Aspects of Phage-Based Vaccines for Protein and Epitope Immunization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection, Inclusion, and Exclusion Criteria
3. Results
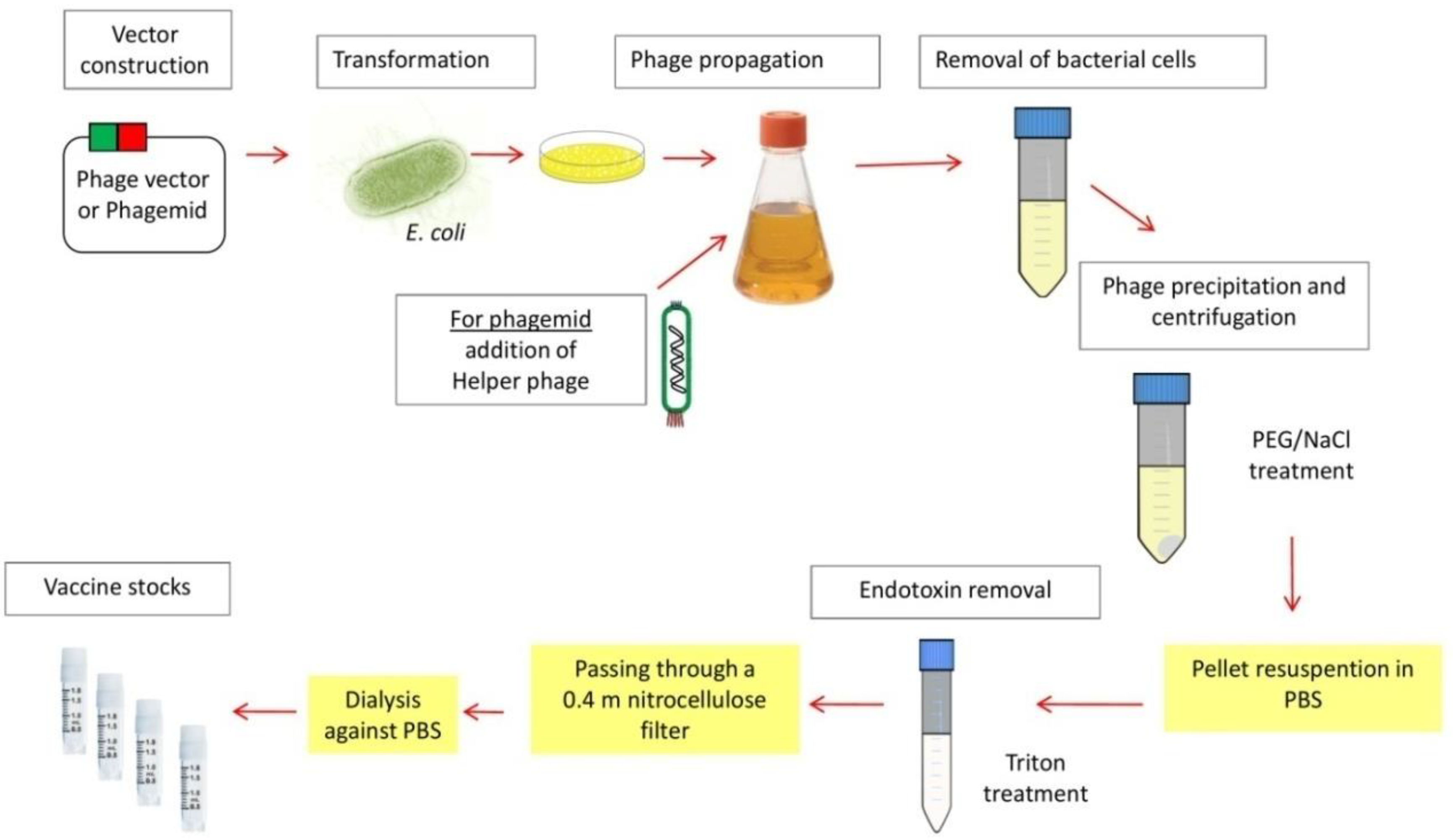
3.1. Description of Phage-Based Vaccines
| Phage | Characteristic | Application | Ref. |
|---|---|---|---|
| Lambda | Icosahedral, dsDNA, E.coli | Display | [15,34,35] |
| T7 | Icosahedral, dsDNA, E.coli | Display | [36,37] |
| T4 | Icosahedral, dsDNA, E.coli | Display | [38,39,40] |
| M13, M13KE | Filamentous, ssDNA, E. coli | Display | [41,42,43] |
| F8 | Filamentous, ssDNA, E. coli | Display | [44,45] |
| Qβ | Icosahedral, ssRNA, E.coli | Conjugated | [46,47] |
| MS2 | Icosahedral, ssRNA, E.coli | Conjugated | [48,49] |
| AP205 | Icosahedral, ssRNA, Acinetobacter | Conjugated | [50,51] |
3.2. Safety of Phage-Based Vaccines for Humans and Animals
3.3. Elicitation of the Immune Response by Phage-Based Vaccines
3.4. Phage-Base Vaccines in Clinical Trials
3.5. Approved Phage-Based Vaccines
3.6. Advantages and Disadvantages of Phage-Based Vaccines
| Condition | Agent | Target Antigen | Phage | Ref. |
|---|---|---|---|---|
| Viral infections | SARS-CoV-2 | S protein, NP | Lambda, AP205, QβBxb1, Bxb2 | [34,39,40,47,67,86,92] |
| Influenza A virus | 3M2e | T4 | [38] | |
| Dengue virus | NS1 | Qβ | [93] | |
| Foot-and-mouth disease | VP1 | MS2, T7 | [36,48] | |
| Zika virus | E protein | Qβ | [36] | |
| Bacterial infections | Chlamydia trachomatis | CT584, MOMP | Qβ, MS2 | [49,94] |
| Escherichia coli | STh | AP205 | [95] | |
| Vibrio Cholera | OSP | Qβ | [96] | |
| Bordetella pertussis | Pentasaccharide | Qβ | [97] | |
| Parasite infections | Rhipicephalus microplus | Sbm746 | M13 | [41] |
| Fasciola hepatic | Cathepsin | M13KE | [42,98] | |
| Plasmodium falciparum | Pfs47 | AP205 | [51] | |
| Yeast infections | Candida albicans | Fba1 | M13 | [99] |
| Cancer | Breast cancer | HER2, xCT | Lambda, M13, MS2 | [35,43,100,101] |
| Leukemia | ASPH | SNS-301 | [15] | |
| General | GD2 | Qβ | [102] | |
| Metastasis and solid tumor | MUC1 | Qβ | [103] | |
| Melanoma | Neoantigens | T7 | [37] | |
| Other conditions | Tauopathies | Tau | Qβ | [104] |
| Hypertension | CaV1.2 | Qβ | [46] | |
| Allergic rhinitis | H4R | M13 | [105] | |
| Cardiovascular diseases | ApoB, PCSK9, and CETP | Qβ | [66] | |
| Fertility control | Gonadotropins | f8 | [45] |
3.7. Phage-Based Vaccines against Viral Infections
3.7.1. SARS-CoV-2
3.7.2. Influenza A Virus
3.7.3. Dengue Virus
3.7.4. Foot-and-Mouth Disease Virus
3.7.5. Zika Virus
3.8. Phage-Based Vaccines against Bacterial Infections
3.8.1. Chlamydia trachomatis
3.8.2. Escherichia coli
3.8.3. Vibrio cholera
3.8.4. Bordetella pertussis
3.9. Phage-Based Vaccines against Parasite Infections
3.9.1. Rhipicephalus microplus
3.9.2. Fasciola hepatic
3.9.3. Plasmodium falciparum
3.10. Phage-Based Vaccines against Yeast Infections
3.11. Phage-Based Vaccines against Cancer
3.11.1. Breast Cancer
3.11.2. Other Types of Cancer
3.12. Phage-Based Vaccines against Other Types of Diseases or Conditions
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Statement
References
- Clokie, M.R.J.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in Nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- Nagano, K.; Tsutsumi, Y. Phage Display Technology as a Powerful Platform for Antibody Drug Discovery. Viruses 2021, 13, 178. [Google Scholar] [CrossRef]
- Molek, P.; Strukelj, B.; Bratkovic, T. Peptide Phage Display as a Tool for Drug Discovery: Targeting Membrane Receptors. Molecules 2011, 16, 857–887. [Google Scholar] [CrossRef] [PubMed]
- Aranaga, C.; Pantoja, L.D.; Martínez, E.A.; Falco, A. Phage Therapy in the Era of Multidrug Resistance in Bacteria: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 4577. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Li, X.; Han, G.; Zhu, Y.; Mao, C.; Yang, M. Phage-Based Vaccines. Adv. Drug Deliv. Rev. 2019, 145, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Mahony, J.; McAuliffe, O.; Ross, R.P.; van Sinderen, D. Bacteriophages as Biocontrol Agents of Food Pathogens. Curr. Opin. Biotechnol. 2011, 22, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Moingeon, P.; de Taisne, C.; Almond, J. Delivery Technologies for Human Vaccines. Br. Med. Bull. 2002, 62, 29–44. [Google Scholar] [CrossRef] [PubMed]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.N.; Rito-Palomares, M.; Benavides, J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef] [PubMed]
- Goulart, L.R.; de Santos, P. Strategies for Vaccine Design Using Phage Display-Derived Peptides. In Vaccine Design: Methods and Protocols, Volume 2: Vaccines for Veterinary Diseases; Springer: Berlin/Heidelberg, Germany, 2016; pp. 423–435. [Google Scholar]
- Aghebati-Maleki, L.; Bakhshinejad, B.; Baradaran, B.; Motallebnezhad, M.; Aghebati-Maleki, A.; Nickho, H.; Yousefi, M.; Majidi, J. Phage Display as a Promising Approach for Vaccine Development. J. Biomed. Sci. 2016, 23, 66. [Google Scholar] [CrossRef]
- de Vries, C.R.; Chen, Q.; Demirdjian, S.; Kaber, G.; Khosravi, A.; Liu, D.; Van Belleghem, J.D.; Bollyky, P.L. Phages in Vaccine Design and Immunity; Mechanisms and Mysteries. Curr. Opin. Biotechnol. 2021, 68, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.L.; Jewell, C.M. Phage Display as a Tool for Vaccine and Immunotherapy Development. Bioeng. Transl. Med. 2020, 5, e10142. [Google Scholar] [CrossRef]
- Roehnisch, T.; Then, C.; Nagel, W.; Blumenthal, C.; Braciak, T.; Donzeau, M.; Böhm, T.; Flaig, M.; Bourquin, C.; Oduncu, F.S. Phage Idiotype Vaccination: First Phase I/II Clinical Trial in Patients with Multiple Myeloma. J. Transl. Med. 2014, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Rajagopala, S.V.; Casjens, S.; Uetz, P. The Protein Interaction Map of Bacteriophage Lambda. BMC Microbiol. 2011, 11, 213. [Google Scholar] [CrossRef]
- Algazi, A.; Smith, W.; Panella, T.; Shin, D.; Fjaellskog, M.-L.; Celebi, J.; Drumheller, A.; Campbell, J.; Pierce, R.; Guarino, M. 349 Early Safety and Efficacy of a Phase 1/2 Open-Label, Multi-Center Trial of SNS-301 Added to Pembrolizumab in Patients with Advanced Squamous Cell Carcinoma of the Head and Neck (SCCHN). In Proceedings of the Regular and Young Investigator Award Abstracts; BMJ Publishing Group Ltd: London, UK, 2020; pp. A213–A214. [Google Scholar]
- Maruyama, I.N.; Maruyama, H.I.; Brenner, S. Lambda Foo: A Lambda Phage Vector for the Expression of Foreign Proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 8273–8277. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wang, L.; You, X.; Dai, P.; Zeng, Y. Advances in the T7 Phage Display System (Review). Mol. Med. Rep. 2017, 17, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Caberoy, N.B.; Zhou, Y.; Jiang, X.; Alvarado, G.; Li, W. Efficient Identification of Tubby-Binding Proteins by an Improved System of T7 Phage Display. J. Mol. Recognit. 2009, 23, 74–83. [Google Scholar] [CrossRef]
- Scott, B.M.; Matochko, W.L.; Gierczak, R.F.; Bhakta, V.; Derda, R.; Sheffield, W.P. Phage Display of the Serpin Alpha-1 Proteinase Inhibitor Randomized at Consecutive Residues in the Reactive Centre Loop and Biopanned with or without Thrombin. PLoS ONE 2014, 9, e84491. [Google Scholar] [CrossRef]
- Li, Q.; Shivachandra, S.B.; Zhang, Z.; Rao, V.B. Assembly of the Small Outer Capsid Protein, Soc, on Bacteriophage T4: A Novel System for High Density Display of Multiple Large Anthrax Toxins and Foreign Proteins on Phage Capsid. J. Mol. Biol. 2007, 370, 1006–1019. [Google Scholar] [CrossRef]
- Ren, Z.J.; Black, L.W.; Lewis, G.K.; Wingfield, P.T.; Locke, E.G.; Steven, A.C. Phage Display of Intact Domains at High Copy Number: A System Based on SOC, the Small Outer Capsid Protein of Bacteriophage T4. Protein Sci. 1996, 5, 1833–1843. [Google Scholar] [CrossRef]
- Jiang, J.; Abu-Shilbayeh, L.; Rao, V.B. Display of a PorA Peptide from Neisseria Meningitidis on the Bacteriophage T4 Capsid Surface. Infect. Immun. 1997, 65, 4770–4777. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, R.; Bradley, R.; Paranchych, W. The Effect of M13 Phage Infection upon the F Pili of E. Coli. Virology 1973, 54, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, V.; Balasubramanian, S.; Sista, S.; Vodyanoy, V.J.; Simonian, A.L. Highly Sensitive Phage-Based Biosensor for the Detection of β-Galactosidase. Anal. Chim. Acta 2007, 589, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Machera, S.J.; Niedziółka-Jönsson, J.; Szot-Karpińska, K. Phage-Based Sensors in Medicine: A Review. Chemosensors 2020, 8, 61. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- O’Rourke, J.P.; Peabody, D.S.; Chackerian, B. Affinity Selection of Epitope-Based Vaccines Using a Bacteriophage Virus-like Particle Platform. Curr. Opin. Virol. 2015, 11, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Gorzelnik, K.V.; Chang, J.-Y.; Langlais, C.; Jakana, J.; Young, R.; Zhang, J. Structures of Qβ Virions, Virus-like Particles, and the Qβ–MurA Complex Reveal Internal Coat Proteins and the Mechanism of Host Lysis. Proc. Natl. Acad. Sci. USA 2017, 114, 11697–11702. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, J. A Novel Delivery Platform Based on Bacteriophage MS2 Virus-like Particles. Virus Res. 2016, 211, 9–16. [Google Scholar] [CrossRef]
- Peabody, D.S.; Manifold-Wheeler, B.; Medford, A.; Jordan, S.K.; do Carmo Caldeira, J.; Chackerian, B. Immunogenic Display of Diverse Peptides on Virus-like Particles of RNA Phage MS2. J. Mol. Biol. 2008, 380, 252–263. [Google Scholar] [CrossRef]
- Maurer, P.; Jennings, G.T.; Willers, J.; Rohner, F.; Lindman, Y.; Roubicek, K.; Renner, W.A.; Müller, P.; Bachmann, M.F. A Therapeutic Vaccine for Nicotine Dependence: Preclinical Efficacy, and Phase I Safety and Immunogenicity. Eur. J. Immunol. 2005, 35, 2031–2040. [Google Scholar] [CrossRef]
- Cornuz, J.; Zwahlen, S.; Jungi, W.F.; Osterwalder, J.; Klingler, K.; van Melle, G.; Bangala, Y.; Guessous, I.; Müller, P.; Willers, J.; et al. A Vaccine against Nicotine for Smoking Cessation: A Randomized Controlled Trial. PLoS ONE 2008, 3, e2547. [Google Scholar] [CrossRef] [PubMed]
- Tissot, A.C.; Renhofa, R.; Schmitz, N.; Cielens, I.; Meijerink, E.; Ose, V.; Jennings, G.T.; Saudan, P.; Pumpens, P.; Bachmann, M.F. Versatile Virus-Like Particle Carrier for Epitope Based Vaccines. PLoS ONE 2010, 5, e9809. [Google Scholar] [CrossRef]
- Davenport, B.J.; Catala, A.; Weston, S.M.; Johnson, R.M.; Ardanuy, J.; Hammond, H.L.; Dillen, C.; Frieman, M.B.; Catalano, C.E.; Morrison, T.E. Phage-like Particle Vaccines Are Highly Immunogenic and Protect against Pathogenic Coronavirus Infection and Disease. NPJ Vaccines 2022, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Nicastro, J.; Slavcev, R.; Razazan, A.; Barati, N.; Nikpoor, A.R.; Brojeni, A.A.M.; Mosaffa, F.; Badiee, A.; Jaafari, M.R.; et al. Lambda Phage Nanoparticles Displaying HER2-Derived E75 Peptide Induce Effective E75-CD8+ T Response. Immunol. Res. 2018, 66, 200–206. [Google Scholar] [CrossRef]
- Wu, P.; Yin, X.; Liu, Q.; Wu, W.; Chen, C. Recombinant T7 Phage with FMDV AKT-III Strain VP1 Protein Is a Potential FMDV Vaccine. Biotechnol. Lett. 2021, 43, 35–41. [Google Scholar] [CrossRef]
- Shukla, G.S.; Sun, Y.-J.; Pero, S.C.; Sholler, G.S.; Krag, D.N. Immunization with Tumor Neoantigens Displayed on T7 Phage Nanoparticles Elicits Plasma Antibody and Vaccine-Draining Lymph Node B Cell Responses. J. Immunol. Methods 2018, 460, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, P.; Chen, C.; Feng, H.; Zhang, W.; Gu, C.; Wen, G.; Rao, V.B.; Tao, P. Bacteriophage T4 Vaccine Platform for Next-Generation Influenza Vaccine Development. Front. Immunol. 2021, 12, 745625. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ananthaswamy, N.; Jain, S.; Batra, H.; Tang, W.-C.; Lewry, D.A.; Richards, M.L.; David, S.A.; Kilgore, P.B.; Sha, J.; et al. A Universal Bacteriophage T4 Nanoparticle Platform to Design Multiplex SARS-CoV-2 Vaccine Candidates by CRISPR Engineering. Sci. Adv. 2021, 7, 1547. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jain, S.; Sha, J.; Batra, H.; Ananthaswamy, N.; Kilgore, P.B.; Hendrix, E.K.; Hosakote, Y.M.; Wu, X.; Olano, J.P.; et al. A Bacteriophage-Based, Highly Efficacious, Needle- and Adjuvant-Free, Mucosal COVID-19 Vaccine. MBio 2022, 13, e1822. [Google Scholar] [CrossRef] [PubMed]
- González-Mora, A.; Calvillo-Rodríguez, K.M.; Hernández-Pérez, J.; Rito-Palomares, M.; Martínez-Torres, A.C.; Benavides, J. Evaluation of the Immune Response of a Candidate Phage-Based Vaccine against Rhipicephalus Microplus (Cattle Tick). Pharmaceutics 2021, 13, 2018. [Google Scholar] [CrossRef] [PubMed]
- Villa-Mancera, A.; Alcalá-Canto, Y.; Olivares-Pérez, J.; Molina-Mendoza, P.; Hernández-Guzmán, K.; Utrera-Quintana, F.; Carreón-Luna, L.; Olmedo-Juárez, A.; Reynoso-Palomar, A. Vaccination with Cathepsin L Mimotopes of Fasciola Hepatica in Goats Reduces Worm Burden, Morphometric Measurements, and Reproductive Structures. Microb. Pathog. 2021, 155, 104859. [Google Scholar] [CrossRef]
- Wang, J.; Lamolinara, A.; Conti, L.; Giangrossi, M.; Cui, L.; Morelli, M.B.; Amantini, C.; Falconi, M.; Bartolacci, C.; Andreani, C.; et al. HER2-Displaying M13 Bacteriophages Induce Therapeutic Immunity against Breast Cancer. Cancers 2022, 14, 4054. [Google Scholar] [CrossRef] [PubMed]
- Samoylov, A.; Cochran, A.; Schemera, B.; Kutzler, M.; Donovan, C.; Petrenko, V.; Bartol, F.; Samoylova, T. Humoral Immune Responses against Gonadotropin Releasing Hormone Elicited by Immunization with Phage-Peptide Constructs Obtained via Phage Display. J. Biotechnol. 2015, 216, 20–28. [Google Scholar] [CrossRef]
- Johnson, A.K.; Jones, R.L.; Kraneburg, C.J.; Cochran, A.M.; Samoylov, A.M.; Wright, J.C.; Hutchinson, C.; Picut, C.; Cattley, R.C.; Martin, D.R.; et al. Phage Constructs Targeting Gonadotropin-Releasing Hormone for Fertility Control: Evaluation in Cats. J. Feline Med. Surg. 2020, 22, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Wang, G.; Qiu, Z.; Hu, X.; Zhang, H.; Yan, X.; Ke, F.; Zou, A.; Wang, M.; et al. A Bivalent Antihypertensive Vaccine Targeting L-type Calcium Channels and Angiotensin AT 1 Receptors. Br. J. Pharmacol. 2020, 177, 402–419. [Google Scholar] [CrossRef]
- Ortega-Rivera, O.A.; Shin, M.D.; Chen, A.; Beiss, V.; Moreno-Gonzalez, M.A.; Lopez-Ramirez, M.A.; Reynoso, M.; Wang, H.; Hurst, B.L.; Wang, J.; et al. Trivalent Subunit Vaccine Candidates for COVID-19 and Their Delivery Devices. J. Am. Chem. Soc. 2021, 143, 14748–14765. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Feng, H.; Chen, Y.; Yang, S.; Wei, Q.; Wang, J.; Liu, D.; Zhang, G. Immunogenicity Evaluation of MS2 Phage-Mediated Chimeric Nanoparticle Displaying an Immunodominant B Cell Epitope of Foot-and-Mouth Disease Virus. PeerJ 2018, 6, e4823. [Google Scholar] [CrossRef]
- Collar, A.L.; Linville, A.C.; Core, S.B.; Frietze, K.M. Epitope-Based Vaccines against the Chlamydia Trachomatis Major Outer Membrane Protein Variable Domain 4 Elicit Protection in Mice. Vaccines 2022, 10, 875. [Google Scholar] [CrossRef]
- Fougeroux, C.; Goksøyr, L.; Idorn, M.; Soroka, V.; Myeni, S.K.; Dagil, R.; Janitzek, C.M.; Søgaard, M.; Aves, K.-L.; Horsted, E.W.; et al. Capsid-like Particles Decorated with the SARS-CoV-2 Receptor-Binding Domain Elicit Strong Virus Neutralization Activity. Nat. Commun. 2021, 12, 324. [Google Scholar] [CrossRef]
- Yenkoidiok-Douti, L.; Williams, A.E.; Canepa, G.E.; Molina-Cruz, A.; Barillas-Mury, C. Engineering a Virus-Like Particle as an Antigenic Platform for a Pfs47-Targeted Malaria Transmission-Blocking Vaccine. Sci. Rep. 2019, 9, 16833. [Google Scholar] [CrossRef]
- Qi, H.; Lu, H.; Qiu, H.-J.; Petrenko, V.; Liu, A. Phagemid Vectors for Phage Display: Properties, Characteristics and Construction. J. Mol. Biol. 2012, 417, 129–143. [Google Scholar] [CrossRef]
- Bordier, C. Phase Separation of Integral Membrane Proteins in Triton X-114 Solution. J. Biol. Chem. 1981, 256, 1604–1607. [Google Scholar] [CrossRef] [PubMed]
- Szermer-Olearnik, B.; Boratyński, J. Removal of Endotoxins from Bacteriophage Preparations by Extraction with Organic Solvents. PLoS ONE 2015, 10, e0122672. [Google Scholar] [CrossRef] [PubMed]
- Bruttin, A.; Brüssow, H. Human Volunteers Receiving Escherichia Coli Phage T4 Orally: A Safety Test of Phage Therapy. Antimicrob. Agents Chemother. 2005, 49, 2874–2878. [Google Scholar] [CrossRef]
- Sarker, S.A.; Berger, B.; Deng, Y.; Kieser, S.; Foata, F.; Moine, D.; Descombes, P.; Sultana, S.; Huq, S.; Bardhan, P.K.; et al. Oral Application of Escherichia Coli Bacteriophage: Safety Tests in Healthy and Diarrheal Children from Bangladesh. Environ. Microbiol. 2017, 19, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral Phage Therapy of Acute Bacterial Diarrhea With Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef]
- Febvre, H.; Rao, S.; Gindin, M.; Goodwin, N.; Finer, E.; Vivanco, J.; Lu, S.; Manter, D.; Wallace, T.; Weir, T. PHAGE Study: Effects of Supplemental Bacteriophage Intake on Inflammation and Gut Microbiota in Healthy Adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The Safety and Toxicity of Phage Therapy: A Review of Animal and Clinical Studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Pattern Recognition Receptors. Cell 2002, 111, 927–930. [Google Scholar] [CrossRef]
- Gaubin, M.; Fanutti, C.; Mishal, Z.; Durrbach, A.; De Berardinis, P.; Sartorius, R.; Del Pozzo, G.; Guardiola, J.; Perham, R.N.; Piatier-Tonneau, D. Processing of Filamentous Bacteriophage Virions in Antigen-Presenting Cells Targets Both HLA Class I and Class II Peptide Loading Compartments. DNA Cell Biol. 2003, 22, 11–18. [Google Scholar] [CrossRef]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding Roles for CD4+ T Cells in Immunity to Viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef]
- Rothenfusser, S.; Tuma, E.; Wagner, M.; Endres, S.; Hartmann, G. Recent Advances in Immunostimulatory CpG Oligonucleotides. Curr. Opin. Mol. Ther. 2003, 5, 98–106. [Google Scholar]
- Krieg, A.M.; Yi, A.-K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. CpG Motifs in Bacterial DNA Trigger Direct B-Cell Activation. Nature 1995, 374, 546–549. [Google Scholar] [CrossRef]
- Mason, K.A.; Ariga, H.; Neal, R.; Valdecanas, D.; Hunter, N.; Krieg, A.M.; Whisnant, J.K.; Milas, L. Targeting Toll-like Receptor 9 with CpG Oligodeoxynucleotides Enhances Tumor Response to Fractionated Radiotherapy. Clin. Cancer Res. 2005, 11, 361–369. [Google Scholar] [CrossRef]
- Ortega-Rivera, O.A.; Pokorski, J.K.; Steinmetz, N.F. A Single-Dose, Implant-Based, Trivalent Virus-like Particle Vaccine against “Cholesterol Checkpoint” Proteins. Adv. Ther. 2021, 4, 2100014. [Google Scholar] [CrossRef]
- van Oosten, L.; Altenburg, J.J.; Fougeroux, C.; Geertsema, C.; van den End, F.; Evers, W.A.C.; Westphal, A.H.; Lindhoud, S.; van den Berg, W.; Swarts, D.C.; et al. Two-Component Nanoparticle Vaccine Displaying Glycosylated Spike S1 Domain Induces Neutralizing Antibody Response against SARS-CoV-2 Variants. MBio 2021, 12, e1813–e1821. [Google Scholar] [CrossRef]
- United States Food and Drug Administration 71 FR 47729 - Food Additives Permitted for Direct Addition to Food for Human Consumption; Bacteriophage Preparation. Available online: https://www.federalregister.gov/documents/2006/08/18/E6-13621/food-additives-permitted-for-direct-addition-to-food-for-human-consumption-bacteriophage-preparation (accessed on 10 December 2022).
- Voelker, R. FDA Approves Bacteriophage Trial. JAMA 2019, 321, 638. [Google Scholar] [CrossRef]
- Phage, A. FDA Clears Expanded Access IND for APT’s Phage Bank Therapy to Combat COVID-19-Related Bacterial Infections. Available online: https://aphage.com/fda-clears-expanded-access-ind-for-apts-phagebank-therapy-to-combat-covid-19-related-bacterial-infections/. (accessed on 10 December 2022).
- Johri, A.V.; Johri, P.; Hoyle, N.; Pipia, L.; Nadareishvili, L.; Nizharadze, D. Case Report: Chronic Bacterial Prostatitis Treated With Phage Therapy After Multiple Failed Antibiotic Treatments. Front. Pharmacol. 2021, 12, 692614. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter Baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Betancourt-Garcia, M.; Kare-Opaneye, Y.O.; Swierczewski, B.E.; Bennett, J.W.; Horne, B.; Fackler, J.; Suazo Hernandez, L.P.; Brownstein, M.J. Critically Ill Patient with Multidrug-Resistant Acinetobacter Baumannii Respiratory Infection Successfully Treated with Intravenous and Nebulized Bacteriophage Therapy. Antimicrob. Agents Chemother. 2022, 66, e00824-21. [Google Scholar] [CrossRef] [PubMed]
- Khatami, A.; Lin, R.C.Y.; Petrovic-Fabijan, A.; Alkalay-Oren, S.; Almuzam, S.; Britton, P.N.; Brownstein, M.J.; Dao, Q.; Fackler, J.; Hazan, R.; et al. Bacterial Lysis, Autophagy and Innate Immune Responses during Adjunctive Phage Therapy in a Child. EMBO Mol. Med. 2021, 13, e936. [Google Scholar] [CrossRef]
- Doub, J.B.; Ng, V.Y.; Johnson, A.J.; Slomka, M.; Fackler, J.; Horne, B.; Brownstein, M.J.; Henry, M.; Malagon, F.; Biswas, B. Salvage Bacteriophage Therapy for a Chronic MRSA Prosthetic Joint Infection. Antibiotics 2020, 9, 241. [Google Scholar] [CrossRef]
- Gainey, A.B.; Burch, A.; Brownstein, M.J.; Brown, D.E.; Fackler, J.; Horne, B.; Biswas, B.; Bivens, B.N.; Malagon, F.; Daniels, R. Combining Bacteriophages with Cefiderocol and Meropenem/Vaborbactam to Treat a Pan-drug Resistant Achromobacter Species Infection in a Pediatric Cystic Fibrosis Patient. Pediatr. Pulmonol. 2020, 55, 2990–2994. [Google Scholar] [CrossRef] [PubMed]
- Gainey, A.B.; Daniels, R.; Burch, A.-K.; Hawn, J.; Fackler, J.; Biswas, B.; Brownstein, M.J. Recurrent ESBL Escherichia Coli Urosepsis in a Pediatric Renal Transplant Patient Treated with Antibiotics and Bacteriophage Therapy. Pediatr. Infect. Dis. J. 2023, 42, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Courtwright, A.M.; Koval, C.; Lehman, S.M.; Morales, S.; Furr, C.L.; Rosas, F.; Brownstein, M.J.; Fackler, J.R.; Sisson, B.M.; et al. Early Clinical Experience of Bacteriophage Therapy in 3 Lung Transplant Recipients. Am. J. Transplant. 2019, 19, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.M.; Burrowes, B.H.; Enright, M.C. The Role of Regulated Clinical Trials in the Development of Bacteriophage Therapeutics. J. Mol. Genet. Med. 2012, 6, e1000050. [Google Scholar] [CrossRef]
- Henry, K.A.; Arbabi-Ghahroudi, M.; Scott, J.K. Beyond Phage Display: Non-Traditional Applications of the Filamentous Bacteriophage as a Vaccine Carrier, Therapeutic Biologic, and Bioconjugation Scaffold. Front. Microbiol. 2015, 6, 755. [Google Scholar] [CrossRef]
- Greenwood, J.; Willis, A.E.; Perham, R.N. Multiple Display of Foreign Peptides on a Filamentous Bacteriophage. J. Mol. Biol. 1991, 220, 821–827. [Google Scholar] [CrossRef] [PubMed]
- El-Awady, M.K.; Tabll, A.A.; Yousif, H.; El-Abd, Y.; Reda, M.; Khalil, S.B.; El-Zayadi, A.R.; Shaker, M.H.; Bader El Din, N.G. Murine Neutralizing Antibody Response and Toxicity to Synthetic Peptides Derived from E1 and E2 Proteins of Hepatitis C Virus. Vaccine 2010, 28, 8338–8344. [Google Scholar] [CrossRef] [PubMed]
- González-Cano, P.; Gamage, L.N.A.; Marciniuk, K.; Hayes, C.; Napper, S.; Hayes, S.; Griebel, P.J. Lambda Display Phage as a Mucosal Vaccine Delivery Vehicle for Peptide Antigens. Vaccine 2017, 35, 7256–7263. [Google Scholar] [CrossRef]
- Bazan, J.; Całkosiński, I.; Gamian, A. Phage Display—A Powerful Technique for Immunotherapy. Hum. Vaccin. Immunother. 2012, 8, 1817–1828. [Google Scholar] [CrossRef]
- Adhya, S.; Merril, C.R.; Biswas, B. Therapeutic and Prophylactic Applications of Bacteriophage Components in Modern Medicine. Cold Spring Harb. Perspect. Med. 2014, 4, a012518. [Google Scholar] [CrossRef] [Green Version]
- Freeman, K.G.; Wetzel, K.S.; Zhang, Y.; Zack, K.M.; Jacobs-Sera, D.; Walters, S.M.; Barbeau, D.J.; McElroy, A.K.; Williams, J.V.; Hatfull, G.F. A Mycobacteriophage-Based Vaccine Platform: SARS-CoV-2 Antigen Expression and Display. Microorganisms 2021, 9, 2414. [Google Scholar] [CrossRef]
- Moon, S.A.; Ki, M.K.; Lee, S.; Hong, M.-L.; Kim, M.; Kim, S.; Chung, J.; Rhee, S.G.; Shim, H. Antibodies against Non-Immunizing Antigens Derived from a Large Immune ScFv Library. Mol. Cells 2011, 31, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Morrison, D.C.; Ulevitch, R.J. The Effects of Bacterial Endotoxins on Host Mediation Systems. A Review. Am. J. Pathol. 1978, 93, 526–618. [Google Scholar]
- Bretaudeau, L.; Tremblais, K.; Aubrit, F.; Meichenin, M.; Arnaud, I. Good Manufacturing Practice (GMP) Compliance for Phage Therapy Medicinal Products. Front. Microbiol. 2020, 11, 61. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Merabishvili, M.; Raemdonck, H.; de Vos, D.; Verbeken, G. Bacteriophage production in compliance with regulatory requirements. In Bacteriophage Therapy. Methods Mol. Biol. 2018, 1693, 233–252. [Google Scholar]
- Staquicini, D.I.; Tang, F.H.F.; Markosian, C.; Yao, V.J.; Staquicini, F.I.; Dodero-Rojas, E.; Contessoto, V.G.; Davis, D.; O’Brien, P.; Habib, N.; et al. Design and Proof-of-Concept for Targeted Phage-Based COVID-19 Vaccination Strategies with a Streamlined Cold-Free Supply Chain. Biorxiv Prepr. Serv. Biol. 2021. [Google Scholar] [CrossRef]
- Warner, N.L.; Frietze, K.M. Development of Bacteriophage Virus-Like Particle Vaccines Displaying Conserved Epitopes of Dengue Virus Non-Structural Protein 1. Vaccines 2021, 9, 726. [Google Scholar] [CrossRef]
- Webster, E.; Seiger, K.W.; Core, S.B.; Collar, A.L.; Knapp-Broas, H.; Graham, J.; Shrestha, M.; Afzaal, S.; Geisler, W.M.; Wheeler, C.M.; et al. Immunogenicity and Protective Capacity of a Virus-like Particle Vaccine against Chlamydia Trachomatis Type 3 Secretion System Tip Protein, CT584. Vaccines 2022, 10, 111. [Google Scholar] [CrossRef]
- Govasli, M.L.; Diaz, Y.; Puntervoll, P. Virus-like Particle-Display of the Enterotoxigenic Escherichia Coli Heat-Stable Toxoid STh-A14T Elicits Neutralizing Antibodies in Mice. Vaccine 2019, 37, 6405–6414. [Google Scholar] [CrossRef] [PubMed]
- Rashidijahanabad, Z.; Kelly, M.; Kamruzzaman, M.; Qadri, F.; Bhuiyan, T.R.; McFall-Boegeman, H.; Wu, D.; Piszczek, G.; Xu, P.; Ryan, E.T.; et al. Virus-like Particle Display of Vibrio Cholerae O -Specific Polysaccharide as a Potential Vaccine against Cholera. ACS Infect. Dis. 2022, 8, 574–583. [Google Scholar] [CrossRef]
- Wang, P.; Huo, C.; Lang, S.; Caution, K.; Nick, S.T.; Dubey, P.; Deora, R.; Huang, X. Chemical Synthesis and Immunological Evaluation of a Pentasaccharide Bearing Multiple Rare Sugars as a Potential Anti-pertussis Vaccine. Angew. Chem. Int. Ed. 2020, 59, 6451–6458. [Google Scholar] [CrossRef]
- Villa-Mancera, A.; Olivares-Pérez, J.; Olmedo-Juárez, A.; Reynoso-Palomar, A. Phage Display-Based Vaccine with Cathepsin L and Excretory-Secretory Products Mimotopes of Fasciola Hepatica Induces Protective Cellular and Humoral Immune Responses in Sheep. Vet. Parasitol. 2021, 289, 109340. [Google Scholar] [CrossRef]
- Shi, H.; Dong, S.; Zhang, X.; Chen, X.; Gao, X.; Wang, L. Phage Vaccines Displaying YGKDVKDLFDYAQE Epitope Induce Protection against Systemic Candidiasis in Mouse Model. Vaccine 2018, 36, 5717–5724. [Google Scholar] [CrossRef]
- Razazan, A.; Nicastro, J.; Slavcev, R.; Barati, N.; Arab, A.; Mosaffa, F.; Jaafari, M.R.; Behravan, J. Lambda Bacteriophage Nanoparticles Displaying GP2, a HER2/Neu Derived Peptide, Induce Prophylactic and Therapeutic Activities against TUBO Tumor Model in Mice. Sci. Rep. 2019, 9, 2221. [Google Scholar] [CrossRef]
- Rolih, V.; Caldeira, J.; Bolli, E.; Salameh, A.; Conti, L.; Barutello, G.; Riccardo, F.; Magri, J.; Lamolinara, A.; Parra, K.; et al. Development of a VLP-Based Vaccine Displaying an XCT Extracellular Domain for the Treatment of Metastatic Breast Cancer. Cancers 2020, 12, 1492. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ye, J.; DeLaitsch, A.T.; Rashidijahanabad, Z.; Lang, S.; Kakeshpour, T.; Zhao, Y.; Ramadan, S.; Saavedra, P.V.; Yuzbasiyan-Gurkan, V.; et al. Chemoenzymatic Synthesis of 9NHAc-GD2 Antigen to Overcome the Hydrolytic Instability of O -Acetylated-GD2 for Anticancer Conjugate Vaccine Development. Angew. Chem. Int. Ed. 2021, 60, 24179–24188. [Google Scholar] [CrossRef]
- Wu, X.; Yin, Z.; McKay, C.; Pett, C.; Yu, J.; Schorlemer, M.; Gohl, T.; Sungsuwan, S.; Ramadan, S.; Baniel, C.; et al. Protective Epitope Discovery and Design of MUC1-Based Vaccine for Effective Tumor Protections in Immunotolerant Mice. J. Am. Chem. Soc. 2018, 140, 16596–16609. [Google Scholar] [CrossRef]
- Maphis, N.M.; Peabody, J.; Crossey, E.; Jiang, S.; Jamaleddin Ahmad, F.A.; Alvarez, M.; Mansoor, S.K.; Yaney, A.; Yang, Y.; Sillerud, L.O.; et al. Qβ Virus-like Particle-Based Vaccine Induces Robust Immunity and Protects against Tauopathy. NPJ Vaccines 2019, 4, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Sha, J.; Wang, H.; An, L.; Liu, T.; Li, L. P-FN12, an H4R-Based Epitope Vaccine Screened by Phage Display, Regulates the Th1/Th2 Balance in Rat Allergic Rhinitis. Mol. Ther. Methods Clin. Dev. 2018, 11, 83–91. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/?gclid=EAIaIQobChMIxYXludnP6gIVVIXVCh1zuQ8iEAAYASAAEgK00fD_BwE (accessed on 23 April 2021).
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human Neutralizing Antibodies Elicited by SARS-CoV-2 Infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide Tag Forming a Rapid Covalent Bond to a Protein, through Engineering a Bacterial Adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, e1115485. [Google Scholar] [CrossRef] [Green Version]
- Janitzek, C.M.; Matondo, S.; Thrane, S.; Nielsen, M.A.; Kavishe, R.; Mwakalinga, S.B.; Theander, T.G.; Salanti, A.; Sander, A.F. Bacterial Superglue Generates a Full-Length Circumsporozoite Protein Virus-like Particle Vaccine Capable of Inducing High and Durable Antibody Responses. Malar. J. 2016, 15, 545. [Google Scholar] [CrossRef]
- Staquicini, D.I.; Barbu, E.M.; Zemans, R.L.; Dray, B.K.; Staquicini, F.I.; Dogra, P.; Cardó-Vila, M.; Miranti, C.K.; Baze, W.B.; Villa, L.L.; et al. Targeted Phage Display-Based Pulmonary Vaccination in Mice and Non-Human Primates. Med 2021, 2, 321–342.e8. [Google Scholar] [CrossRef]
- Pintos Pascual, I.; Muñez Rubio, E.; Alarcón Tomás, A.; Ramos Martínez, A. Infecciones Por Virus de La Gripe y Virus Respiratorios. Med. Programa. Form. Médica Contin. Acreditado 2018, 12, 3291–3297. [Google Scholar] [CrossRef]
- Grubman, M.J.; Baxt, B. Foot-and-Mouth Disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Bittle, J.L.; Houghten, R.A.; Alexander, H.; Shinnick, T.M.; Sutcliffe, J.G.; Lerner, R.A.; Rowlands, D.J.; Brown, F. Protection against Foot-and-Mouth Disease by Immunization with a Chemically Synthesized Peptide Predicted from the Viral Nucleotide Sequence. Nature 1982, 298, 30–33. [Google Scholar] [CrossRef]
- Dai, L.; Song, J.; Lu, X.; Deng, Y.-Q.; Musyoki, A.M.; Cheng, H.; Zhang, Y.; Yuan, Y.; Song, H.; Haywood, J.; et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe 2016, 19, 696–704. [Google Scholar] [CrossRef]
- Basu, R.; Zhai, L.; Contreras, A.; Tumban, E. Immunization with Phage Virus-like Particles Displaying Zika Virus Potential B-Cell Epitopes Neutralizes Zika Virus Infection of Monkey Kidney Cells. Vaccine 2018, 36, 1256–1264. [Google Scholar] [CrossRef]
- Mohseni, M.; Sung, S.; Takov, V. Chlamydia; Starpearls: Tampa, FL, USA, 2022. [Google Scholar]
- Barta, M.L.; Hickey, J.; Kemege, K.E.; Lovell, S.; Battaile, K.P.; Hefty, P.S. Structure of CT584 from Chlamydia Trachomatis Refined to 3.05 Å Resolution. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Bulir, D.C.; Liang, S.; Lee, A.; Chong, S.; Simms, E.; Stone, C.; Kaushic, C.; Ashkar, A.; Mahony, J.B. Immunization with Chlamydial Type III Secretion Antigens Reduces Vaginal Shedding and Prevents Fallopian Tube Pathology Following Live C. Muridarum Challenge. Vaccine 2016, 34, 3979–3985. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.X.; Watkins, N.G.; Caldwell, H.D. Nucleotide and Deduced Amino Acid Sequences for the Four Variable Domains of the Major Outer Membrane Proteins of the 15 Chlamydia Trachomatis Serovars. Infect. Immun. 1989, 57, 1040–1049. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E.M.; Cheng, X.; Markoff, B.A.; Fielder, T.J.; de la Maza, L.M. Functional and Structural Mapping of Chlamydia Trachomatis Species-Specific Major Outer Membrane Protein Epitopes by Use of Neutralizing Monoclonal Antibodies. Infect. Immun. 1991, 59, 4147–4153. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, T.; Yamasaki, S.; Takeda, Y.; Nair, G.B. Vibrio Cholerae O139 Bengal: Odyssey of a Fortuitous Variant. Microbes Infect. 2003, 5, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.L. Pertussis: The Disease and New Diagnostic Methods. Clin. Microbiol. Rev. 1988, 1, 365–376. [Google Scholar] [CrossRef]
- Maclennan, A.P. Specific Lipopolysaccharides of Bordetella. Biochem. J. 1960, 74, 398–409. [Google Scholar] [CrossRef]
- Caroff, M.; Brisson, J.-R.; Martin, A.; Karibian, D. Structure of the Bordetella Pertussis 1414 Endotoxin. FEBS Lett. 2000, 477, 8–14. [Google Scholar] [CrossRef]
- Niedziela, T.; Letowska, I.; Lukasiewicz, J.; Kaszowska, M.; Czarnecka, A.; Kenne, L.; Lugowski, C. Epitope of the Vaccine-Type Bordetella Pertussis Strain 186 Lipooligosaccharide and Antiendotoxin Activity of Antibodies Directed against the Terminal Pentasaccharide-Tetanus Toxoid Conjugate. Infect. Immun. 2005, 73, 7381–7389. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zheng, J. Prospects for Liver Fluke Vaccines. Exp. Parasitol. 2021, 230, 108170. [Google Scholar] [CrossRef] [PubMed]
- Villa-Mancera, A.; Alcalá-Canto, Y.; Reynoso-Palomar, A.; Olmedo-Juárez, A.; Olivares-Pérez, J. Vaccination with Cathepsin L Phage-Exposed Mimotopes, Single or in Combination, Reduce Size, Fluke Burden, Egg Production and Viability in Sheep Experimentally Infected with Fasciola Hepatica. Parasitol. Int. 2021, 83, 102355. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.W. Global Malaria Eradication and the Importance of Plasmodium Falciparum Epidemiology in Africa. BMC Med. 2015, 13, 23. [Google Scholar] [CrossRef]
- Canepa, G.E.; Molina-Cruz, A.; Yenkoidiok-Douti, L.; Calvo, E.; Williams, A.E.; Burkhardt, M.; Peng, F.; Narum, D.; Boulanger, M.J.; Valenzuela, J.G.; et al. Antibody Targeting of a Specific Region of Pfs47 Blocks Plasmodium Falciparum Malaria Transmission. Npj. Vaccines 2018, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderone, R.A.; Fonzi, W.A. Virulence Factors of Candida Albicans. Trends Microbiol. 2001, 9, 327–335. [Google Scholar] [CrossRef]
- Rodaki, A.; Young, T.; Brown, A.J.P. Effects of Depleting the Essential Central Metabolic Enzyme Fructose-1,6-Bisphosphate Aldolase on the Growth and Viability of Candida Albicans: Implications for Antifungal Drug Target Discovery. Eukaryot. Cell 2006, 5, 1371–1377. [Google Scholar] [CrossRef]
- Medrano-Díaz, C.L.; Vega-González, A.; Ruiz-Baca, E.; Moreno, A.; Cuéllar-Cruz, M. Moonlighting Proteins Induce Protection in a Mouse Model against Candida Species. Microb. Pathog. 2018, 124, 21–29. [Google Scholar] [CrossRef]
- Xin, H.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Synthetic Glycopeptide Vaccines Combining β-Mannan and Peptide Epitopes Induce Protection against Candidiasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13526–13531. [Google Scholar] [CrossRef]
- Tovey, S.M.; Brown, S.; Doughty, J.C.; Mallon, E.A.; Cooke, T.G.; Edwards, J. Poor Survival Outcomes in HER2-Positive Breast Cancer Patients with Low-Grade, Node-Negative Tumours. Br. J. Cancer 2009, 100, 680–683. [Google Scholar] [CrossRef]
- Maadi, H.; Soheilifar, M.H.; Choi, W.-S.; Moshtaghian, A.; Wang, Z. Trastuzumab Mechanism of Action; 20 Years of Research to Unravel a Dilemma. Cancers 2021, 13, 540. [Google Scholar] [CrossRef]
- Donofrio, G.; Tebaldi, G.; Lanzardo, S.; Ruiu, R.; Bolli, E.; Ballatore, A.; Rolih, V.; Macchi, F.; Conti, L.; Cavallo, F. Bovine Herpesvirus 4-Based Vector Delivering the Full Length XCT DNA Efficiently Protects Mice from Mammary Cancer Metastases by Targeting Cancer Stem Cells. Oncoimmunology 2018, 7, e1494108. [Google Scholar] [CrossRef]
- Dong, X.; Pan, P.; Ye, J.-J.; Zhang, Q.-L.; Zhang, X.-Z. Hybrid M13 Bacteriophage-Based Vaccine Platform for Personalized Cancer Immunotherapy. Biomaterials 2022, 289, 121763. [Google Scholar] [CrossRef]
- Rajaram, P.; Chandra, P.; Ticku, S.; Pallavi, B.; Rudresh, K.; Mansabdar, P. Epidermal Growth Factor Receptor: Role in Human Cancer. Indian J. Dent. Res. 2017, 28, 687. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, B.S.; Pestana, E.S.; Hidalgo, G.G.; García, T.H.; Rodríguez, R.P.; Ullrich, A.; Férnandez, L.E. Active Antimetastatic Immunotherapy in Lewis Lung Carcinoma with Self EGFR Extracellular Domain Protein in VSSP Adjuvant. Int. J. Cancer 2006, 119, 2190–2199. [Google Scholar] [CrossRef]
- Asadi-Ghalehni, M.; Rasaee, M.J.; Namvar Asl, N.; Khosravani, M.; Rajabibazl, M.; Khalili, S.; Modjtahedi, H.; Sadroddiny, E. Construction of a Recombinant Phage-Vaccine Capable of Reducing the Growth Rate of an Established LL2 Tumor Model. Iran. J. Allergy. Asthma. Immunol. 2018, 17, 240–249. [Google Scholar]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.Y.; Cheung, N.-K.V.; Modak, S.; Mauguen, A.; Feng, Y.; Basu, E.; Roberts, S.S.; Ragupathi, G.; Kushner, B.H. Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients With High-Risk Neuroblastoma With Prior Disease Progression. J. Clin. Oncol. 2021, 39, 215–226. [Google Scholar] [CrossRef]
- Sjoberg, E.R.; Manzi, A.E.; Khoo, K.H.; Dell, A.; Varki, A. Structural and Immunological Characterization of O-Acetylated GD2. Evidence That GD2 Is an Acceptor for Ganglioside O-Acetyltransferase in Human Melanoma Cells. J. Biol. Chem. 1992, 267, 16200–16211. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bandyopadhyay, D. MUC1: A Target Molecule for Cancer Therapy. Cancer Biol. Ther. 2007, 6, 481–486. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Suehiro, Y.; Fukui, M.; Shikichi, K.; Imai, K.; Hinoda, Y. Circulating Anti-MUC1 IgG Antibodies as a Favorable Prognostic Factor for Pancreatic Cancer. Int. J. Cancer 2003, 103, 97–100. [Google Scholar] [CrossRef]
- Qadir, J.; Majid, S. Immunopharmacogenomics: Clinical Applications, Challenges, and Future Prospects. In A Molecular Approach to Immunogenetics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 255–276. [Google Scholar]
- Billingsley, M.L.; Kincaid, R.L. Regulated Phosphorylation and Dephosphorylation of Tau Protein: Effects on Microtubule Interaction, Intracellular Trafficking and Neurodegeneration. Biochem. J. 1997, 323, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.K.; Rafiq, T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can. J. Cardiol. 2021, 37, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Tajada, S.; Cidad, P.; Colinas, O.; Santana, L.F.; López-López, J.R.; Pérez-García, M.T. Down-Regulation of CaV1.2 Channels during Hypertension: How Fewer CaV1.2 Channels Allow More Ca2+ into Hypertensive Arterial Smooth Muscle. J. Physiol. 2013, 591, 6175–6191. [Google Scholar] [CrossRef]
- Kakli, H.A.; Riley, T.D. Allergic Rhinitis. Prim. Care Clin. Off. Pract. 2016, 43, 465–475. [Google Scholar] [CrossRef]
- Kundu, J.; Kundu, S. Cardiovascular Disease (CVD) and Its Associated Risk Factors among Older Adults in India: Evidence from LASI Wave 1. Clin. Epidemiol. Glob. Health 2022, 13, 100937. [Google Scholar] [CrossRef]
- Willer, C.J.; Sanna, S.; Jackson, A.U.; Scuteri, A.; Bonnycastle, L.L.; Clarke, R.; Heath, S.C.; Timpson, N.J.; Najjar, S.S.; Stringham, H.M.; et al. Newly Identified Loci That Influence Lipid Concentrations and Risk of Coronary Artery Disease. Nat. Genet. 2008, 40, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Holesh, J.E.; Bass, A.N.; Lord, M. Physiology, Ovulation; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK441996/ (accessed on 10 December 2022).
- Benka, V.A.W.; Levy, J.K. Vaccines for Feline Contraception. J. Feline Med. Surg. 2015, 17, 758–765. [Google Scholar] [CrossRef]
- Dąbrowska, K. Phage Therapy: What Factors Shape Phage Pharmacokinetics and Bioavailability? Systematic and Critical Review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.-C.; Roach, D.R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, M. Aspects of Phage-Based Vaccines for Protein and Epitope Immunization. Vaccines 2023, 11, 436. https://doi.org/10.3390/vaccines11020436
Palma M. Aspects of Phage-Based Vaccines for Protein and Epitope Immunization. Vaccines. 2023; 11(2):436. https://doi.org/10.3390/vaccines11020436
Chicago/Turabian StylePalma, Marco. 2023. "Aspects of Phage-Based Vaccines for Protein and Epitope Immunization" Vaccines 11, no. 2: 436. https://doi.org/10.3390/vaccines11020436
APA StylePalma, M. (2023). Aspects of Phage-Based Vaccines for Protein and Epitope Immunization. Vaccines, 11(2), 436. https://doi.org/10.3390/vaccines11020436






