Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status
Abstract
:1. Introduction
2. Adenoviruses (AdVs) as a Vector for Vaccine Delivery
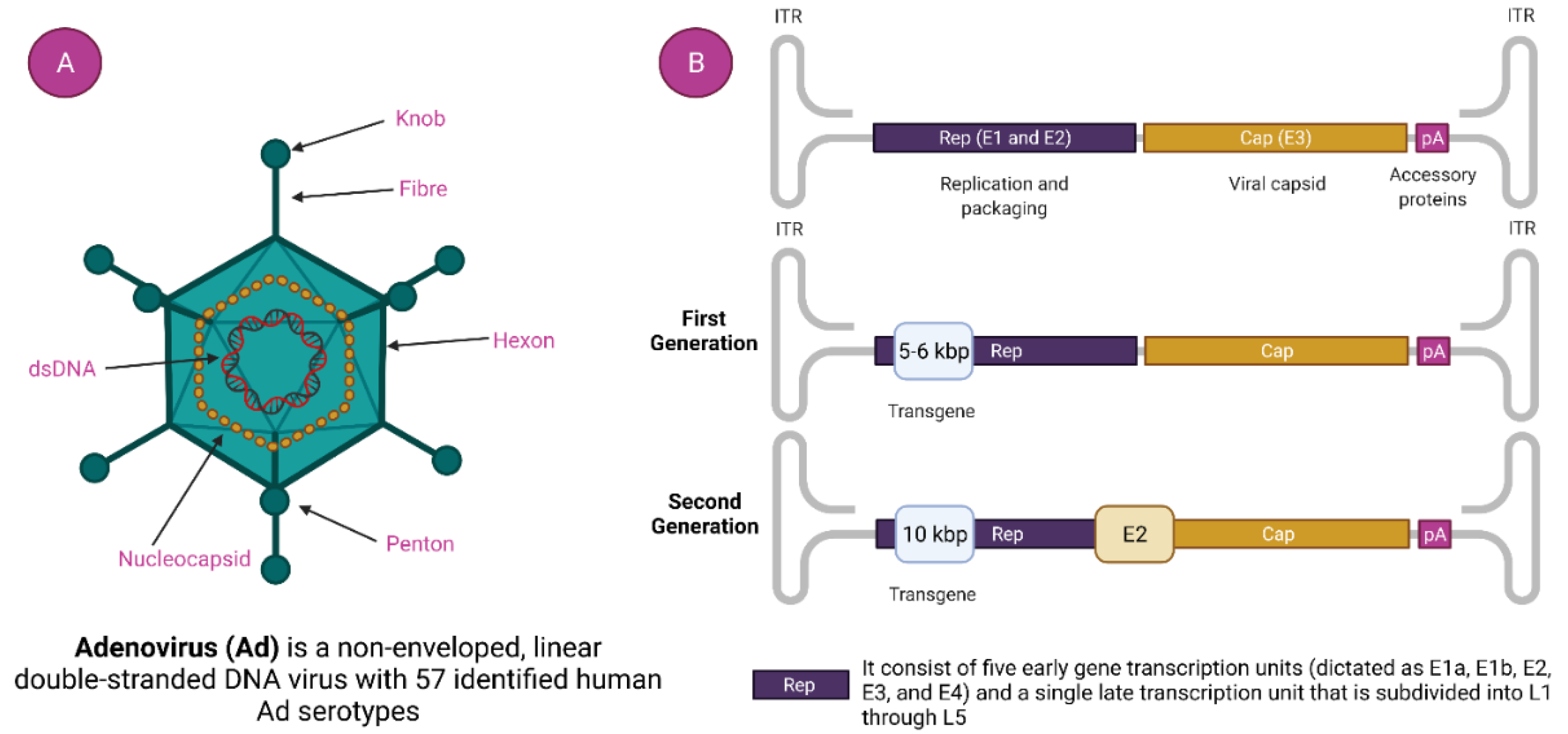
2.1. Characteristics of Adenovirus (AdVs)
2.2. Generation of Ad Viral Vector
2.2.1. First-generation Ad Viral Vector
2.2.2. Second Generation Ad Viral Vector
2.2.3. Third Generation (Gutless or High-Capacity Adenovirus Vector) Ad Viral Vector
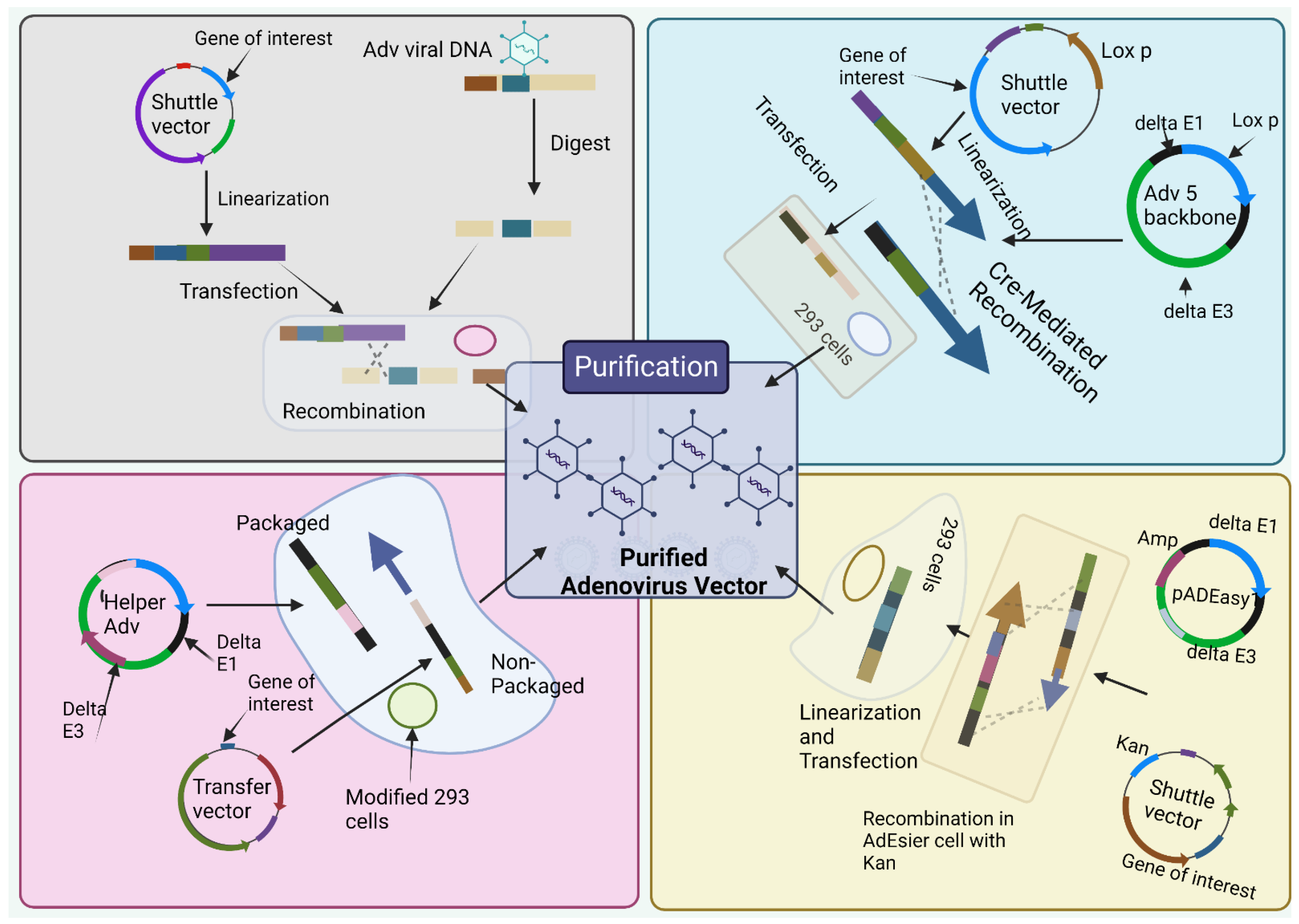
2.3. Recombinant Adenoviral Vector (rAdVs) Production
2.3.1. The Traditional Method
2.3.2. Cre/LoxP-Mediated Recombination
2.3.3. The AdEasy System
2.3.4. The Usage of Helper Adenovirus for the Construction of HC
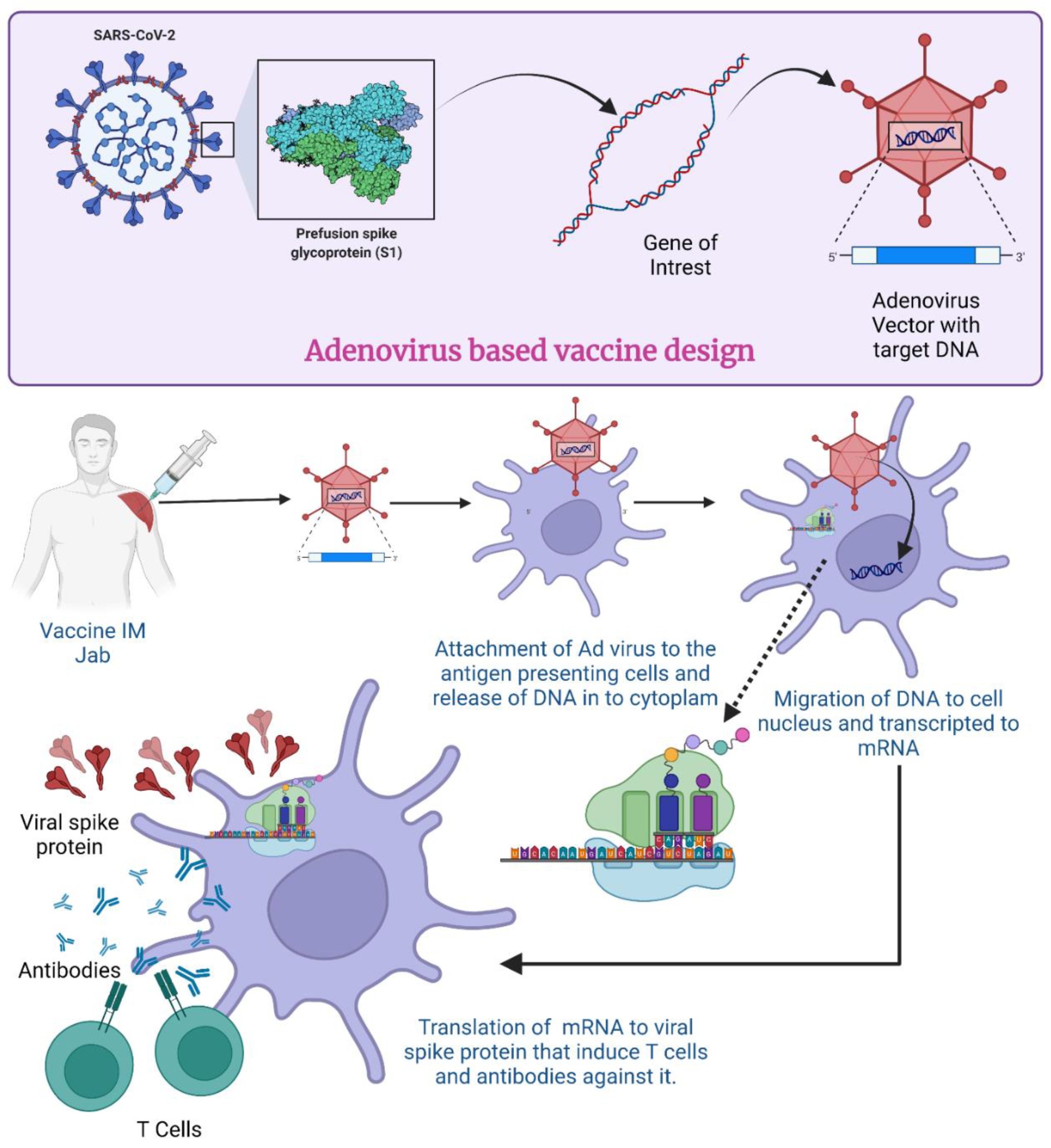
2.4. Vaccine Design/Process Development for COVID-19
Transgene Design
2.5. Mechanism of Action for Adenovirus Vector-Based Vaccine
3. Adeno Viral Vector-Based Vaccine-Based Platform for COVID-19: Intramuscular Injection
4. Ad vector-Based Vaccine Platform for COVID-19: Intranasal Delivery
4.1. Altimmune
4.2. AstraZeneca
4.3. Bharat Biotech-Washington University
4.4. CanSino Biologics Inc./Beijing Institute of Biotechnology
4.5. Ad Vaccines for SARS-CoV-2 Variants
5. Challenges to Adenoviral Vector Use for Vaccine Delivery
5.1. Pre-Existing Immunity
5.1.1. Use Rare Viruses as a Vector
5.1.2. Use Different Virus Vectors for Priming and Booster/Additional Dose
5.1.3. Different Routes of Immunization
5.1.4. Modification in Vector
5.2. Heterologous Immunity
5.3. Thrombocytopenia
6. Booster Dose Strategy
Importance of Anti-SARS-CoV2 Vaccination in Patients with Autoimmune Diseases
7. Concluding Remarks and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chavda, V.P.; Gajjar, N.; Shah, N.; Dave, D.J. Darunavir ethanolate: Repurposing an anti-HIV drug in COVID-19 treatment. Eur. J. Med. Chem. Rep. 2021, 3, 100013. [Google Scholar] [CrossRef]
- Gralinski, L.E.; Menachery, V.D. Return of the coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 2020, 20, 669–677. [Google Scholar] [CrossRef]
- Gupta, D.; Sahoo, A.K.; Singh, A. Ivermectin: Potential candidate for the treatment of COVID-19. Braz. J. Infect. Dis. 2020, 24, 369–371. [Google Scholar] [CrossRef]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Rahimi, A.; Mirzazadeh, A.; Tavakolpour, S. Genetics and genomics of SARS-CoV-2: A review of the literature with the special focus on genetic diversity and SARS-CoV-2 genome detection. Genomics 2021, 113, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.; Isom, D.G. One Year of SARS-CoV-2: How Much Has the Virus Changed? Biology 2021, 10, 91. [Google Scholar] [CrossRef]
- Mirtaleb, M.S.; Mirtaleb, A.H.; Nosrati, H.; Heshmatnia, J.; Falak, R.; Zolfaghari Emameh, R. Potential therapeutic agents to COVID-19: An update review on antiviral therapy, immunotherapy, and cell therapy. Biomed. Pharmacother. 2021, 138, 111518. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef]
- Basu, D.; Chavda, V.P.; Mehta, A.A. Therapeutics for COVID-19 and post COVID-19 complications: An update. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100086. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Kapadia, C.; Soni, S.; Prajapati, R.; Chauhan, S.C.; Yallapu, M.M.; Apostolopoulos, V. A global picture: Therapeutic perspectives for COVID-19. Immunotherapy 2022, 14, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, P.; Sargolzaei, S.; Bhardwaj, S.K.; Bhardwaj, V.; Dixit, C.; Kaushik, A. Grand Challenges in Bio-Nanotechnology to Manage the COVID-19 Pandemic. Front. Nanotechnol. 2020, 2, 5. [Google Scholar] [CrossRef]
- Tiwari, S.; Juneja, S.; Ghosal, A.; Bandara, N.; Khan, R.; Wallen, S.; Ramakrishna, S.; Kaushik, A. Antibacterial and antiviral high-performance nano-systems to mitigate new SARS-CoV-2 variants of concerns. Curr. Opin. Biomed. Eng. 2021, 21, 100363. [Google Scholar] [CrossRef]
- WHO COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 26 January 2022).
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Chavda, V.P.; Apostolopoulos, V. Omicron Variant (B.1.1.529) of SARS-CoV-2: Threat for the elderly? Maturitas 2022, 158, 78–81. [Google Scholar] [CrossRef]
- Chavda, V.P.; Apostolopoulos, V. Is Booster Dose Strategy Sufficient for Omicron Variant of SARS-CoV-2? Vaccines 2022, 10, 367. [Google Scholar] [CrossRef]
- Chavda, V.P.; Apostolopoulos, V. Global impact of delta plus variant and vaccination. Expert Rev. Vaccines 2022, 21, 597–600. [Google Scholar] [CrossRef]
- Petersen, E.; Ntoumi, F.; Hui, D.S.; Abubakar, A.; Kramer, L.D.; Obiero, C.; Tambyah, P.A.; Blumberg, L.; Yapi, R.; Al-Abri, S.; et al. Emergence of new SARS-CoV-2 Variant of Concern Omicron (B.1.1.529)—highlights Africa’s research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. Int. J. Infect. Dis. 2022, 114, 268–272. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Athalye, M.; Parikh, P.K.; Chhipa, A.S.; Patel, S.; Apostolopoulos, V. Replicating Viral Vector-Based Vaccines for COVID-19: Potential Avenue in Vaccination Arena. Viruses 2022, 14, 759. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. Npj Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Dahlke, C.; Addo, M.M. Recombinant vesicular stomatitis virus vector vaccines for WHO blueprint priority pathogens. Hum. Vaccines Immunother. 2019, 15, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Fast, P.E.; Modjarrad, K.; Clarke, D.K.; Martin, B.K.; Fusco, J.; Nichols, R.; Heppner, D.G.; Simon, J.K.; Dubey, S.; et al. rVSVΔG-ZEBOV-GP (also designated V920) recombinant vesicular stomatitis virus pseudotyped with Ebola Zaire Glycoprotein: Standardized template with key considerations for a risk/benefit assessment. Vaccine X 2019, 1, 100009. [Google Scholar] [CrossRef]
- Rollier, C.S.; Reyes-Sandoval, A.; Cottingham, M.G.; Ewer, K.; Hill, A.V.S. Viral vectors as vaccine platforms: Deployment in sight. Curr. Opin. Immunol. 2011, 23, 377–382. [Google Scholar] [CrossRef]
- Ramezanpour, B.; Haan, I.; Osterhaus, A.; Claassen, E. Vector-based genetically modified vaccines: Exploiting Jenner’s legacy. Vaccine 2016, 34, 6436–6448. [Google Scholar] [CrossRef]
- Robert-Guroff, M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 2007, 18, 546–556. [Google Scholar] [CrossRef]
- COVID-19 Vaccines with WHO Emergency Use Listing. Available online: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued (accessed on 19 December 2022).
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zeng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Crystal, R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef]
- Lukashev, A.N.; Zamyatnin, A.A. Viral vectors for gene therapy: Current state and clinical perspectives. Biochem. Mosc. 2016, 81, 700–708. [Google Scholar] [CrossRef]
- Coughlan, L. Factors Which Contribute to the Immunogenicity of Non-replicating Adenoviral Vectored Vaccines. Front. Immunol. 2020, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.; Sebastian, S.; Spencer, A.J.; Gilbert, S.; Hill, A.V.S.; Lambe, T. Chimpanzee adenoviral vectors as vaccines for outbreak pathogens. Hum. Vaccines Immunother. 2017, 13, 3020–3032. [Google Scholar] [CrossRef] [PubMed]
- Ghebremedhin, B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.R.; Poland, G.A. Critical aspects of packaging, storage, preparation, and administration of mRNA and adenovirus-vectored COVID-19 vaccines for optimal efficacy. Vaccine 2021, 39, 457. [Google Scholar] [CrossRef] [PubMed]
- Schlimgen, R.; Howard, J.; Wooley, D.; Thompson, M.; Baden, L.R.; Yang, O.O.; Christiani, D.C.; Mostoslavsky, G.; Diamond, D.V.; Duane, E.G.; et al. Risks Associated With Lentiviral Vector Exposures and Prevention Strategies. J. Occup. Environ. Med. 2016, 58, 1159–1166. [Google Scholar] [CrossRef]
- Raikwar, S.P.; Kao, C.H.; Gardner, T.A. Targeted Adenoviral Vectors II: Transcriptional Targeting. In Adenoviral Vectors for Gene Therapy; Academic Press: Cambridge, MA, USA, 2002; pp. 247–286. [Google Scholar] [CrossRef]
- Barry, M. Single-cycle adenovirus vectors in the current vaccine landscape. Expert Rev. Vaccines 2018, 17, 163–173. [Google Scholar] [CrossRef]
- Shaw, A.R.; Suzuki, M. Immunology of Adenoviral Vectors in Cancer Therapy. Mol. Ther. Methods Clin. Dev. 2019, 15, 418–429. [Google Scholar] [CrossRef]
- Tapia, M.D.; Sow, S.O.; Mbaye, K.D.; Thiongane, A.; Ndiaye, B.P.; Ndour, C.T.; Mboup, S.; Keshinro, B.; Kinge, T.N.; Vernet, G.; et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in children in Africa: A randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020, 20, 719–730. [Google Scholar] [CrossRef]
- Harro, C.; Sun, X.; Stek, J.E.; Leavitt, R.Y.; Mehrotra, D.V.; Wang, F.; Bett, A.J.; Casimiro, D.R.; Shiver, J.W.; DiNubile, M.J.; et al. Safety and immunogenicity of the merck adenovirus serotype 5 (MRKAd5) and MRKAd6 human immunodeficiency virus type 1 trigene vaccines alone and in combination in healthy adults. Clin. Vaccine Immunol. 2009, 16, 1285–1292. [Google Scholar] [CrossRef]
- Steffen, T.; Hassert, M.; Hoft, S.; Stone, E.T.; Zhang, J.; Geerling, E.; Grimberg, B.T.; Roberts, M.S.; Pinto, A.K.; Brien, J.D. Immunogenicity and efficacy of a recombinant human adenovirus type 5 vaccine against Zika virus. Vaccines 2020, 8, 170. [Google Scholar] [CrossRef]
- Shiratsuchi, T.; Rai, U.; Kaneko, I.; Zhang, M.; Iwanaga, S.; Yuda, M.; Tsuji, M. A potent malaria vaccine based on adenovirus with dual modifications at Hexon and pVII. Vaccine 2017, 35, 6990–7000. [Google Scholar] [CrossRef] [PubMed]
- Fausther-Bovendo, H.; Kobinger, G. Vaccine innovation spurred by the long wait for an Ebola virus vaccine. Lancet Infect. Dis. 2021, 21, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Daussy, C.F.; Pied, N.; Wodrich, H. Understanding Post Entry Sorting of Adenovirus Capsids; A Chance to Change Vaccine Vector Properties. Viruses 2021, 13, 1221. [Google Scholar] [CrossRef]
- Chandler, M.; de la Cruz, F.; Dyda, F.; Hickman, A.B.; Moncalian, G.; Ton-Hoang, B. Breaking and joining single-stranded DNA: The HUH endonuclease superfamily. Nat. Rev. Microbiol. 2013, 11, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; Rutten, L.; van der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. Npj Vaccines 2020, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Fehrmann, F.; Laimins, L.A. Role of the E1--E4 protein in the differentiation-dependent life cycle of human papillomavirus type 31. J. Virol. 2005, 79, 6732–6740. [Google Scholar] [CrossRef] [PubMed]
- Ritter, T.; Lehmann, M.; Volk, H.-D. Improvements in gene therapy: Averting the immune response to adenoviral vectors. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2002, 16, 3–10. [Google Scholar] [CrossRef]
- Gorziglia, M.I.; Lapcevich, C.; Roy, S.; Kang, Q.; Kadan, M.; Wu, V.; Pechan, P.; Kaleko, M. Generation of an adenovirus vector lacking E1, e2a, E3, and all of E4 except open reading frame 3. J. Virol. 1999, 73, 6048–6055. [Google Scholar] [CrossRef]
- Ricobaraza, A.; Gonzalez-Aparicio, M.; Mora-Jimenez, L.; Lumbreras, S.; Hernandez-Alcoceba, R. High-Capacity Adenoviral Vectors: Expanding the Scope of Gene Therapy. Int. J. Mol. Sci. 2020, 21, 3643. [Google Scholar] [CrossRef]
- Alba, R.; Bosch, A.; Chillon, M. Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Ther. 2005, 12, S18–S27. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Wold, W.S.M.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Danthinne, X.; Imperiale, M.J. Production of first generation adenovirus vectors: A review. Gene Ther. 2000, 7, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Subramanian, G.; Silayeva, L.; Newkirk, I.; Doctor, D.; Chawla, K.; Chattopadhyay, S.; Chandra, D.; Chilukuri, N.; Betapudi, V. Gene Therapy Leaves a Vicious Cycle. Front. Oncol. 2019, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Yue, Y.; Yan, Z.; Engelhardt, J.F. DNA Virus Vectors II. Mol. Ther. 2000, 1, S169–S184. [Google Scholar] [CrossRef]
- Zhang, P.; Miao, D.; Zhang, Y.; Wang, M.; Hu, Z.; Lü, P.; Yao, Q. Cloning and rescue of the genome of Bombyx mori bidensovirus, and characterization of a recombinant virus. Virol. J. 2016, 13, 126. [Google Scholar] [CrossRef]
- Dong, J.Y.; Fan, P.D.; Frizzell, R.A. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum. Gene Ther. 1996, 7, 2101–2112. [Google Scholar] [CrossRef]
- Rosewell, A.; Vetrini, F.; Ng, P. Helper-Dependent Adenoviral Vectors. J. Genet. Syndr. Gene Ther. 2011, (Suppl. 5), 1. [Google Scholar] [CrossRef]
- Phan, Q.V.; Contzen, J.; Seemann, P.; Gossen, M. Site-specific chromosomal gene insertion: Flp recombinase versus Cas9 nuclease. Sci. Rep. 2017, 7, 17771. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Vaghasiya, D.D. SARS-CoV-2 variants and vulnerability at the global level. J. Med. Virol. 2022, 94, 2986–3005. [Google Scholar] [CrossRef]
- See, R.H.; Zakhartchouk, A.N.; Petric, M.; Lawrence, D.J.; Mok, C.P.Y.; Hogan, R.J.; Rowe, T.; Zitzow, L.A.; Karunakaran, K.P.; Hitt, M.M.; et al. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J. Gen. Virol. 2006, 87, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.; Wong, M.U.; Huffman, A.; He, Y. COVID-19 Coronavirus Vaccine Design Using Reverse Vaccinology and Machine Learning. Front. Immunol. 2020, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, S.; Yao, Y.; Xing, Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol. Ther. Methods Clin. Dev. 2016, 3, 16030. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.K.; Rivera-Soto, R.; Gray, S.J. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov. Med. 2015, 19, 49–57. [Google Scholar] [PubMed]
- Chavda, V.P.; Pandya, R.; Apostolopoulos, V. DNA vaccines for SARS-CoV-2: Towards third generation vaccination era. Expert Rev. Vaccines 2021, 20, 1549–1560. [Google Scholar] [CrossRef]
- Chavda, V.P.; Hossain, M.K.; Beladiya, J.; Apostolopoulos, V. Nucleic Acid Vaccines for COVID-19: A Paradigm Shift in the Vaccine Development Arena. Biologics 2021, 1, 20. [Google Scholar] [CrossRef]
- Duan, L.; Zheng, Q.; Zhang, H.; Niu, Y.; Lou, Y.; Wang, H. The SARS-CoV-2 Spike Glycoprotein Biosynthesis, Structure, Function, and Antigenicity: Implications for the Design of Spike-Based Vaccine Immunogens. Front. Immunol. 2020, 11, 576622. [Google Scholar] [CrossRef]
- Xie, Y.; Karki, C.B.; Du, D.; Li, H.; Wang, J.; Sobitan, A.; Teng, S.; Tang, Q.; Li, L. Spike Proteins of SARS-CoV and SARS-CoV-2 Utilize Different Mechanisms to Bind With Human ACE2. Front. Mol. Biosci. 2020, 7, 392. [Google Scholar] [CrossRef]
- Majhen, D.; Calderon, H.; Chandra, N.; Fajardo, C.A.; Rajan, A.; Alemany, R.; Custers, J. Adenovirus-based vaccines for fighting infectious diseases and cancer: Progress in the field. Hum. Gene Ther. 2014, 25, 301–317. [Google Scholar] [CrossRef]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Bezbaruah, R.; Borah, P.; Kakoti, B.B.; Al-Shar’I, N.A.; Chandrasekaran, B.; Jaradat, D.M.M.; Al-Zeer, M.A.; Abu-Romman, S. Developmental Landscape of Potential Vaccine Candidates Based on Viral Vector for Prophylaxis of COVID-19. Front. Mol. Biosci. 2021, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.W.; Fisher, K.D. Adenovirus: Teaching an old dog new tricks. Hum. Gene Ther. 2011, 22, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, I. Revisiting the Role of Clathrin-Mediated Endoytosis in Synaptic Vesicle Recycling. Front. Cell Neurosci. 2018, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef] [PubMed]
- Fay, N.; Panté, N. Nuclear entry of DNA viruses. Front. Microbiol. 2015, 6, 467. [Google Scholar] [CrossRef]
- Pishesha, N.; Harmand, T.J.; Rothlauf, P.W.; Praest, P.; Alexander, R.K.; van den Doel, R.; Liebeskind, M.J.; Vakaki, M.A.; McCaul, N.; Wijne, C.; et al. A class II MHC-targeted vaccine elicits immunity against SARS-CoV-2 and its variants. Proc. Natl. Acad. Sci. USA 2021, 118, e2116147118. [Google Scholar] [CrossRef]
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. Npj Vaccines 2021, 6, 97. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Common. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Case, J.B.; Rothlauf, P.W.; Chen, R.E.; Kafai, N.M.; Fox, J.M.; Smith, B.K.; Shrihari, S.; McCune, B.T.; Harvey, I.B.; Keeler, S.P.; et al. Replication-Competent Vesicular Stomatitis Virus Vaccine Vector Protects against SARS-CoV-2-Mediated Pathogenesis in Mice. Cell Host Microbe 2020, 28, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, I.R.; Sebastian, S. Novel viral vectors in infectious diseases. Immunology 2018, 153, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Roig, J.; Núñez-Manchón, E.; Alemany, R.; Villanueva, E.; Fillat, C. Codon Usage and Adenovirus Fitness: Implications for Vaccine Development. Front. Microbiol. 2021, 12, 633946. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, G.T.; Weterings, N.; Obregon-Henao, A.; Lepolder, M.; Dutt, T.S.; van Overveld, F.J.; Henao-Tamayo, M. Antigen Presentation of mRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines. Vaccines 2021, 9, 848. [Google Scholar] [CrossRef]
- Shah, V.K.; Firmal, P.; Alam, A.; Ganguly, D.; Chattopadhyay, S. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Front. Immunol. 2020, 11, 1949. [Google Scholar] [CrossRef] [PubMed]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Worldo meter. COVID-19: CORONAVIRUS PANDEMIC. 2022. Available online: https://www.worldometers.info/coronavirus/ (accessed on 22 December 2022).
- Lundstrom, K. Coronavirus Pandemic—Therapy and Vaccines. Biomedicines 2020, 8, 109. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines 2022, 10, 513. [Google Scholar] [CrossRef]
- Kumar, V.M.; Pandi-Perumal, S.R.; Trakht, I.; Thyagarajan, S.P. Strategy for COVID-19 vaccination in India: The country with thesecond highest population and number of cases. Npj Vaccines 2021, 6, 60. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet Lond. Engl. 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Voysey, M.; Ann, S.; Clemens, C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- US. National Library of Medicine. Phase III Double-Blind, Placebo-Controlled Study of AZD1222 for the Prevention of COVID-19 in Adults; ClinicalTrials.gov: Bethesda, MD, USA, 2020. [Google Scholar]
- Kashte, S.; Gulbake, A.; El-Amin III, S.F.; Gupta, A. COVID-19 vaccines: Rapid development, implications, challenges and future prospects. Hum. Cell 2021, 34, 711–733. [Google Scholar] [CrossRef] [PubMed]
- McGill COVID19 Vaccine Tracker Team COVID-19 VACCINE TRACKER. 2022. Available online: https://covid19.trackvaccines.org/ (accessed on 22 December 2022).
- Simpson, C.R.; Shi, T.; Vasileiou, E.; Katikireddi, S.V.; Kerr, S.; Moore, E.; McCowan, C.; Agrawal, U.; Shah, S.A.; Ritchie, L.D.; et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021, 27, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Charmet, T.; Schaeffer, L.; Galmiche, S.; Madec, Y.; Von Platen, C.; Chény, O.; Omar, F.; David, C.; Rogoff, A.; et al. Impact of SARS-CoV-2 Delta variant on incubation, transmission settings and vaccine effectiveness: Results from a nationwide case-control study in France. Lancet Reg. Health Eur. 2021, 13, 100278. [Google Scholar] [CrossRef]
- Ikegame, S.; Siddiquey, M.N.A.; Hung, C.-T.; Haas, G.; Brambilla, L.; Oguntuyo, K.Y.; Kowdle, S.; Chiu, H.-P.; Stevens, C.S.; Vilardo, A.E.; et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Nat. Commun. 2021, 12, 4598. [Google Scholar] [CrossRef]
- Babira, V.F.; Borisevich, S.V.; Naroditsky, B.S.; Gintsburg, A.L. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Cross Mark 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Second Interim Analysis of Clinical Trial Data Showed a 91.4% Efficacy for the Sputnik V Vaccine on Day 28 after the First Dose; Vaccine Efficacy Is over 95% 42 Days after the First Dose. Official Website Vaccine against COVID-19 Sputnik V. Available online: https://sputnikvaccine.com/newsroom/pressreleases/second-interim-analysis-of-clinical-trial-data-showed-a-91-4-efficacy-for-the-sputnik-v-vaccine-on-d/ (accessed on 22 December 2022).
- González, S.; Olszevicki, S.; Salazar, M.; Calabria, A.; Regairaz, L.; Marín, L.; Campos, P.; Varela, T.; Martínez, V.V.G.; Ceriani, L.; et al. Effectiveness of the first component of Gam-COVID-Vac (Sputnik V) on reduction of SARS-CoV-2 confirmed infections, hospitalisations and mortality in patients aged 60–79: A retrospective cohort study in Argentina. EClinicalMedicine 2021, 40, 101126. [Google Scholar] [CrossRef]
- FDA. Janssen COVID-19 Vaccine; FDA: Silver Spring, ML, USA, 2022. [Google Scholar]
- Zhu, F.; Li, Y.; Guan, X.; Hou, L.; Wang, W.; Li, J.; Wu, S.; Wang, B.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine . Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Zhu, F.; Guan, X.; Li, Y.; Huang, J.; Jiang, T.; Hou, L.; Li, J.; Yang, B.; Wang, L.; Wang, W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo- controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- US. National Library of Medicine. Phase III Trial of A COVID-19 Vaccine of Adenovirus Vector in Adults 18 Years Old and Above; ClinicalTrials.gov: Bethesda, MD, USA, 2020. [Google Scholar]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- US. National Library of Medicine. Safety, Tolerability and Immunogenicity of the Candidate Vaccine MVA-SARS-2-S against COVID-19; ClinicalTrials.gov: Bethesda, MD, USA, 2021. [Google Scholar]
- US. National Library of Medicine. A Synthetic MVA-Based SARS-CoV-2 Vaccine, COH04S1, for the Prevention of COVID-19—Full Text View; ClinicalTrials.gov: Bethesda, MD, USA, 2021. [Google Scholar]
- US. National Library of Medicine. Clinical Trial to Evaluate the Safety and Immunogenicity of the COVID-19 Vaccine; ClinicalTrials.gov: Bethesda, MD, USA, 2020. [Google Scholar]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet Lond. Engl. 2021, 397, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D. V Data discrepancies and substandard reporting of interim data of Sputnik V phase 3 trial – Authors’ reply. Lancet 2021, 397, 1883–1884. [Google Scholar] [CrossRef]
- US. National Library of Medicine. GRAd-COV2 Vaccine AGAINST COVID-19; ClinicalTrials.gov: Bethesda, MD, USA, 2020. [Google Scholar]
- US. National Library of Medicine. Study of GRAd-COV2 for the Prevention of COVID-19 in Adults; ClinicalTrials.gov: Bethesda, MD, USA, 2021. [Google Scholar]
- US. National Library of Medicine. An Open Study on the Safety, Tolerability, and Immunogenicity of “Sputnik Light” Vaccine; ClinicalTrials.gov: Bethesda, MD, USA, 2021. [Google Scholar]
- US. National Library of Medicine. Study to Evaluate Efficacy, Immunogenicity and Safety of the Sputnik-Light; ClinicalTrials.gov: Bethesda, MD, USA, 2021. [Google Scholar]
- Ramvikas, M.; Arumugam, M.; Chakrabarti, S.R.; Jaganathan, K.S. Nasal Vaccine Delivery. In Micro and Nanotechnology in Vaccine Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 279–301. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 3221. [Google Scholar] [CrossRef]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Vihol, D.R. COVAX-19® Vaccine: Completely blocks virus transmission to non-immune individuals. Clin. Complement. Med. Pharmacol. 2021, 1, 100004. [Google Scholar] [CrossRef]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Purushotham, J.N.; Schulz, J.E.; Holbrook, M.G.; Bushmaker, T.; Carmody, A.; Port, J.R.; Yinda, C.K.; Okumura, A.; Saturday, G.; et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 2021, 13, eabh0755. [Google Scholar] [CrossRef]
- Hassan, A.O.; Shrihari, S.; Gorman, M.J.; Ying, B.; Yaun, D.; Raju, S.; Chen, R.E.; Dmitriev, I.P.; Kashentseva, E.; Adams, L.J.; et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 2021, 36, 109452. [Google Scholar] [CrossRef] [PubMed]
- King, R.G.; Silva-Sanchez, A.; Peel, J.N.; Botta, D.; Meza-Perez, S.; Allie, R.; Schultz, M.D.; Liu, M.; Bradley, J.E.; Qiu, S.; et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 in mice. BioRxiv Prepr. Serv. Biol. 2020. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Purushotham, J.; Schulz, J.; Holbrook, M.; Bushmaker, T.; Carmody, A.; Port, J.; Yinda, K.C.; Okumura, A.; Saturday, G.; et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. BioRxiv Prepr. Serv. Biol. 2021. [Google Scholar] [CrossRef]
- Rubin, R. COVID-19 Vaccine Nasal Spray. JAMA 2021, 326, 1138. [Google Scholar] [CrossRef]
- Hassan, A.O.; Feldmann, F.; Zhao, H.; Curiel, D.T.; Okumura, A.; Tang-Huau, T.-L.; Case, J.B.; Meade-White, K.; Callison, J.; Chen, R.E.; et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep. Med. 2021, 2, 100230. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef]
- NMPA Approves the Application for Conditional Marketing. Available online: https://www.lillyasiaventures.com/blog/nmpa-approves-the-application-for-conditional-marketing-authorization-of (accessed on 6 November 2022).
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Hanage, W.P.; Rasmussen, A.L. Making Sense of Mutation: What D614G Means for the COVID-19 Pandemic Remains Unclear. Cell 2020, 182, 794–795. [Google Scholar] [CrossRef]
- Dutta, N.K.; Mazumdar, K.; Gordy, J.T. The Nucleocapsid Protein of SARS–CoV-2: A Target for Vaccine Development. J. Virol. 2020, 94, e00647-20. [Google Scholar] [CrossRef]
- Rice, A.; Verma, M.; Shin, A.; Zakin, L.; Sieling, P.; Tanaka, S.; Adisetiyo, H.; Taft, J.; Patel, R.; Buta, S.; et al. A Next Generation Bivalent Human Ad5 COVID-19 Vaccine Delivering Both Spike and Nucleocapsid Antigens Elicits Th1 Dominant CD4+, CD8+ T-cell and Neutralizing Antibody Responses. bioRxiv 2020. [Google Scholar] [CrossRef]
- Suntronwong, N.; Kanokudom, S.; Auphimai, C.; Assawakosri, S.; Thongmee, T.; Vichaiwattana, P.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; Chansaenroj, J.; et al. Effects of boosted mRNA and adenoviral-vectored vaccines on immune responses to omicron BA.1 and BA.2 following the heterologous CoronaVac/AZD1222 vaccination. J. Med. Virol. 2022, 94, 5713–5722. [Google Scholar] [CrossRef]
- Heterologous Booster with Inhaled Adenovirus Vector Vaccine Expected to Be an Effective Strategy in Preventing Omicron Variant BA.5: Research—Global Times. Available online: https://www.globaltimes.cn/page/202210/1277116.shtml (accessed on 6 November 2022).
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bergelson, J.M. Adenovirus receptors. J. Virol. 2005, 79, 12125–12131. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.E.; Timares, L.; Matthews, Q.L. Strategies to overcome host immunity to adenovirus vectors in vaccine development. Expert Rev. Vaccines 2009, 8, 761–777. [Google Scholar] [CrossRef]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Zuniga, A.; Wang, Z.L.; Liniger, M.; Hangartner, L.; Caballero, M.; Pavlovic, J.; Wild, P.; Viret, J.F.; Glueck, R.; Billeter, M.A.; et al. Attenuated measles virus as a vaccine vector. Vaccine 2007, 25, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Mühlebach, M.D. Vaccine platform recombinant measles virus. Virus Genes 2017, 53, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Ramsauer, K.; Schwameis, M.; Firbas, C.; Müllner, M.; Putnak, R.J.; Thomas, S.J.; Desprès, P.; Tauber, E.; Jilma, B.; Tangy, F. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: A randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect. Dis. 2015, 15, 519–527. [Google Scholar] [CrossRef]
- Kremer, E.J.; Perricaudet, M. Adenovirus and adeno-associated virus mediated gene transfer. Br. Med. Bull. 1995, 51, 31–44. [Google Scholar] [CrossRef]
- Echavarría, M. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 2008, 21, 704–715. [Google Scholar] [CrossRef]
- Barouch, D.H.; Kik, S.V.; Weverling, G.J.; Dilan, R.; King, S.L.; Maxfield, L.F.; Clark, S.; Ng’ang’a, D.; Brandariz, K.L.; Abbink, P.; et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 2011, 29, 5203–5209. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.J.; Xiang, Z.Q.; Gao, G.P.; Ertl, H.C.J.; Wilson, J.M.; Bergelson, J.M. Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J. Gen. Virol. 2002, 83, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Farina, S.F.; Gao, G.; Xiang, Z.Q.; Rux, J.J.; Burnett, R.M.; Alvira, M.R.; Marsh, J.; Ertl, H.C.J.; Wilson, J.M. Replication-Defective Vector Based on a Chimpanzee Adenovirus. J. Virol. 2001, 75, 11603–11613. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, C.R.; Lachapelle, A.; Delgado, C.; Parkes, V.; Wadsworth, S.C.; Smith, A.E.; Francis, G.E. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999, 10, 1349–1358. [Google Scholar] [CrossRef]
- Kim, J.; Kim, P.H.; Kim, S.W.; Yun, C.O. Enhancing the therapeutic efficacy of adenovirus in combination with biomaterials. Biomaterials 2012, 33, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, G.; HogenEsch, H.; North, A.; Hays, J.; Mittal, S.K. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector specific immune response. Gene Ther. 2002, 9, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, M.; Vignier, N.; Pitard, B.; Botelho-Nevers, E.; Wyplosz, B.; Cohen, R.; Epaulard, O. COVID-19 vaccines: Frequently asked questions and updated answers. Infect. Dis. Now 2021, 51, 319–333. [Google Scholar] [CrossRef]
- Xiang, Z.Q.; Gao, G.P.; Reyes-Sandoval, A.; Li, Y.; Wilson, J.M.; Ertl, H.C.J. Oral Vaccination of Mice with Adenoviral Vectors Is Not Impaired by Preexisting Immunity to the Vaccine Carrier. J. Virol. 2003, 77, 10780–10789. [Google Scholar] [CrossRef]
- Higgins, T.S.; Wu, A.W.; Illing, E.A.; Sokoloski, K.J.; Weaver, B.A.; Anthony, B.P.; Hughes, N.; Ting, J.Y. Intranasal Antiviral Drug Delivery and Coronavirus Disease 2019 (COVID-19): A State of the Art Review. Otolaryngol. Head Neck Surg. 2020, 163, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Vigna, E.; Naldini, L. Lentiviral vectors: Excellent tools for experimental gene transfer and promising candidates for gene therapy. J. Gene Med. 2000, 2, 308–316. [Google Scholar] [CrossRef]
- Izumida, M.; Togawa, K.; Hayashi, H.; Matsuyama, T.; Kubo, Y. Production of Vesicular Stomatitis Virus Glycoprotein-Pseudotyped Lentiviral Vector Is Enhanced by Ezrin Silencing. Front. Bioeng. Biotechnol. 2020, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.-W.; Bourgine, M.; Authié, P.; Lopez, J.; Nemirov, K.; Moncoq, F.; Noirat, A.; Vesin, B.; Nevo, F.; Blanc, C.; et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe 2021, 29, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, B. Heterologous Immunity: Role in Natural and Vaccine-Induced Resistance to Infections. Front. Immunol. 2019, 10, 2631. [Google Scholar] [CrossRef] [PubMed]
- Reche, P.A. Potential Cross-Reactive Immunity to SARS-CoV-2 From Common Human Pathogens and Vaccines. Front. Immunol. 2020, 11, 2694. [Google Scholar] [CrossRef]
- Möller, J.; Kraner, M.E.; Burkovski, A. More than a toxin: Protein inventory of Clostridium tetani toxoid vaccines. Proteomes 2019, 7, 15. [Google Scholar] [CrossRef]
- Möller, J.; Kraner, M.; Sonnewald, U.; Sangal, V.; Tittlbach, H.; Winkler, J.; Winkler, T.H.; Melnikov, V.; Lang, R.; Sing, A.; et al. Proteomics of diphtheria toxoid vaccines reveals multiple proteins that are immunogenic and may contribute to protection of humans against Corynebacterium diphtheriae. Vaccine 2019, 37, 3061–3070. [Google Scholar] [CrossRef]
- Liu, J.; Xu, K.; Xing, M.; Zhuo, Y.; Guo, J.; Du, M.; Wang, Q.; An, Y.; Li, J.; Gao, P.; et al. Heterologous prime-boost immunizations with chimpanzee adenoviral vectors elicit potent and protective immunity against SARS-CoV-2 infection. Cell Discov. 2021, 7, 123. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine–Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef]
- Ahmed, I.; Majeed, A.; Powell, R. Heparin Induced Thrombocytopenia: Diagnosis and Management Update. Postgrad. Med. J. 2007, 83, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Selleng, K.; Mayerle, J.; Palankar, R.; Wesche, J.; Reiche, S.; Aebischer, A.; Warkentin, T.E.; Muenchhoff, M.; Hellmuth, J.C.; et al. Anti–platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood 2021, 138, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Towards Understanding ChAdOx1 nCov-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT)—ISTH Congress Abstracts. Available online: https://abstracts.isth.org/abstract/towards-understanding-chadox1-ncov-19-vaccine-induced-immune-thrombotic-thrombocytopenia-vitt/ (accessed on 6 November 2022).
- Krutzke, L.; Roesler, R.; Wiese, S.; Kochanek, S. Process-related impurities in the ChAdOx1 nCov-19 vaccine. eLife 2021, 11, e78513. [Google Scholar] [CrossRef] [PubMed]
- Vaccine Safety Datalink (VSD)|VSD|Monitoring|Ensuring Safety|Vaccine Safety|CDC. Available online: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html (accessed on 21 January 2023).
- Ruggiero, R.; Balzano, N.; Di Napoli, R.; Mascolo, A.; Berrino, P.M.; Rafaniello, C.; Sportiello, L.; Rossi, F.; Capuano, A. Capillary leak syndrome following COVID-19 vaccination: Data from the European pharmacovigilance database Eudravigilance. Front. Immunol. 2022, 13, 956825. [Google Scholar] [CrossRef]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 NCoV-19) Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Selected Adverse Events Reported after COVID-19 Vaccination | CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html (accessed on 21 January 2023).
- See, I.; Lale, A.; Marquez, P.; Streiff, M.B.; Wheeler, A.P.; Tepper, N.K.; Woo, E.J.; Broder, K.R.; Edwards, K.M.; Gallego, R.; et al. Case Series of Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination—United States, December 2020 to August 2021. Ann. Intern. Med. 2022, 175, 513–522. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef]
- Tiboni, M.; Casettari, L.; Illum, L. Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines ? Int. J. Pharm. 2021, 603, 120686. [Google Scholar] [CrossRef]
- Kwan, A.C.; Ebinger, J.E.; Wei, J.; Le, C.N.; Oft, J.R.; Zabner, R.; Teodorescu, D.; Botting, P.G.; Navarrette, J.; Ouyang, D.; et al. Apparent risks of postural orthostatic tachycardia syndrome diagnoses after COVID-19 vaccination and SARS-Cov-2 Infection. Nat. Cardiovasc. Res. 2022, 1, 1187–1194. [Google Scholar] [CrossRef]
- Ewen Callaway, The coronavirus is mutating—Does it matter? Available online: https://www.nature.com/articles/d41586-020-02544-6 (accessed on 20 December 2022).
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Bernal, J.L. Effectiveness of COVID-19 booster vaccines against covid-19 related symptoms, hospitalisation and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Gower, C.; Ramsay, M.; Bernal, J.L. Effectiveness of BNT162b2 (Comirnaty, Pfizer-BioNTech) COVID-19 booster vaccine against covid-19 related symptoms in England: Test negative case-control study. medRxiv 2021. [Google Scholar] [CrossRef]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Daian e Silva, D.S.D.O.; da Fonseca, F.G. The Rise of Vectored Vaccines: A Legacy of the COVID-19 Global Crisis. Vaccines 2021, 9, 1101. [Google Scholar] [CrossRef]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Antibody boost after third dose varies greatly by vaccine, study finds. BMJ 2021, 375, n3011. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.H.; Schelde, A.B.; Moustsen-Helm, I.R.; Emborg, H.-D.; Krause, T.G.; Mølbak, K.; Valentiner-Branth, P. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: A Danish cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Lu, L.; Mok, B.W.-Y.; Chen, L.; Chan, J.M.-C.; Tsang, O.T.-Y.; Lam, B.H.-S.; Chuang, V.W.-M.; Chu, A.W.-H.; Chan, W.-M.; Ip, J.D.; et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. medRxiv 2021. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Pajon, R.; Doria-Rose, N.A.; Shen, X.; Schmidt, S.D.; O’Dell, S.; McDanal, C.; Feng, W.; Tong, J.; Eaton, A.; Maglinao, M.; et al. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. N. Engl. J. Med. 2022, 386, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- D’amelio, R.; Asero, R.; Cassatella, M.A.; Laganà, B.; Lunardi, C.; Migliorini, P.; Nisini, R.; Parronchi, P.; Quinti, I.; Racanelli, V.; et al. Anti-COVID-19 vaccination in patients with autoimmune-autoinflammatory disorders and primary/secondary immunodeficiencies: The position of the task force on behalf of the italian immunological societies. Biomedicines 2021, 9, 1163. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Olson, S.M.; Self, W.H.; Talbot, H.K.; Lindsell, C.J.; Steingrub, J.S.; Shapiro, N.I.; Ginde, A.A.; Douin, D.J.; Prekker, M.E.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years—United States, January–March 2021. MMWR Recomm. Rep. 2021, 70, 674–679. [Google Scholar] [CrossRef]
- Naveen, R.; Parodis, I.; Joshi, M.; Sen, P.; Lindblom, J.; Agarwal, V.; Lilleker, J.B.; Tan, A.L.; Nune, A. COVID-19 vaccination in autoimmune diseases (COVAD) study: Vaccine safety and tolerance in rheumatoid arthritis. Rheumatology 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Chi, W.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vuppu, S.; Mishra, T.; Kamaraj, S.; Patel, A.B.; Sharma, N.; Chen, Z.-S. Recent Review of COVID-19 Management: Diagnosis, Treatment and Vaccination. Pharmacol. Rep. 2022, 74, 1120–1148. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Kumari, R.; Mittal, S.K. Current use of adenovirus vectors and their production methods. Methods Mol. Biol. 2019, 1937, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Kitney, R.I.; Bell, J.; Philp, J. Build a Sustainable Vaccines Industry with Synthetic Biology. Trends Biotechnol. 2021, 39, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors. Hum. Vaccines Immunother. 2015, 10, 2875–2884. [Google Scholar] [CrossRef]
- Dangi, T.; Sanchez, S.; Lew, M.H.; Visvabharathy, L.; Richner, J.; Koralnik, I.J.; Penaloza-MacMaster, P. Pre-existing immunity modulates responses to mRNA boosters. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hasanpourghadi, M.; Novikov, M.; Ertl, H.C.J. COVID-19 Vaccines Based on Adenovirus Vectors. Trends Biochem. Sci. 2021, 46, 429–430. [Google Scholar] [CrossRef]
- Hwang, S.S.; Lim, J.; Yu, Z.; Kong, P.; Sefik, E.; Xu, H.; Harman, C.C.D.; Kim, L.K.; Lee, G.R.; Li, H.B.; et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1255–1260. [Google Scholar] [CrossRef]

| Innovator | Vaccine | Vector Type | Phase | Formulation | References |
|---|---|---|---|---|---|
| AstraZeneca/University of Oxford | AZD1222 (Covishield and Vaxzevria) | ChAdOx1 | III NCT05293665 NCT04973449 NCT05236491 and 14 more trials are ongoing. | Active Component:
| [94,95,96] |
| Gamaleya Research Institute/Acellena Contract Drug Research And Development | GamCOVID-Vac (frozen) and GamCOVID-Vac Lyo (lyophilized) (Sputnik V) | rAd26 and rAd5 | III NCT04640233 NCT04564716 NCT04530396 NCT04642339 NCT04656613 NCT04954092 | Active Component:
| [114,115] |
| Janssen Pharmaceutical | Ad26Cov2-S (JNJ-78436735) | rAd26 | III NCT05047640 NCT04505722 NCT04614948 NCT04838795 NCT05220397 and 3 more trials are ongoing. | Active Component:
| [106] |
| CanSino Biologics/Beijing Institute of Biotechnology | Ad5-nCoV (Convidecia) | rAd5 | III NCT05169008 NCT04540419 NCT04526990 | Active Component:
| [107,108,109] |
| Gamaleya Research Institute/Acellena Contract Drug Research And Development | Gam-COVID-Vac | rAd5 | II NCT05248373; Phase III is ongoing | Active Component:
| [116,117] |
| Gamaleya Research Institute/ Acellena Contract Drug Research And Development | Sputnik Light | rAd26 | III NCT04741061 and 5 more trials are ongoing. | Active ingredients:
| [118,119] |
| Vaccine Name and Innovator | Ad Vector Type | Phase | Clinical Trial Number | Nasal Delivery Device | Remarks |
|---|---|---|---|---|---|
| AZD1222 (ChAdOx1) And The University of Oxford (UK) with AstraZeneca (Cambridge, UK) | Non-replicating rChAd vector | I | NCT04816019 | Mucosal atomization Device (MAD Nasal™) |
|
| ChAd-SARS-CoV-2-S/BBV154 And Bharat Biotech (Genome Valley, India)-Washington University (USA) | Non-replicating rChAd vector | I | NCT04751682 | Currently, Pipette droppers |
|
| AdCOVIDTM And Altimmune | Non-replicating Ad5 vector | I | NCT04679909 | Pipette droppers |
|
| SC-Ad6-1 And Tetherex Pharmaceuticals Corporation | Non-replicating single cycle rAd6 vector | I | NCT04839042 | Direct inoculation into the nose |
|
| Ad5-nCoV And CanSino/Beijing Institute of Biotechnology (China) | Non-replicating rAd5 vector | I/II | NCT04840992 | Aerogen Ultra Device |
|
| Adverse Event | Vaccine Reported | Explanation | References |
|---|---|---|---|
| Guillain-Barré syndrome (GBS) | J&J/Janssen | In the uncommon illness known as GBS, the immune system of the body damages nerve cells, leading to muscular weakness and occasionally paralysis. Men 50 years of age and older make up the majority of GBS cases reported. | [174] |
| Capillary leak syndrome (CLS) | mRNA COVID-19 Vaccines | The COVID-19 vaccination-related adverse event after immunization (AEFI) known as capillary leak syndrome (CLS) has recently appeared. Increased capillary permeability in CLS, a rare disorder that mostly affects the upper and lower limbs, causes hypoalbuminemia, hypotension, and edema. | [175] |
| Anaphylaxis | Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccines), A tetravalent cold-adapted live-attenuated influenza vaccine (LAIV) produced by Medimmune/AstraZeneca and Nasovac® | Approximately five incidences of anaphylaxis have been reported after receiving the COVID-19 vaccine for every million doses of the vaccine. Any sort of immunization might result in anaphylaxis, a severe allergic response. | [176,177,178] |
| Thrombosis with thrombocytopenia syndrome (TTS) | J&J/Janssen, Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccines) | Approximately four incidences of thrombosis with thrombocytopenia syndrome (TTS) following J&J/Janssen COVID-19 vaccine have been reported per million doses given. TTS is an uncommon but dangerous adverse effect that results in low platelets and blood clots in big blood arteries. | [178,179] |
| Myocarditis and pericarditis | Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccines) | Pericarditis is an inflammation of the heart’s outer membrane, whereas myocarditis is an inflammation of the heart muscle. The majority of people who had myocarditis or pericarditis after receiving the COVID-19 vaccine reacted favorably to treatment, rest, and improved swiftly. The majority of instances, notably in male teenagers and young adults, have been linked to Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccinations). | [180] |
| Reports of death | J&J/Janssen | Clinicians from the CDC and FDA examined death reports submitted to Vaccine Adverse Event Reporting System (VAERS), which may include death certificates, autopsies, and medical records. Nine deaths that can be directly linked to the J&J/Janssen COVID-19 vaccine have been found via ongoing surveillance. The CDC and FDA keep track of reports of fatalities following COVID-19 vaccinations and update data when it becomes available. | [178] |
| Bell’s palsy | (Nasalflu, Berna Biotech, Leiden, The Netherlands) | Bell’s palsy is a disorder that causes the muscles on one side of the face to suddenly weaken. The weakness often subsides over a few weeks and is only transitory. The weakening makes the lower portion of the face look sagging. One-sided smiles causes the afflicted eye to resist closing. | [181,182] |
| Postural orthostatic tachycardia syndrome (POTS) | mRNA-based vaccines | After standing or sitting up, a condition known as postural tachycardia syndrome (PoTS) causes an unnatural rise in heart rate. Consistent signs include fainting and dizziness. Postural orthostatic tachycardia syndrome (POTS) is another name for it. | [183] |
| Viral Vector Vaccine | RNA Vaccine | References |
|---|---|---|
| Enhanced immunological reaction | Lower immunogenicity. | [202] |
| Ad vaccines can be kept at 2–8 °C for three–six times longer than the mRNA vaccine made by Moderna | The Moderna vaccine needs to be stored at −20 °C while the Pfizer-BioNTech vaccine needs to be kept at −70 °C. Despite the fact that each vaccine can be kept at 2–8 °C for 5 and 30 days, respectively, these rigorous long-term storage specifications will make distribution difficult, particularly in places lacking a cold-chain infrastructure. | [203] |
| The manufacturing process is more complicated. | In comparison to a facility handling viral particles, mRNA vaccine may offer a low-cost, cell-free production method that is highly scalable and simpler to establish and run. | [204,205] |
| Risk of genomic integration. | mRNAs do not pose a risk for genome integration. | [202,206] |
| Response dampened by pre-existing immunity against vector. | Responses to mRNA boosters are influenced by pre-existing immunity. | [207,208] |
| Emergency Use listing Adenoviral vector-based mostly contains Full-length Spike protein, however Ad26Cov2-S vaccine express S protein contains K986P and V987P alterations (2P) in a loop that abuts the S2’ membrane fusion machinery’s core helix. | BioNTech-Pfizer and Moderna’s mRNA vaccines have the two stabilizing mutations in S2 (K986P and V987P), which have been shown to inhibit the conformational transition of the pre-fusion into the post-fusion structure of S. | [209,210] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. https://doi.org/10.3390/vaccines11020432
Chavda VP, Bezbaruah R, Valu D, Patel B, Kumar A, Prasad S, Kakoti BB, Kaushik A, Jesawadawala M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines. 2023; 11(2):432. https://doi.org/10.3390/vaccines11020432
Chicago/Turabian StyleChavda, Vivek P., Rajashri Bezbaruah, Disha Valu, Bindra Patel, Anup Kumar, Sanjay Prasad, Bibhuti Bhusan Kakoti, Ajeet Kaushik, and Mariya Jesawadawala. 2023. "Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status" Vaccines 11, no. 2: 432. https://doi.org/10.3390/vaccines11020432
APA StyleChavda, V. P., Bezbaruah, R., Valu, D., Patel, B., Kumar, A., Prasad, S., Kakoti, B. B., Kaushik, A., & Jesawadawala, M. (2023). Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines, 11(2), 432. https://doi.org/10.3390/vaccines11020432








