COVID-19 Vaccine-Induced Lichenoid Eruptions—Clinical and Histopathologic Spectrum in a Case Series of Fifteen Patients with Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. COVID-19 VILE: Case Series of Fifteen Patients
2.1.2. Review of the Literature
Search Strategy
3. Results
3.1. Case Series of 15 Patients
3.1.1. Case 1—BLAISE—Unilateral—Left Upper Extremity
3.1.2. Case 2—BLAISE—Bilateral—Lower Extremities
3.1.3. Case 3—BLAISE, Lichen Planus—Unilateral—Left-Sided Trunk and Thigh
3.1.4. Case 4—BLAISE, Lichen Striatus-Like—Unilateral—Left Side of Neck
3.1.5. Case 5—V-ILE with Inverse Component
3.1.6. Case 6—V-ILE with Multiple Lichen Planus-like Keratosis (LPLKs)
3.1.7. Case 7—V-ILE, Extensive
3.1.8. Case 8—V-ILE, Unilateral Left-Sided
3.1.9. Case 9—V-ILE Limited to Trunk with Psoriasiform Clinical Presentation
3.1.10. Case 10—V-ILE with Solitary Lesion, Forme Fruste Presentation
3.1.11. Cases 11, 12, 13—V-ILE—Typical-Appearing Papular Eruptions
3.1.12. Case 14—V-ILE with Lichenoid Plaques on Chest and Thoracolumbar Areas
3.1.13. Case 15—V-ILE with Extensive Post Inflammatory Hyperpigmentation
3.1.14. Summary of Clincal and Atypical Histopthologic Features of Case Series
3.2. Results—Literature Review
COVID-19 Vaccine-Associated Lichenoid Eruptions
3.3. Data from Literature Review Compared to That of from Case Series
3.3.1. The COVID-19 Vaccines
3.3.2. V-ILE – First Eruption and Vaccine Dose Number
3.3.3. Time to Onset of First Eruption
3.3.4. Treatment
3.3.5. Clinical Course
4. Discussion
4.1. Immunopathogenesis—Nexus between Vaccination and Autoimmune Phenomena
4.2. Timing of the Lichenoid Eruption in Relation to Vaccine Dose or Prior History
4.3. Etiology
4.4. Discussion of V-ILE, BLAISE: Cases 1,2,3,4
4.5. Variability of the Blaschkoid Clinical Presentation: Symptoms, Distribution, Laterality, Treatment and Resolution
5. Conclusions
Variability of Pathology within the V-ILE Case Series
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, J.; Wang, N.; Corbett, K.S.; Wrapp, D.; Kirchdoerfer, R.N.; Turner, H.L.; Cottrell, C.A.; Becker, M.M.; Wang, L.; Shi, W.; et al. Immunogenicity and structures of a rationally designed perfusion. MERS-Cov spike antigen. Proc. Natl. Acad. Sci. USA 2017, 114, E7348–E7357. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Goldsmith, J.A.; Schaub, J.M.; Divenere, A.M.; Kuo, H.-C.; Javanmardi, K.; Le, K.C.; Wrapp, D.; Lee, A.G.; Liu, Y.; et al. Structure-based design of perfusion-stabilized SARS-COV-2 spikes. Science 2020, 23, eabd0826. [Google Scholar] [CrossRef]
- Corbett, K.S.; Nason, M.C.; Flach, B.; Gagne, M.; O’Connell, S.; Johnston, T.S.; Shah, S.N.; Edara, V.V.; Floyd, K.; Lai, L.; et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-Co-V-2 in nonhuman primates. Science 2021, 373, eabj0299. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. mRNASG. An mRNA vaccine against SARS-CoV-2-preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Guler, A.; et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2S vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef]
- Kaplonek, P.; Cizmeci, D.; Fischinger, S.; Collier, A.R.; Suscovich, T.; Linde, C.; Broge, T.; Mann, C.; Amanat, F.; Dayal, D.; et al. mRNA-1273 and BNT162b COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions. Sci. Transl. Med. 2022, 14, eabm2311. [Google Scholar] [CrossRef]
- Sontheimer, R.D. Lichenoid tissue reaction / interface dermatitis: Clinical and histological perspectives. J. Investig. Dermatol. 2009, 129, 1088–1099. [Google Scholar] [CrossRef]
- Yasukawa, M.; Ohminami, A.; Arai, J.; Kasahara, Y.; Ishida, Y.; Fujita, S. Granule exocytosis, and not the Fas/Fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4(+) as well as CD8(+) cytotoxic T lymphocytes in humans. Blood 2000, 95, 2352–2355. [Google Scholar] [CrossRef]
- Shiohara, T.; Mizukawa, Y. Lichen Planus and Lichenoid Dermatoses. In Dermatology, 4th ed.; Bologna, J.L., Schaffer, J.V., Cerroni, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 191–192. [Google Scholar]
- Tziotzios, C.; BChir, M.B.; Lee, J.Y.W.; Brier, T.; Stefanato, C.M.; Fenton, D.; McGrath, J.A. Lichen planus and lichenoid dermatoses: Clinical overview and molecular basis. J. Am. Acad. Dermatol. 2018, 79, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Fishman, M.; Wattenberg, D.; Gordon, M.; Lebwohl, M. “COVID arm”: A reaction to the Moderna vaccine. JAAD Case Rep. 2021, 10, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.S.; Galan, A.; Watsky, K.L.; Little, A.J. Delayed localized hypersensitivity reactions to the Moderna COVID-19 vaccine. JAMA Dermatol. 2021, 157, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Bolognia, J.L.; Orlow, S.J.; Glick, S. Lines of Blaschko. J. Am. Acad. Dermatol. 1994, 31, 157–190. [Google Scholar] [CrossRef] [PubMed]
- Patrizi, A.; Neri, I.; Fiorentini, C.; Bonci, A.; Ricci, G. Lichen striatus: Clinical and laboratory features of 115 children. Pediatr. Dermatol. 2004, 21, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Grosshans, E.; Marot, L. Blaschkitis in adults. Ann. Dermatol. Venereol. 1990, 117, 9–15. [Google Scholar] [PubMed]
- Taieb, A.; El Youbi, A.; Grosshans, E.; Maleville, J. Lichen striatus: A Blaschko linear acquired inflammatory skin eruption. J. Am. Acad. Dermatol. 1991, 25, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Halevy, S.; Shai, A. Lichenoid drug eruptions. J. Am. Acad. Dermatol. 1993, 29, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Lodi, G.; Pellicano, R.; Carrozzo, M. Hepatitis C virus infection and lichen planus: A systematic review with meta analysis. Oral Dis. 2010, 16, 601–612. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Tampa, M.; Mitran, M.I.; Mitran, C.I.; Sarbu, M.I.; Nicolae, I.; Matei, C.; Caruntu, C.; Neagu, M.; Popa, M.I. Potential pathogenic mechanisms involved in the association between lichen planus and hepatitis C infection. Exp. Ther. Med. 2019, 17, 1045–1051. [Google Scholar] [CrossRef]
- Daramola, O.O.; George, A.O.; Ogunbiyi, A.O.; Otegbayo, J.A. Hepatitis, B virus in Nigerians with lichen planus. West Afr. J. Med. 2004, 23, 104–106. [Google Scholar] [CrossRef]
- Mizukawa, Y.; Horie, C.; Yamazaki, Y.; Shiohara, T. Detection of varicella-zoster virus antigens in lesional skin of zosteriform lichen planus but not in that of linear lichen planus. Dermatology 2012, 225, 22–26. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.J.; van Marle, J.; Teunissen, M.B.; Picavet, D.; Zorgdrager, F.; Bos, J.D.; Weel, J.; Cornelissen, M. Lichen planus is associated with human herpesvirus type 7 replication and infiltration of plasmacytoid dendritic cells. Br. J. Dermatol. 2006, 154, 361–364. [Google Scholar] [CrossRef]
- Jackson, G.; Samolitis, N.J.; Harris, R.M. Lichenoid eruption in a patient with AIDS. Arch. Dermatol. 2006, 142, 385–390. [Google Scholar]
- Ashraf, S.; Al-Maweri, S.A.; Alaizari, N.; Umair, A.; Ariffin, Z.; Alhajj, M.N.; Kassim, S.; Awan, K.H. The association between Epstein-Barr virus and oral lichen planus: A systematic review and meta-analysis. J. Oral Pathol. Med. 2020, 49, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Guimaraens, B.; Dominguez-Santas, M.; Suarez-Valle, A.; Fernandez-Nieto, D.; Jimenez-Cauhe, J.; Ballester, A. Annular lichen planus associated with coronavirus SARS-CoV-2 disease (COVID-19). Int. J. Dermatol. 2020, 60, 246–247. [Google Scholar] [CrossRef]
- Lai, Y.C.; Yew, Y.W. Lichen planus and lichenoid drug eruption after vaccination. Cutis 2017, 100, E6–E20. [Google Scholar]
- Calista, D.; Morri, M. Lichen planus induced by hepatitis B vaccination: A new case and review of the literature. Int. J. Dermatol. 2004, 43, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.B.; Briggs, P.L.; Bravo, F. Lichen planus versus lichenoid drug eruption. In Differential Diagnosis in Dermatopathology III; Lea & Febiger: London, UK, 1993; pp. 18–21. [Google Scholar]
- Hiltun, I.; Sarriugarte, J.; Martínez-De-Espronceda, I.; Garcés, A.; Llanos, C.; Vives, R.; Yanguas, J.I. Lichen planus arising after COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e414–e415. [Google Scholar] [CrossRef]
- Merhy, R.; Sarkis, A.-S.; Kaikati, J.; El Khoury, L.; Ghosn, S.; Stephan, F. New-onset cutaneous lichen planus triggered by COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e729–e730. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef]
- McMahon, D.E.; Kovarik, C.L.; Damsky, W.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Chamberlin, G.; Fathy, R.; Nazarian, R.M.; Desai, S.R.; et al. Clinical and pathologic correlation of COVID-19 vaccine reactions including V-REPP: A registry based study. J. Am. Acad. Dermatol. 2022, 86, 113–121. [Google Scholar] [CrossRef]
- Herzum, A.; Burlando, M.; Molle, M.F.; Micalizzi, C.; Cozzani, E.; Parodi, A. Lichen planus flare following COVID-19 vaccination: A case report. Clin. Case Rep. 2021, 9, e05092. [Google Scholar] [CrossRef]
- Piccolo, V.; Mazzatenta, C.; Bassi, A.; Argenziano, G.; Cutrone, M.; Grimalt, R.; Russo, T. COVID vaccine-induced lichen planus on areas previously affected by vitiligo. J. Eur. Acad. Dermatol. Venereol. 2021, 36, e28–e30. [Google Scholar] [CrossRef] [PubMed]
- Bularca, E.; Monte-Serrano, J.; Villagrasa-Boli, P.; Lapena-Casado, A.; de-la-Fuente, S. Reply to “COVID vaccine-induced lichen planus on areas previously affected by vitiligo”. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e423–e425. [Google Scholar] [CrossRef]
- Diab, R.; Araghi, F.; Gheisari, M.; Kani, Z.A.; Moravvej, H. Lichen planus and lichen planopilaris flare after COVID-19 vaccination. Dermatol. Ther 2021, 35, e15283. [Google Scholar] [CrossRef]
- Paolino, G.; Rongioletti, F. Palmoplantar lichenoid drug eruption following the administration of Pfizer-BioNTech COVID-19 vaccine. J. Am. Acad. Dermatol. 2022, 21, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Fernandes, S.; Soares-de-Almeida, L.; Filipe, P. Exuberant lichenoid eruption after Oxford-AstraZeneca COVID-19 vaccine: A singular case. J. Eur. Acad. Dermatol. Venereol. 2021, 36, e268–e270. [Google Scholar] [CrossRef]
- Onn, P.-Y.; Chang, C.L. Lichenoid cutaneous skin eruption and associated inflammatory response following Pfizer-BioNTech mRNA COVID-19 vaccine administration. Respirol. Case Rep. 2021, 9, e0860. [Google Scholar] [CrossRef]
- Ziraldo, M.; Theate, I.; Vanhooteghem, O. Drug-induced lichenoid exanthema by a vaccine against COVID-19 (Vaxzevira). Dermatol. Rep. 2021, 13, 9358. [Google Scholar] [CrossRef]
- Babazadeh, A.; Miladi, R.; Barary, M.; Shirvani, M.; Ebrahimpour, S.; Aryanian, Z.; Afshar, Z.M. COVID-19 vaccine-related new-onset lichen planus. Clin. Case Rep. 2022, 10, e05323. [Google Scholar] [CrossRef]
- Zagaria, O.; Villani, A.; Ruggiero, A.; Potestio, L.; Fabbrocini, G.; Gallo, L. New-onset lichen planus arising after COVID-19 vaccination. Dermatol. Ther. 2022, 35, e15374. [Google Scholar] [CrossRef]
- Camela, E.; Guerrasio, G.; Patruno, C.; Scalvenzi, M.; DiCaprio, N.; Fabbrocini, G.; Napolitano, M. Reply to ‘New-onset cutaneous lichen planus triggered by COVID-19 vaccination’ by Merhy et al. J. Eur. Acad. Dermatol. Venereol. 2021, 36, e249–e251. [Google Scholar] [CrossRef] [PubMed]
- Awada, B.; Abdullah, L.; Kurban, M.; Abbas, O. Inverse lichen planus post Oxford-AstraZeneca COVID-19 vaccine. J. Cosmetic Dermatol. 2022, 21, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Satilmis Kaya, A.; Cemsitoglu, N.; Adisen, E.; Erdem, O. Lichen planus after CoronaVac: A rare complication of vaccines. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e326–e327. [Google Scholar] [CrossRef] [PubMed]
- Alrawashdeh, H.; AL-Habahbeh, O.; Naser, A.Y.; Serhan, H.A.; Hamdan, O.; Sweiss, K.; Aldalameh, Y. Lichen planus eruption following Oxford-AstraZeneca COVID-19 vaccine administration: A case report and review of the literature. Cureus 2022, 14, e22669. [Google Scholar] [CrossRef]
- Sun, L.; Duarte, S.; Soares-de-Almeida, L. Case of lichen planus pigmentosus-inversus after Oxford-AstraZeneca COVID-19 vaccine: Cause or coincidence? J. Eur. Acad. Dermatol. Venereol. 2022, 36, e514–e516. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, Y.; Ishitsuka, Y.; Kato, Y.; Arase, N.; Fujimoto, M. Blistering papulosquamous erythema with arthralgia: A commentary. Acta Derm Venereol. 2022, 102, adv00690. [Google Scholar] [CrossRef] [PubMed]
- Picone, V.; Fabbrocini, G.; Martora, L.; Martora, F. A case of new-onset lichen planus after CVID-19 vaccination. Dermatol. Ther. 2022, 12, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Zengarini, C.; Piraccini, B.M.; La-Placa, M. Lichen ruber planus occurring after SARS-CoV-2 vaccination. Dermatol. Ther. 2022, 35, e15389. [Google Scholar] [CrossRef] [PubMed]
- Masseran, C.; Calugareanu, A.; Caux, F.; Bohelay, G. Extensive cutaneous lichen planus triggered by viral vector COVID-19 vaccination (ChAdOx1 nCoV-19). J. Eur. Acad. Dermatol. Venereol 2022, 36, e263–e265. [Google Scholar] [CrossRef] [PubMed]
- Gamonal, S.B.L.; Gamonal, A.C.C.; Adario, C.L. Lichen planus and vitiligo occurring after ChAdOx1 nCoV-19 vaccination against SARS-CoV-2. Dermatol. Ther. 2022, 35, e15422. [Google Scholar] [CrossRef]
- Alotaibi, S.H.; Alharithy, R.; Alsharif, F.A.; Alkhayal, N. Lichen planus after COVID-19 vaccination: A report of 2 cases. J. Dermatol. Dermatol. Surg. 2022, 26, 57–60. [Google Scholar]
- Belina, M.E.; Sarver, M.M.; Al-Rohil, R.; Fresco, A. Lichen striatus post-COVID-19 vaccination. JAAD Case Rep. 2021, 16, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Kamiya, T.; Handa, T.; Kobayashi, E.; Hida, T.; Yamashita, T.; Uhara, H. Linear lichen planus after COVID-19 vaccination. Australia J. Dermatol. 2022, 63, e385–e387. [Google Scholar] [CrossRef] [PubMed]
- Rovira-Lopez, R.; Pujol, R.M. Blaschkolinear acquired inflammatory skin eruption (blaschkitis) following COVID-19 vaccination. JAAD Case Rep. 2022, 26, 35–37. [Google Scholar] [CrossRef]
- Hali, F.; Bousmara, R.; Marnissi, F.; Deddah, M.A.; Meftah, A.; Chiheb, S. Lichenoid drug eruption following COVID-19 vaccination: A series of four cases. Arch. Clin. Exp. Dermatol. 2022, 4, 129. [Google Scholar]
- Sharda, P.; Mohta, A.; Ghiya, B.C.; Mehta, R.D. Development of oral lichen planus after COVID-19 vaccination—a rare case report. J. Eur. Acad. Dermatol. Venereol. 2021, 36, e82–e83. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Sollecito, T. COVID-19 vaccination: Possible short-term exacerbations of oral mucosal diseases. Int. J. Dermatol. 2021, 60, e335–e336. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Paz, Z.; Israeli, E.; Shoenfeld, Y. Vaccines and autoimmunity. Nat. Rev. Rheumatol. 2009, 5, 648–652. [Google Scholar] [CrossRef]
- Wraith, D.C.; Goldman, M.; Lambert, P.H. Vaccination and autoimmune disease: What is the evidence? Lancet 2003, 362, 1659–1666. [Google Scholar] [CrossRef]
- Schonberger, L.B.; Bregman, D.J.; Sullivan-Bolyai, J.Z.; Keenlyside, R.A.; Ziegler, D.W.; Retailliau, H.F.; Eddins, D.L.; Bryan, J.A. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am. J. Epidemiol. 1979, 110, 105–123. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Pfeifer, D.; Schonberger, L.B. Guillain-barre syndrome following influenza vaccination: Causal or coincidental? Curr. Infect. Dis. Rep. 2011, 13, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.; Krikorian, K.S. The neuro-paralytic accidents of anti-rabies treatment. Ann. Trop. Med. Parasitol. 1928, 22, 327–377. [Google Scholar] [CrossRef]
- Hemachudha, T.; Griffin, D.E.; Giffels, J.J.; Johnson, R.T.; Moser, A.B.; Phanuphak, P. Myelin basic protein as an encephalitogen in encephalomyelitis and polyneuritis following rabies vaccination. N Engl. J. Med. 1987, 316, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Piyasirisilp, S.; Schmeckpeper, B.J.; Chandanayingyong, D.; Hemachudha, T.; Griffin, D.E. Association of HLA and T-Cell receptor gene polymorphisms with Semple rabies vaccine-induced autoimmune encephalomyelitis. Ann. Neurol. 1999, 45, 595–600. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jimenez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramirez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef] [PubMed]
- Albert, L.J.; Inman, R.D. Molecular mimicry and autoimmunity. New Eng. J. Med. 1999, 341, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Shukla, S.; Mahajan, S.N.; Diwan, S.K. Molecular mimicry in human diseases-phenomena or epiphenomena? JAPI 2010, 58, 163–168. [Google Scholar] [PubMed]
- Niebel, D.; Novak, N.; Wilhelmi, J.; Ziob, J.; Wilsmann-Theis, D.; Bieber, T.; Wenzel, J.; Braegelmann, C. Cutaneous adverse reactions to COVID-19 vaccines: Insights from an immuno-dermatological perspective. Vaccines 2021, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.; Acosta-Ampudia, Y.; Monsalve, D.M.; Chang, C.; Gershwin, M.E.; Anaya, J.-M. Bystander activation of autoimmunity. J. Autoimmun. 2019, 103, 102301. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-S.; Shin, E.-C. The activation of bystander CD8+ T cells and their roles in viral infection. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bonamente, D.; Foti, C.; Vestita, M.; Angelini, G. Noneczematous contact dermatitis. ISRN Allergy 2013, 2013, 361746. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.; Bahmer, F.A. Oral lesions and symptoms related to metals used in dental restorations: A clinical, allergological, and histologic study. J. Am. Acad. Dermatol. 1999, 41, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Hafner, C.; Landthaler, M.; Vogt, T. Lichen striatus (Blaschkitis) following varicella infection. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 1345–1347. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ohashi, T.; Yamamoto, T. Lichen striatus following influenza infection. J. Dermatol. 2014, 41, 1133–1134. [Google Scholar] [CrossRef] [PubMed]
- Richarz, N.A.; Fusta-Novell, X.; Fatsini-Blanch, V.; Fortuny, C.; Gonzalez-Ensenat, M.A.; Vicente, A. Lichen striatus following scarlet fever in a 3-year-old female patient. Int. J. Dermatol. 2018, 57, 1118–1119. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Marquart, J.D.; Logemann, N.F.; DiBlasi, D.R. Lichen striatus-like eruption in an adult following hepatitis B vaccination: A case report and review of the literature. Dermatol. Online J. 2018, 24, 1–4. [Google Scholar] [CrossRef]
- Herzum, A.; Viglizzo, G.; Gariazzo, L.; Ferro, J.; Vellone, V.G.; Occella, C. Lichen striatus following COVID-19. Clin. Dermatol. 2022, 40, 744–746. [Google Scholar] [CrossRef]
- Thappa, D.M.; Srinivasulu, K.N. Unilateral linear lichen planus along the lines of Blaschko. Ind. J. Dermatol. 2003, 48, 170–171. [Google Scholar]
- Bonito, F.; Alves, J.; Antonio, A.M.; Sequeira, P.; Nogueira, J. A case of blaschkoid lichenoid drug eruption. J. Cutan Pathol. 2022, 49, 1–109. [Google Scholar] [CrossRef]
- Sato, N.A.; Kano, Y.; Shiohara, T. Lichen planus occurring after influenza vaccination: Report of three cases and review of the literature. Dermatology 2010, 221, 296–299. [Google Scholar] [CrossRef]
- Hardy, C.; Glass, J. Linear lichen planus in the setting of annual vaccination. Mil. Med. 2019, 184, e467–e469. [Google Scholar] [CrossRef]
- Garcia-Martinez, F.J.; Salgado-Boquete, L.; Vazquez-Osorio, I.; Toribio, J. Unilateral blaschkoid lichen planus due to influenza vaccination. Med. Cutan Iber. Lat. Am. 2015, 43, S38–S40. [Google Scholar]
- Arora, A.; Mohta, A.; Soni, P. A case of Blaschkoid pityriasis rosea following COVID-19 vaccination: A rare exceptional occurrence. Our Dermatol. Online 2022, 13, 286–288. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Gogl, M.; Berndt, R.; Troeltzsch, M. Oral lichen planus following the administration of vector-based COVID-19 vaccine (Ad26.COV2.S). Oral Dis. 2022, 28, 2595–2596. [Google Scholar] [CrossRef]
- Van den Haute, V.; Antoine, J.L.; Lachapelle, J.M. Histopathological discriminant criteria between lichenoid drug eruption and idiopathic lichen planus: Retrospective study on selected samples. Dermatologica 1989, 179, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Long, C.C.; Finlay, A.Y. Multiple linear lichen planus in the lines of Blaschko. Br. J. Dermatol. 1996, 135, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.; Saad El-Din, S.A. Bilateral linear lichen planus along the lines of Blaschko: Report of a rare case and brief review. Our Dermatol. Online 2017, 8, 322–325. [Google Scholar] [CrossRef]
- Criscito, M.C.; Brinster, N.K.; Skopicki, D.L.; Seidenberg, R.; Cohen, J.M. Blaschkoid lichen planus: Throwing a “curve” in the nomenclature of linear lichen planus. J. Am. Acad. Dermatol. 2020, 6, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, R.R.; Cockerell, C.J.; Belsito, D.; Ostreicher, R. Allergic contact dermatitis from color film developers: Clinical and histologic features. J. Am. Acad. Dermatol. 1993, 28, 827–830. [Google Scholar] [CrossRef]







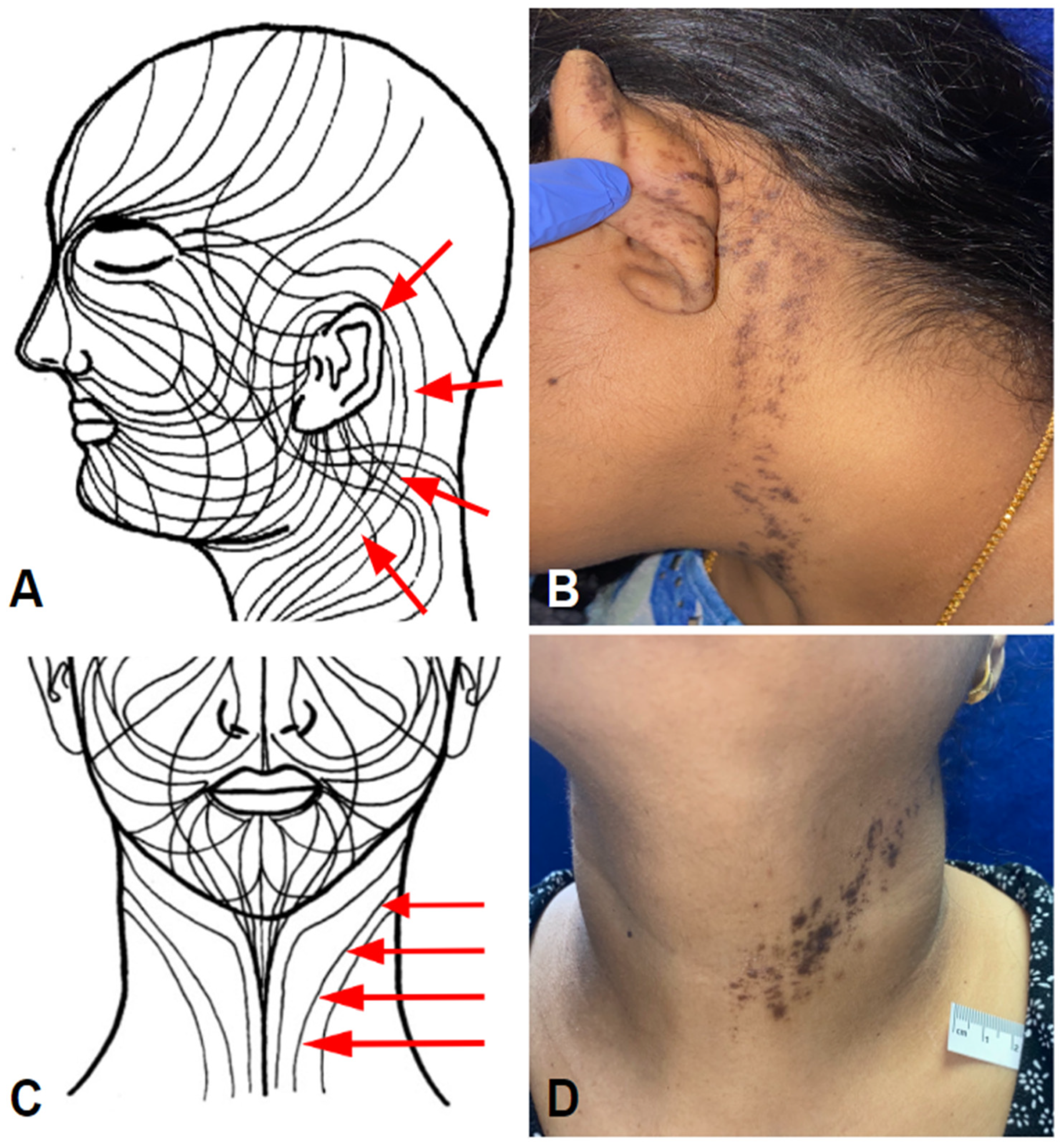


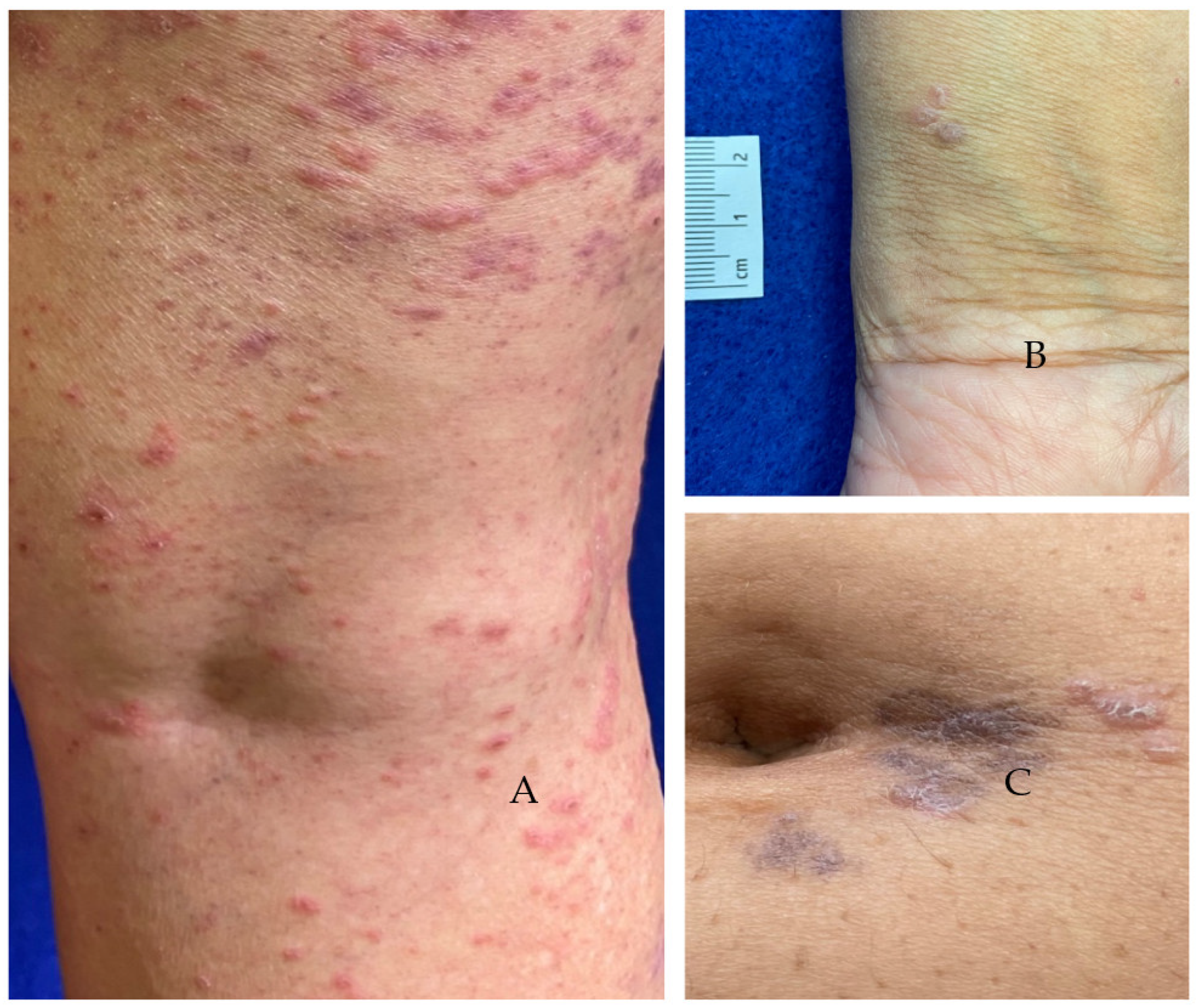









| Pt # | Age | Sex | Race | Medical History | Vaccine | Interval to 1st Eruption | Presentation | Distribution | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | F | Asian | Asthma | Pfizer | 2 weeks after V2 | LE-B | Unilateral/Left forearm | TAC oint, oral AH | Resolved with PIH |
| 2 | 54 | F | Hispanic | Asthma, oral LP | Pfizer | 8 days after V1; RA 2 weeks after V2 | LE-B | Bilateral/Anterior and posterior legs | TAC oint, prednisone | Resolved |
| 3 | 69 | M | Asian | HTN, hypothyroid, pre-DM | Moderna | 1 week after V2 | LE-B: LP | Unilateral/Left chest, abdomen, back, groin | TAC oint | Resolved with PIH |
| 4 | 42 | F | Asian | None | Pfizer | 10 days after V2 | LE-B: LS-like | Unilateral/Left post-auricular and neck | TAC oint | Resolved with PIH |
| 5 | 68 | M | Hispanic | HTN, COVID-19 | Pfizer | 2 weeks after V2; RA months after B1 | LP with inverse lesion | Bilateral/Axilla, forearms, thigh | TAC oint, oral AH | Resolved with PIH |
| 6 | 80 | F | Caucasian | Anxiety | Pfizer | 4 days after V1 | LP with LPLKs | Bilateral/Lower extremities and forearms | TAC oint, NBUVB | Resolving |
| 7 | 81 | M | Asian | Prostate CA | Moderna | 3 weeks after V2 | Extensive LP | Bilateral/Trunk and extremities | Oral AH, NBUVB, acitretin | Resolved |
| 8 | 62 | F | Asian | None | Moderna | 2–3 weeks after V2 | LP | Unilateral/Left forearm, periumbilical | TAC oint | Resolved with PIH |
| 9 | 65 | M | Caucasian | GIST | Pfizer | 10 days after V2 | Psoriasiform | Bilateral/Trunk | TAC oint | Resolved |
| 10 | 40 | M | Hispanic | None | Moderna | 2 weeks after V2 | Solitary lichenoid lesion | Unilateral/Left ACF | TAC oint | Resolved with PIH |
| 11 | 62 | M | Caucasian | HTN, stroke | Pfizer | 11 days after V1; RA 4 days after V2 and 3 weeks after B1 | Papular LP | Bilateral/Flexural forearms and lower back | TAC oint, prednisone | Resolved |
| 12 | 66 | M | Asian | HTN, T2DM, HLD | Moderna | 3 weeks after V2 | Papular LP | Bilateral/Flexural forearms and pretibial legs | Clobetastol oint | Resolved |
| 13 | 36 | F | Hispanic | LP | Pfizer | 12 days after V2 | Papular LP | Bilateral/Upper and lower back, flexural wrists | Clobetastol oint | Resolved |
| 14 | 59 | F | Black | Breast CA lumpectomy | Janssen | 1 weeks after V1 | Truncal plaques | Bilateral R chest lower back | Clobetastol oint | Resolved with PIH |
| 15 | 68 | F | Black | HTN, hypothyroid | Moderna | 4 days after B2 | Extensive PIH | Bilateral/Forearms, back, legs | Clobetastol oint | Resolved with PIH |
| Authors [REF #] | A G | Type of Vaccine/MFR | Type of Eruption | Time to Onset of Eruption | Treatment, Clinical Course, Distribution |
|---|---|---|---|---|---|
| Hiltun [35] 2021 | 56 F | mRNA/P-B | Reactivation of lichen planus | 2 days after V2 | Topical steroids; NR ankles, flexural wrist, forearms, periumbilical, mammary and axillary folds |
| Mehry [36] 2021 | 56 F | mRNA/P-B | New onset LP | 1 week after V1 | NR;NR Trunk |
| McMahon [38] 2021 V-REPP | NR | mRNA P-B n = 3 mRNA Moderna n = 1 | Lichen planus-like NOTE: 4/58 cases with biopsy reports | NR | NR:NR NR |
| Herzum [39] 2021 | 59 F | mRNA/P-B | Flare of LP—papular | 2 weeks after V2 | Topical steroids; Resolved after 3 weeks. Ankles, feet |
| Piccolo [40] | 64 F | mRNA/P-B | LP on areas of vitiligo | 5 days after V1 1 day after V2 | Topical and systemic corticosteroids/NR Dorsa of hands—Bilat |
| Bularca [41] 2022 | 29 F | mRNA/P-B | After V1: LP on areas of vitiligo. After V2 LP extended to areas not affected by vitiligo. | 1 week after V1 LP after V2 | Methotrexate Clinical course: NR Dorsa of hands, wrists, eyelids, submammary region, legs. Oral mucosa |
| Diab [42] | 60 F 55 F | Vector/AstraZeneca Sinopharm | (1) Flare of lichen planopilaris and new lesions of LP (2) Flare of LP— previously had only a solitary lesion | (1) 2 weeks after V2 (2) 3 days after V1, more severe after V2 | ILTAC, Tofacitinib Gradual improvement Face, scalp Metronidazole 500 mg bid Lesions improved Lower extremities and buttocks |
| Paolino [43] 2022 | 63 F | mRNA P/B | Palmoplantar | 3 days after V2 | Acitretin 25mg/day × 2 months and topical calcipotriene/betamethasone foam After 4 weeks: Total clearing of acral lesions, residual hyperpigmentation of palms. Palms, wrists and soles |
| Correia [44] 2021 | 66 M | Vector Oxford-AstraZeneca | Exuberant Generalized | 5 days after V1 | Topical steroid; Resolved after 4 months. Back, scalp, trunk, extremities |
| Onn [45] 2021 | 53 F | mRNA/P-B | Generalized Lichenoid skin reaction and SIRS | 12 days after V1 | Topical steroid, cetirizine; oral prednisone then IV hydrocortisone Abdomen, chest, back, scalp |
| Ziraldo [46] 2021 | 66 F | Vector/AstraZeneca | Lichenoid exanthema— EM-like lesions | 3 weeks after V1 | Oral steroids Resolved in 10 days. Entire body involved |
| Babazadeh [47] 2021 | 52 F | Sinopharm | New onset LP Clinical DX—NO BX | 1 week after V2 | Topical steroids, Antihistamines; Favorable response Arms, legs |
| Zagaria [48] 2022 | 54 M | mRNA/P-B | New onset LP | 10 days after V1 | Oral prednisone, 25 mg daily with rapid taper over 4 weeks. Rapid resolution. Trunk, arms, legs |
| Camela [49] 2021 | 59 M | mRNA/P-B | Lichenoid eruption | 2 weeks after V1 | NR;NR Trunk, extremities |
| Awada [50] 2022 | 44 M | Vector Oxford-AstraZeneca | Inverse LP | 2 weeks after V2 | Betamethasone cream Resolved after 4 weeks Axillae |
| Satilmis [51] 2022 | 60 F | Inactivated virus CoronaVac | Lichen planus | 6 days after V1 | Treatment: NR Flexural wrists, dorsa of hands and feet |
| Alrawashdeh [52] 2022 | 46 M | Vector Oxford-AstraZeneca | Lichen planus | 5 days after V1 | Prednisone was refused. Hydroxychloroquine 200 mg bid, minimal improvement after 2 months. Face, abdomen, back and legs |
| Sun [53] 2022 | 64 F | Vector Oxford-AstraZeneca | Lichen planus pigmentosus- inversus | 2 weeks after V1 | Topical steroid; Minor improvement after 2 months Inframammary, axillae, lower back, groin |
| Kurosaki [54] 2022 | 54 F | mRNA Pfizer | Lichen planus pemphigoides | 1 day after V2 | Topical steroid Blisters appeared Trunk and xtremities |
| Picone [55] 2022 | 81 M | mRNA Moderna | New-onset LP with oral LP | 7 days after V1 | Topical steroid and cetirizine oral AH Exam after 25 days: Resolved Flexural wrists, lower back, posterior thighs, dorsa of feet |
| Zengarini [56] 2022 | 49 M | Vector AstraZeneca | Eruptive LP | 11 days after V2 | Topical steroids, Oral AH Resolved with no itch and only mild erythema after 1 month. Trunk and extremities |
| Masseran [57] 2022 | 65 F | Vector ChAdOx1 nCoV-19 | Extensive LP | 10 days after V1 7 days after V2 which were 3 months apart. | Clobetasol cream x 4 weeks—nearly complete remission but remains with diffuse hyperpigmentation. Arms, legs, buttocks, abdomen |
| Gamonal [58] 2022 | 86 M | Vector ChAdOx1 nCoV-19 | Eruptive LP | 7 days after V1 Worse after V2 which was 3 months after V1. | Topical steroid cream Clinical course: NR Arms, legs, trunk, buttocks |
| Alotaibi [59] 2022 | 57 F | mRNA/Pfizer | LP | 3 weeks after third dose | Tacrolimus ointment, steroid ointment Clinical course: NR Chest, axillae, arms, legs |
| Belina [60] 2021 | 42 F | mRNA P-B | Lichen Striatus | 3 days after V2 | Tacrolimus ointment 0.1% Result: NR |
| Kato [61] 2022 | 57 F | mRNA P/B | Linear lichen planus | 2 weeks after V1 (3rd dose of the vaccine) | Topical steroids Improvement with only mild residual hyperpigmentation |
| Rovira-Lopez [62] 2022 | 46 F | mRNA/P-B | BLAISE | A few days after V1. No flare after V2. | Topical steroids No improvement |
| Hali [63] 2022 | 67 M | Vector/Astrazeneca | Lichenoid eruption | 3 days after V2 | Topical steroid Improved thigh, neck, upper chest, forearms |
| 20 F | Inactivated virus Sinopharm | Lichenoid Eeuption | 1 day after V1 | Topical steroid; Started to heal. Entire body | |
| 28 M | Inactivated virus Sinopharm | Lichenoid eruption | 15 days after V2 | Topical steroid and antihistamines Some improvement legs, arms | |
| 65 F | Inactivated virus Sinopharm | Lichenoid eruption | 30 days after V3 | Topical steroids Some improvement entire body |
| Literature Review (n = 31) | Case Series (n = 15) | |
|---|---|---|
| Mean age (Range) Median age (Range) | 56 [20–86] | 59 [36–81] |
| Gender F, n (%) | 21 (68) | 8 (53) |
| Race, n (%) Asian Hispanic Caucasian Black | N/A N/A N/A N/A | 6 (40) 4 (27) 3 (20) 2 (13) |
| Blaschkoid distribution, n (%) | 03 (10) | 04 (27) |
| First eruption, n (%) After V1 After V2 After V3 After V4 | 17 (55) 11 (35) 03 (10) None | 04 (27) 10 (67) None 01 (07) |
| Time to onset of eruption (mean days; range) After V1 After V2 After V3 After V4 | 8.4 (1–21) 7.9 (1–15) 21.7 (14–30) N/A | 7.5 (4–11) 14.2 (7–21) N/A 4.0 (N/A) |
| Eruptions after successive vaccinations: n (%) | 06 (19) | 02 (13) |
| Idiopathic Lichen Planus (ILP) | Lichenoid Drug Eruption (LDE) | Vaccine-Induced Lichenoid Eruption (V-ILE) | |
|---|---|---|---|
| Clinical | |||
| Mean age | Fifth or sixth decade | Sixty-six years [23] | Sixth decade |
| Latent Period | N/A | Often several months or longer | Several days to several weeks in most published reports. |
| Pruritus, Burning | Intense pruritus, common | Pruritus may or may not be present. | Intensity varies among cases. Often intense but may be completely absent in some cases. Burning sensation is possible. |
| Location | Flexural wrists and forearms, presacral, shins, ankles, intraoral, genitals, hair, nails | More generalized. Less likely to involve hair, nails. Intraoral, in some cases | May or may not involve classic sites like flexural wrists. Peripheral distribution, often. May be generalized. |
| Mucous Membranes | Commonly involved | May be involved | May be involved |
| Photodistribution | Not characteristic | Frequent, depending on the drug. | Not characteristic |
| Blaschkoid Distribution | 0.24–0.62% of all cases [86] | Has been reported [87] | Several case reports [60,61,62,88,89,90,91] Cases 1–4 herein. Likely more common in V-ILE than in ILP. Present in 04/15 cases reported herein. |
| Morphology | Classic is “6 Ps”: Pruritic purple, planar, polygonal, papules and plaques | May show classic morphology. May show larger plaques. May appear psoriasiform or eczematous. | May show classic morphology. Papules may be skin-colored or erythematous. May be psoriasiform. |
| Wickham Striae | Classic | Often absent | Present in some cases [41,57,58,92] |
| Hyperpigmentation | Common | Very common | Very common |
| Number of Lesions | Most often multiple; may be solitary in lichen planus-like keratosis | Most often multiple | Most often multiple, but may be solitary (case 9) |
| Histopathologic | |||
| Lichenoid Interface Dermatitis | Present | Present | Present |
| Compact Orthokeratosis | Characteristic | May be present | May be present |
| Wedge-shaped Hypergranulosis | Characteristic | May be present | May be present |
| Focal Parakeratosis | Not characteristic | May be present | May be present |
| Focal Interruption of Granular Layer | Not characteristic | May be present | May be present |
| Sawtoothing of Rete Ridges | Characteristic | May be present | May be present |
| Focal Spongiosis | Not characteristic | May be present | May be present |
| Necrotic Keratinocytes Number At All Levels of Epidermis | Fewer than LDE, V-ILE Not characteristic. | Larger number [93]. May cluster. May be seen at all levels. | May be more than seen in ILP. This feature was seen in 5/15 (33%) cases reported herein. |
| Location of Cytoid Bodies in Epidermis | Lower spinous layer | Lower spinous layer; may also be seen in upper spinous, granular, cornified layers. | Lower spinous layer; may also be seen in upper spinous, granular, cornified layers. |
| Lymphocytic Infiltrate Throughout Epidermis | Not characteristic | May be present | Present in 6/15 (40%) cases in series reported herein. |
| Location of Lymphocytic Infiltrate in Dermis | Superficial (papillary) | Superficial and may extend deeper | Superficial and may extend deeper |
| Deep Perivascular Infiltrate | Rarely seen | May be present | Often present in lichen striatus Occasionally seen in other lichenoid reactions |
| Focally lichenoid or patchy infiltrate | Dense band-like infiltrate is characteristic. | May be present | Present in 7/15 (47%) of cases reported herein. |
| Perieccrine or Periadnexal Infiltrate | Not characteristic. May be seen in adnexotrophic variants. | Uncommon | Often seen in vaccine-induced LS. |
| Eosinophils in Infiltrate | Not characteristic | May be present. Found in 2/15 cases (13.4%) in study directly comparing LDE to ILP [93]. | May be present. Found in 1/15 (6.7%) cases reported herein. |
| Plasma Cells in Infiltrate | Not characteristic | May be present | May be present |
| Granuloma Formation | Not characteristic | May rarely be present | Seen in 1/15 cases in series reported herein. |
| Author/YEAR Diagnosis | Age M/F Injection Site | Location of Eruption | Type of Vaccine Manufacturer | Interval | Treatment | Clinical Course |
|---|---|---|---|---|---|---|
| Sato [88] 2010 Case 1 Linear Lichen Planus | 71 F L Arm | L Buttock + L Thigh/Leg | Influenza Kaketsuken Astelas | 7 days after vaccination | Topical Corticosteroids | Resolved with slight pigmentation. Recurrence 1 week after influenza vaccination following year. |
| Sato 2010 Case 2 Linear Lichen Planus | 70F Site NR | L Leg | Influenza NR | 2 weeks after vaccination | Topical Corticosteroids | Resolved completely after 6 months. |
| Hardy [89] 2019 Linear Lichen Planus | 43 M L Arm | L Side of Trunk | Influenza NR | 8–10 days after vaccination | Topical Corticosteroids | Progression noticed at 1 year follow-up. |
| Garcia-Martinez [90] 2015 Blaschkoid LP | 50 F L Arm | Left Side of Body Leg, Lumbar, Abdomen, Arm | Influenza NR | 2 weeks after vaccination | Topical Corticosteroid Oral Corticosteroids | Resolved over 6 months with residual hyperpigmentation. |
| Bellina [60] 2021 Lichen striatus | 42 F R Deltoid | R Arm | COVID-19 Pfizer-BioN Tech | 3 days after V2 | Tacrolimus Ointment 0.1% | NR Not reported |
| Kato [61] 2022 Linear Lichen Planus | 57 F L Deltoid | L Arm | COVID-19 Pfizer-BioN Tech | 2 weeks after third dose | Topical Corticosteroids Short course of Oral Corticosteroids | Improvement with mild residual hyperpigmentation. |
| Rovira-Lopez [62] 2022 BLAISE | 46 F L Deltoid | L-Sided Eruption on Trunk | COVID-19 Pfizer-BioN Tech | “A few days” after V1 No flare after V2 | Oral Corticosteroids and potent Topical Corticosteroids | Resolved completely with residual hyperpigmentation. |
| Case 1 BLAISE | 38 F L Deltoid | L Arm | COVID-19 Pfizer-BioN Tech | 2 weeks after V2 | TAC Ointment 0.1% Oral AH | Resolved with residual mild hyperpigmentation. |
| Case 2 BLAISE | 54 F L Deltoid | Bilateral Legs | COVID-19 Pfizer-BioN Tech | 8 days after V1 2 weeks after V2 | TAC Ointment 0.1% Prednisone | Resolved |
| Case 3 BLAISE, LP-like | 69 M L Deltoid | L-Sided Trunk | COVID-19 Moderna | 1 week after V2 | TAC Ointment 0.1% | Resolved with residual hyperpigmentation. |
| Case 4 BLAISE, LS-like | 42 F L Deltoid | L Side of Neck | COVID-19 Pfizer-BioN Tech | 10 days after V2 | TAC Ointment 0.1% | Resolved with residual hyperpigmentation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapadin, Y.K.; Mermelstein, E.; Phelps, R.G.; Basler, C.F.; Tufariello, J.M.; Lebwohl, M.G. COVID-19 Vaccine-Induced Lichenoid Eruptions—Clinical and Histopathologic Spectrum in a Case Series of Fifteen Patients with Review of the Literature. Vaccines 2023, 11, 438. https://doi.org/10.3390/vaccines11020438
Sapadin YK, Mermelstein E, Phelps RG, Basler CF, Tufariello JM, Lebwohl MG. COVID-19 Vaccine-Induced Lichenoid Eruptions—Clinical and Histopathologic Spectrum in a Case Series of Fifteen Patients with Review of the Literature. Vaccines. 2023; 11(2):438. https://doi.org/10.3390/vaccines11020438
Chicago/Turabian StyleSapadin, Yonatan K., Elazar Mermelstein, Robert G. Phelps, Christopher F. Basler, JoAnn M. Tufariello, and Mark G. Lebwohl. 2023. "COVID-19 Vaccine-Induced Lichenoid Eruptions—Clinical and Histopathologic Spectrum in a Case Series of Fifteen Patients with Review of the Literature" Vaccines 11, no. 2: 438. https://doi.org/10.3390/vaccines11020438
APA StyleSapadin, Y. K., Mermelstein, E., Phelps, R. G., Basler, C. F., Tufariello, J. M., & Lebwohl, M. G. (2023). COVID-19 Vaccine-Induced Lichenoid Eruptions—Clinical and Histopathologic Spectrum in a Case Series of Fifteen Patients with Review of the Literature. Vaccines, 11(2), 438. https://doi.org/10.3390/vaccines11020438






