, D.M.; Valentini Zacarias, J.M.; Laguila Visentainer, J.E.; Bahls-Pinto, L.D. Vaccine Protection Through Placenta and Breastfeeding: The Unmet Topic in COVID-19 Pandemic. Front. Immunol. 2022, 13, 910138. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, C.; Cantarutti, A.; Costenaro, P.; Donà, D.; Bonfante, F.; Cosma, C.; Ferrarese, M.; Cozzani, S.; Petrara, M.R.; Carmona, F.; et al. Long-term Immune Response to SARS-CoV-2 Infection Among Children and Adults After Mild Infection. JAMA Netw. Open 2022, 5, e2221616. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Novak, T.; Hecker, J.; Grubbs, G.; Zahra, F.T.; Bellusci, L.; Pourhashemi, S.; Chou, J.; Moffitt, K.; Halasa, N.B.; et al. Cross-reactive immunity against the SARS-CoV-2 Omicron variant is low in pediatric patients with prior COVID-19 or MIS-C. Nat. Commun. 2022, 13, 2979. [Google Scholar] [CrossRef]
- Wang, L.; Berger, N.A.; Kaelber, D.C.; Davis, P.B.; Volkow, N.D.; Xu, R. Incidence Rates and Clinical Outcomes of SARS-CoV-2 Infection With the Omicron and Delta Variants in Children Younger Than 5 Years in the US. JAMA Pediatr. 2022, 176, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.Y.; Jeong, H.; Kim, Y. Identifying susceptibility of children and adolescents to the Omicron variant (B.1.1.529). BMC Med. 2022, 20, 451. [Google Scholar] [CrossRef] [PubMed]
- Taytard, J.; Prevost, B.; Schnuriger, A.; Aubertin, G.; Berdah, L.; Bitton, L.; Dupond-Athenor, A.; Thouvenin, G.; Nathan, N.; Corvol, H. SARS-CoV-2 B.1.1.529 (Omicron) Variant Causes an Unprecedented Surge in Children Hospitalizations and Distinct Clinical Presentation Compared to the SARS-CoV-2 B.1.617.2 (Delta) Variant. Front. Pediatr. 2022, 10, 932170. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.A.; Preston-Hurlburt, P.; Dai, Y.; Aschner, C.B.; Cheshenko, N.; Galen, B.; Garforth, S.J.; Herrera, N.G.; Jangra, R.K.; Morano, N.C.; et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 2020, 12, eabd5487. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.A.; Sy, S.; Galen, B.; Goldstein, D.Y.; Orner, E.; Keller, M.J.; Herold, K.C.; Herold, B.C. Natural mucosal barriers and COVID-19 in children. JCI Insight 2021, 6, e148694. [Google Scholar] [CrossRef]
- Yoshida, M.; Worlock, K.B.; Huang, N.; Lindeboom, R.G.H.; Butler, C.R.; Kumasaka, N.; Dominguez Conde, C.; Mamanova, L.; Bolt, L.; Richardson, L.; et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature 2022, 602, 321–327. [Google Scholar] [CrossRef]
- Loske, J.; Röhmel, J.; Lukassen, S.; Stricker, S.; Magalhães, V.G.; Liebig, J.; Chua, R.L.; Thürmann, L.; Messingschlager, M.; Seegebarth, A.; et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2022, 40, 319–324. [Google Scholar] [CrossRef]
- Rowntree, L.C.; Nguyen, T.H.O.; Kedzierski, L.; Neeland, M.R.; Petersen, J.; Crawford, J.C.; Allen, L.F.; Clemens, E.B.; Chua, B.; McQuilten, H.A.; et al. SARS-CoV-2-specific T cell memory with common TCRαβ motifs is established in unvaccinated children who seroconvert after infection. Immunity 2022, 55, 1299–1315.e1294. [Google Scholar] [CrossRef]
- Peeples, L. Understanding Kids and COVID. Proc. Natl. Acad. Sci. USA 2022, 119, e2203753119. [Google Scholar] [CrossRef]
- Kaaijk, P.; Olivo Pimentel, V.; Emmelot, M.E.; Poelen, M.C.M.; Cevirgel, A.; Schepp, R.M.; den Hartog, G.; Reukers, D.F.M.; Beckers, L.; van Beek, J.; et al. Children and Adults With Mild COVID-19: Dynamics of the Memory T Cell Response up to 10 Months. Front. Immunol. 2022, 13, 307. [Google Scholar] [CrossRef]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Moss, P.; Ladhani, S. Cross-reactive adaptive immunity against coronaviruses in young children. Nat. Immunol. 2022, 23, 11–12. [Google Scholar] [CrossRef]
- Cohen, C.A.; Li, A.P.Y.; Hachim, A.; Hui, D.S.C.; Kwan, M.Y.W.; Tsang, O.T.Y.; Chiu, S.S.; Chan, W.H.; Yau, Y.S.; Kavian, N.; et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat. Commun. 2021, 12, 4678. [Google Scholar] [CrossRef]
- Fonte, L.; Ginori, M.; García, G.; Hernández, Y.; de Armas, Y.; Calderón, E.J. Nonspecific Effects of Infant Vaccines Make Children More Resistant to SARS-CoV-2 Infection. Children 2022, 9, 1858. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, C.; Clemmensen, A.; Sparrewath, C.; Tetens, M.; Krogfelt, K.A. Children Naturally Evading COVID-19—Why Children Differ from Adults. COVID 2022, 2, 369–378. [Google Scholar] [CrossRef]
- Fonte, L.; Acosta, A.; Sarmiento, M.E.; Ginori, M.; García, G.; Norazmi, M.N. COVID-19 Lethality in Sub-Saharan Africa and Helminth Immune Modulation. Front. Immunol. 2020, 11, 574910. [Google Scholar] [CrossRef]
- Tschan, S.L.; Bolliger, D. Coagulation and Aging: Implications for the Anesthesiologist. Curr. Anesthesiol. Rep. 2021, 11, 387–395. [Google Scholar] [CrossRef]
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the human innate immune system. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef]
- Tizazu, A.M.; Mengist, H.M.; Demeke, G. Aging, inflammaging and immunosenescence as risk factors of severe COVID-19. Immun. Ageing 2022, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, J.R.; Pettifor, A.; Janes, H.; Brown, E.R.; Kublin, J.G.; Stephenson, K.E. COVID-19 Vaccines and SARS-CoV-2 Transmission in the Era of New Variants: A Review and Perspective. Open Forum Infect. Dis. 2022, 9, ofac124. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.; Thompson, L.A.; Filipp, S.L.; Ryan, K. COVID-19 vaccine perceptions and hesitancy amongst parents of school-aged children during the pediatric vaccine rollout. Vaccine 2022, 40, 6680–6687. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Orr, C.J.; Delamater, A.M.; Flower, K.B.; Heerman, W.J.; Perrin, E.M.; Rothman, R.L.; Yin, H.S.; Sanders, L. COVID-19 vaccine hesitancy among low-income, racially and ethnically diverse US parents. Patient Educ. Couns. 2022, 105, 2771–2777. [Google Scholar] [CrossRef]
- Ruiz, J.B.; Bell, R.A. Parental COVID-19 Vaccine Hesitancy in the United States. Public Health Rep. 2022, 137, 1162–1169. [Google Scholar] [CrossRef]
- Yigit, M.; Ozkaya-Parlakay, A.; Senel, E. Evaluation of COVID-19 Vaccine Refusal in Parents. Pediatr. Infect. Dis. J. 2021, 40, e134–e136. [Google Scholar] [CrossRef] [PubMed]
- Skafle, I.; Nordahl-Hansen, A.; Quintana, D.S.; Wynn, R.; Gabarron, E. Misinformation About COVID-19 Vaccines on Social Media: Rapid Review. J. Med. Internet Res. 2022, 24, e37367. [Google Scholar] [CrossRef]
- Lee, S.K.; Sun, J.; Jang, S.; Connelly, S. Misinformation of COVID-19 vaccines and vaccine hesitancy. Sci. Rep. 2022, 12, 13681. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.M.; Kennedy, A.; Basket, M.; Sheedy, K. Exploring the choice to refuse or delay vaccines: A national survey of parents of 6- through 23-month-olds. Acad. Pediatr. 2012, 12, 375–383. [Google Scholar] [CrossRef]
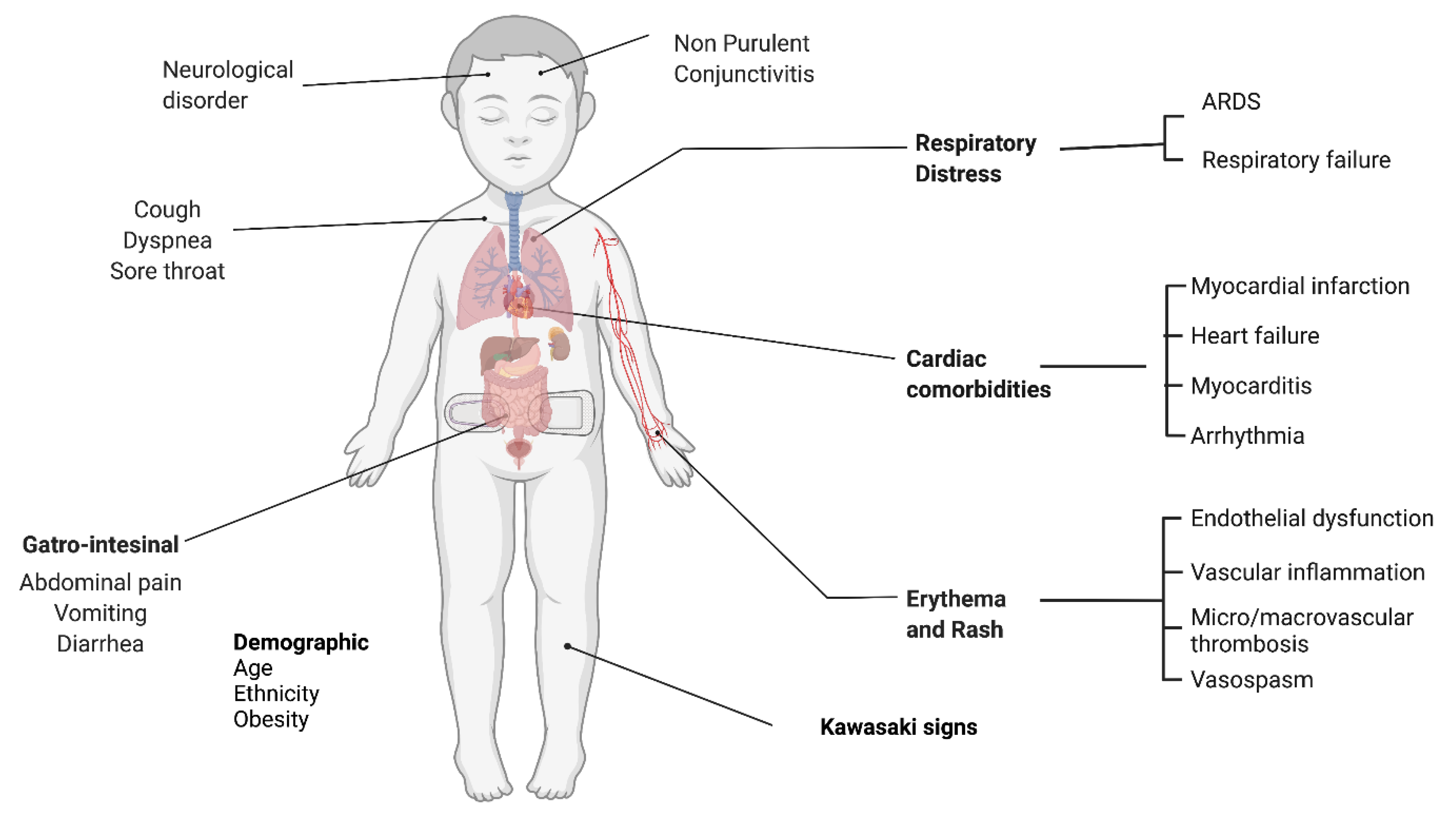
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef]
- Santos, M.O.; Gonçalves, L.C.; Silva, P.A.N.; Moreira, A.L.E.; Ito, C.R.M.; Peixoto, F.A.O.; Wastowski, I.J.; Carneiro, L.C.; Avelino, M.A.G. Multisystem inflammatory syndrome (MIS-C): A systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. J. Pediatr. 2022, 98, 338–349. [Google Scholar] [CrossRef]
- Abrams, J.Y.; Godfred-Cato, S.E.; Oster, M.E.; Chow, E.J.; Koumans, E.H.; Bryant, B.; Leung, J.W.; Belay, E.D. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review. J. Pediatr. 2020, 226, 45–54.e41. [Google Scholar] [CrossRef]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Thomas, P.G.; Randolph, A.G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 2022, 23, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.A.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Diorio, C.; Kuri-Cervantes, L.; Alanio, C.; Pampena, M.B.; Wu, J.E.; Chen, Z.; et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci. Immunol. 2021, 6, eabf7570. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020, 183, 968–981.e967. [Google Scholar] [CrossRef]
- Holm, M.; Espenhain, L.; Glenthøj, J.; Schmidt, L.S.; Nordly, S.B.; Hartling, U.B.; Nygaard, U. Risk and Phenotype of Multisystem Inflammatory Syndrome in Vaccinated and Unvaccinated Danish Children Before and During the Omicron Wave. JAMA Pediatr. 2022, 176, 821–823. [Google Scholar] [CrossRef]
- Levy, N.; Koppel, J.H.; Kaplan, O.; Yechiam, H.; Shahar-Nissan, K.; Cohen, N.K.; Shavit, I. Severity and Incidence of Multisystem Inflammatory Syndrome in Children During 3 SARS-CoV-2 Pandemic Waves in Israel. JAMA 2022, 327, 2452–2454. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2021, 386, 35–46. [Google Scholar] [CrossRef]
- Fang, Z.; Monteiro, V.S.; Hahn, A.M.; Grubaugh, N.D.; Lucas, C.; Chen, S. Bivalent mRNA vaccine booster induces robust antibody immunity against Omicron lineages BA.2, BA.2.12.1, BA.2.75 and BA.5. Cell Discov. 2022, 8, 108. [Google Scholar] [CrossRef]
- Davis-Gardner, M.E.; Lai, L.; Wali, B.; Samaha, H.; Solis, D.; Lee, M.; Porter-Morrison, A.; Hentenaar, I.T.; Yamamoto, F.; Godbole, S.; et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA Bivalent Booster. N. Engl. J. Med. 2022, 388, 183–185. [Google Scholar] [CrossRef]
- Gupta, S.L.; Jaiswal, R.K. An Assessment of the Bivalent Vaccine as a Second Booster for COVID-19. Vaccines 2023, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Karron, R.A.; Garcia Quesada, M.; Schappell, E.A.; Schmidt, S.D.; Deloria Knoll, M.; Hetrich, M.K.; Veguilla, V.; Doria-Rose, N.; Dawood, F.S. Binding and neutralizing antibody responses to SARS-CoV-2 in very young children exceed those in adults. JCI Insight 2022, 7, e157963. [Google Scholar] [CrossRef]
- Gupta, S.L.; Mantus, G.; Manning, K.E.; Ellis, M.; Patel, M.; Ciric, C.R.; Lu, A.; Turner, J.S.; O’Halloran, J.A.; Presti, R.M.; et al. Loss of Pfizer (BNT162b2) Vaccine-Induced Antibody Responses against the SARS-CoV-2 Omicron Variant in Adolescents and Adults. J. Virol. 2022, 96, e0058222. [Google Scholar] [CrossRef]
- González, S.; Olszevicki, S.; Gaiano, A.; Baino, A.N.V.; Regairaz, L.; Salazar, M.; Pesci, S.; Marín, L.; Martínez, V.V.G.; Varela, T.; et al. Effectiveness of BBIBP-CorV, BNT162b2 and mRNA-1273 vaccines against hospitalisations among children and adolescents during the Omicron outbreak in Argentina: A retrospective cohort study. Lancet Reg. Health Am. 2022, 13, 100316. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; González, C.; Acevedo, J.; Pizarro, A.; Vergara, V.; Soto-Marchant, M.; Gilabert, R.; Flores, J.C.; et al. Effectiveness of CoronaVac in children 3–5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat. Med. 2022, 28, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Kasi, S.G.; Dhir, S.K.; Shah, A.; Shivananda, S.; Verma, S.; Marathe, S.; Chatterjee, K.; Agarwalla, S.; Srirampur, S.; Kalyani, S.; et al. Coronavirus Disease 2019 (COVID-19) Vaccination for Children: Position Statement of Indian Academy of Pediatrics Advisory Committee on Vaccination and Immunization Practices. Indian Pediatr. 2022, 59, 51–57. [Google Scholar] [CrossRef]
- Mariatulqabtiah, A.R.; Buttigieg, K.R. COVID-19 vaccinations for children. Lancet Infect. Dis. 2022, 22, 1255–1256. [Google Scholar] [CrossRef]
- Tian, F.; Yang, R.; Chen, Z. Safety and efficacy of COVID-19 vaccines in children and adolescents: A systematic review of randomized controlled trials. J. Med. Virol. 2022, 94, 4644–4653. [Google Scholar] [CrossRef]
- Tan, S.H.X.; Cook, A.R.; Heng, D.; Ong, B.; Lye, D.C.; Tan, K.B. Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 387, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Greish, K.; Alawadhi, A.; Jaradat, A.; Almarabheh, A.; AlMadhi, M.; Jawad, J.; Alsaffar, B.; Alalawi, E.; Alsayyad, A.; Merza, A.; et al. Safety and Immunogenicity of COVID-19 BBIBP-CorV Vaccine in Children 3-12 Years Old. Vaccines 2022, 10, 586. [Google Scholar] [CrossRef]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: A double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 1645–1653. [Google Scholar] [CrossRef]
- Zhu, F.; Jin, P.; Zhu, T.; Wang, W.; Ye, H.; Pan, H.; Hou, L.; Li, J.; Wang, X.; Wu, S.; et al. Safety and Immunogenicity of a Recombinant Adenovirus Type-5-Vectored Coronavirus Disease 2019 (COVID-19) Vaccine With a Homologous Prime-Boost Regimen in Healthy Participants Aged ≥6 Years: A Randomized, Double-Blind, Placebo-Controlled, Phase 2b Trial. Clin. Infect. Dis. 2022, 75, e783–e791. [Google Scholar] [CrossRef] [PubMed]
- Vadrevu, K.M.; Reddy, S.; Jogdand, H.; Ganneru, B.; Mirza, N.; Tripathy, V.N.; Singh, C.; Khalatkar, V.; Prasanth, S.; Rai, S.; et al. Immunogenicity and reactogenicity of an inactivated SARS-CoV-2 vaccine (BBV152) in children aged 2-18 years: Interim data from an open-label, non-randomised, age de-escalation phase 2/3 study. Lancet Infect. Dis. 2022, 22, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Thuluva, S.; Paradkar, V.; Gunneri, S.; Yerroju, V.; Mogulla, R.R.; Suneetha, P.V.; Turaga, K.; Kyasani, M.; Manoharan, S.K.; Adabala, S.; et al. Safety, tolerability and immunogenicity of Biological E’s CORBEVAX™ vaccine in children and adolescents: A prospective, randomised, double-blind, placebo controlled, phase-2/3 study. Vaccine 2022, 40, 7130–7140. [Google Scholar] [CrossRef]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Li, G.; Cappuccini, F.; Marchevsky, N.G.; Aley, P.K.; Aley, R.; Anslow, R.; Bibi, S.; Cathie, K.; Clutterbuck, E.; Faust, S.N.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine in children aged 6–17 years: A preliminary report of COV006, a phase 2 single-blind, randomised, controlled trial. Lancet 2022, 399, 2212–2225. [Google Scholar] [CrossRef]
| Fully Vaccinated People | Percent of US Population |
|---|---|
| Total | 68.7% |
| Population ≥ 5 Years of Age | 72.8% |
| Population ≥ 12 Years of Age | 76.9% |
| Population ≥ 18 Years of Age | 78.5% |
| Population ≥ 65 Years of Age | 93.7% |
| Fully Vaccinated People | Per 100 Persons |
|---|---|
| World | 64.51 |
| USA | 68.42 |
| India | 68.94 |
| France | 78.9 |
| Germany | 76.37 |
| Brazil | 79.52 |
| Japan | 81.54 |
| Republic of Korea | 87.17 |
| Italy | 82.95 |
| The United Kingdom | 74.59 |
| Russian Federation | 53.6 |
| Pfizer-BioNTech Vaccine Authorized Age | Dose | Usage Status |
|---|---|---|
| 16 years and older (Comirnaty brand name) | 2 dose primary series (30 µg/dose) | Fully licensed |
| 12–16 years old | 2 dose primary series. (30 µg/dose) | Fully approved |
| 5–11 years old | 2 dose primary series. (10 µg/dose) | Under EUA and not fully approved |
| 6 months- 4 years old | 3 dose primary series (3 µg/dose) | Under EUA and not fully approved |
| Moderna vaccine authorized age | ||
| 17 years and older (Spikevax brand name) | 2 dose primary series (100 µg/dose) | Fully licensed |
| 12–16 years old | 2 dose primary series (100 µg/dose) | Under EUA |
| 6–11 years old | 2 dose primary series (50 µg/dose) | Under EUA |
| Novovax vaccine authorized age | ||
| 12 years and older | 2 dose primary series (0.5 mL/dose) | Under EUA |
| Coronavac-Sinovac and BBIBP-CorV (Sinopharm) | ||
| 3–17 years | 2 doses (0.5 mL/dose) | Approved by China officials [92,93,94] |
| Ad5-nCoV (CanSino) | ||
| 6–17 years | Phase 2b clinical trial in China [95] | |
| Covavax from Novavax company | ||
| 12–17 years | 10 doses (0.5 mL/dose) | |
| 6 months to 11 years | 2 doses of 5 μg | https://clinicaltrials.gov/ct2/show/NCT05468736, accessed on 30 November 2022 |
| Covaxin (BB152) by Bharat Biotech | ||
| 12–17 Years | Approved by Indian Officials | |
| 2–18 | Phase 2–3 clinical trial in India [96] | |
| Corbevax | 2 doses (0.5 mL/dose) | [97] |
| 5–17-year-old | ||
| ZyCoV-D (Zydus Cadila) | Phase 3 clinical trial in India [98] | |
| 12–17 years old | ||
| ChAdOx1 nCov-19 (AZD1222) | Phase 2 clinical trial in UK | |
| 6–17 years old | 2 doses (5 × 1010) viral particle | [99] |
| Pfizer-BioNTech Bivalent Vaccine Authorized Age | Dose | Usage Status |
|---|---|---|
| 16 years and older | 1 dose (30 µg/dose) | Under EUA |
| 12–16 years old | 1 dose (30 µg/dose) | Under EUA |
| 5–11 years old | Under EUA | |
| 6 months–4 years old | Not approved yet | |
| Moderna vaccine bivalent authorized age | ||
| 12 years and older | 1 dose (50 µg/dose) | Under EUA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.L.; Tyagi, R.; Dhar, A.; Oswal, N.; Khandelwal, A.; Jaiswal, R.K. Children’s SARS-CoV-2 Infection and Their Vaccination. Vaccines 2023, 11, 418. https://doi.org/10.3390/vaccines11020418
Gupta SL, Tyagi R, Dhar A, Oswal N, Khandelwal A, Jaiswal RK. Children’s SARS-CoV-2 Infection and Their Vaccination. Vaccines. 2023; 11(2):418. https://doi.org/10.3390/vaccines11020418
Chicago/Turabian StyleGupta, Sneh Lata, Rohit Tyagi, Atika Dhar, Neelam Oswal, Ankita Khandelwal, and Rishi Kumar Jaiswal. 2023. "Children’s SARS-CoV-2 Infection and Their Vaccination" Vaccines 11, no. 2: 418. https://doi.org/10.3390/vaccines11020418
APA StyleGupta, S. L., Tyagi, R., Dhar, A., Oswal, N., Khandelwal, A., & Jaiswal, R. K. (2023). Children’s SARS-CoV-2 Infection and Their Vaccination. Vaccines, 11(2), 418. https://doi.org/10.3390/vaccines11020418







