Analysis of Antibodies Induced after SARS-CoV-2 Vaccination Using Antigen Coded Bead Array Luminex Technology
Abstract
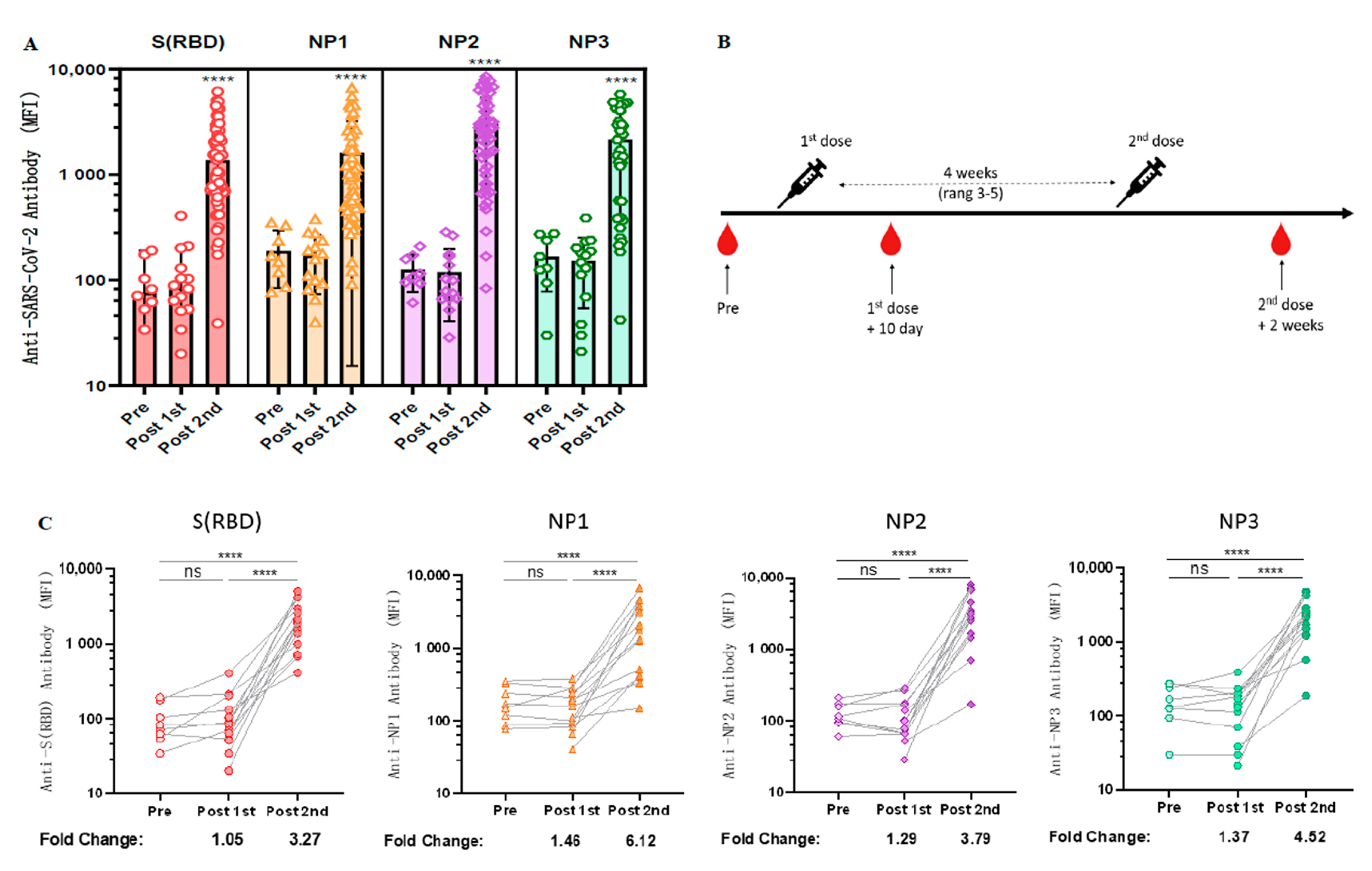
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Antigens and Antibodies
2.3. Construction of the Recombinant Expression Vectors
2.4. Expression and Purification of the Recombinant Protein
2.5. Coomassie Brilliant Blue Staining
2.6. Western Blot Analysis
2.7. Luminex Bead-Based Immunoassay
2.8. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Study Participants
3.2. Generation of the ACE-2 Receptor-Binding Domain Soluble Antigenic Protein from Wild-Type and Delta Variant Coronaviruses
3.3. Longitudinal Observation of Changes in Anti-RBD Antibody Levels after CoronaVac Vaccination
3.4. Cross-Sectional Observation of Anti-SARS-CoV-2 Antibodies Induced by Different Vaccinations
3.5. Linear Regression Analysis of Factors Related to the Influence of Antibody Level
3.6. Antibodies Induced by Vaccination Can Cross-React with the RBD Antigen of VOCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Palacios, R.; Patiño, E.G.; Piorelli, R.D.O.; Conde, M.T.R.P.; Batista, A.P.; Zeng, G.; Xin, Q.; Kallas, E.G.; Flores, J.; Ockenhouse, C.F.; et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac – PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 1–3. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Dai, L.; Gao, L.; Tao, L.; Hadinegoro, S.R.; Erkin, M.; Ying, Z.; He, P.; Girsang, R.T.; Vergara, H.; Akram, J.; et al. Efficacy and Safety of the RBD-Dimer–Based Covid-19 Vaccine ZF2001 in Adults. N. Engl. J. Med. 2022, 386, 2097–2111. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Li, C.; Huang, A.; Xia, S.; Lu, S.; Shi, Z.; Lu, L.; Jiang, S.; Yang, Z.; Wu, Y.; et al. Potent Binding of 2019 Novel Coronavirus Spike Protein by a SARS Coronavirus-Specific Human Monoclonal Antibody. Emerg. Microbes Infect. 2020, 9, 382–385. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Agoramoorthy, G.; Lee, S.-S. Evolution, Mode of Transmission, and Mutational Landscape of Newly Emerging SARS-CoV-2 Variants. Mbio 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Hobson, P.; Hatipoglu, E.; Ngai, Y.; et al. Neutralising antibody activity against SARS-CoV-2 VOCs, B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021, 397, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Casner, R.G.; Nair, M.S.; Wang, M.; Yu, J.; Cerutti, G.; Liu, L.; Kwong, P.D.; Huang, Y.; Shapiro, L.; et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe 2021, 29, 747–751.e4. [Google Scholar] [CrossRef]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Zeng, C.; Evans, J.P.; Qu, P.; Faraone, J.; Zheng, Y.-M.; Carlin, C.; Bednash, J.S.; Zhou, T.; Lozanski, G.; Mallampalli, R.; et al. Neutralization and Stability of SARS-CoV-2 Omicron Variant. J. BioRxiv 2021. 2021.2012.2016.472934. [Google Scholar] [CrossRef]
- Riou, C.; Keeton, R.; Moyo-Gwete, T.; Hermanus, T.; Kgagudi, P.; Baguma, R.; Valley-Omar, Z.; Smith, M.; Tegally, H.; Doolabh, D.; et al. Escape from recognition of SARS-CoV-2 variant spike epitopes but overall preservation of T cell immunity. Sci. Transl. Med. 2022, 14, eabj6824. [Google Scholar] [CrossRef]
- Malato, J.; Ribeiro, R.M.; Leite, P.P.; Casaca, P.; Fernandes, E.; Antunes, C.; Fonseca, V.R.; Gomes, M.C.; Graca, L. Risk of BA.5 Infection among Persons Exposed to Previous SARS-CoV-2 Variants. N. Engl. J. Med. 2022, 387, 953–954. [Google Scholar] [CrossRef]
- Guo, J.; Guo, X.; Wang, Y.; Tian, F.; Luo, W.; Zou, Y. Cytokine response to Hantaan virus infection in patients with hemorrhagic fever with renal syndrome. J. Med. Virol. 2017, 89, 1139–1145. [Google Scholar] [CrossRef]
- Guo, X.; Hu, J.; Luo, W.; Luo, Q.; Guo, J.; Tian, F.; Ming, Y.; Zou, Y. Analysis of Sera of Recipients with Allograft Rejection Indicates That Keratin 1 Is the Target of Anti-Endothelial Antibodies. J. Immunol. Res. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Stastny, P.; Süsal, C.; Döhler, B.; Opelz, G. Antibodies against MICA Antigens and Kidney-Transplant Rejection. N. Engl. J. Med. 2007, 357, 1293–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.-W.; Li, Y.; Zhang, H.-N.; Wang, W.; Yang, X.; Qi, H.; Li, H.; Men, D.; Zhou, J.; Tao, S.-C. SARS-CoV-2 proteome microarray for global profiling of COVID-19 specific IgG and IgM responses. Nat. Commun. 2020, 11, 3581. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714.e11. [Google Scholar] [CrossRef]
- Wang, G.-L.; Wang, Z.-Y.; Duan, L.-J.; Meng, Q.-C.; Jiang, M.-D.; Cao, J.; Yao, L.; Zhu, K.-L.; Cao, W.-C.; Ma, M.-J. Susceptibility of Circulating SARS-CoV-2 Variants to Neutralization. N. Engl. J. Med. 2021, 384, 2354–2356. [Google Scholar] [CrossRef]
- Mariën, J.; Ceulemans, A.; Michiels, J.; Heyndrickx, L.; Kerkhof, K.; Foque, N.; Widdowson, M.-A.; Mortgat, L.; Duysburgh, E.; Desombere, I.; et al. Evaluating SARS-CoV-2 spike and nucleocapsid proteins as targets for antibody detection in severe and mild COVID-19 cases using a Luminex bead-based assay. J. Virol. Methods. 2020, 288, 114025. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, R.; Wang, Y.; Zhao, M.; Liu, N.; Li, S.; Huang, H.; Yang, D.; Au, K.-K.; Wang, H.; et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine 2022, 77, 103904. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Lassaunière, R.; Frische, A.; Harboe, Z.B.; Nielsen, A.C.Y.; Fomsgaard, A.; Krogfelt, K.A.; Jørgensen, C.S. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv 2020. [Google Scholar] [CrossRef]
- Ong, D.S.; Fragkou, P.C.; Schweitzer, V.A.; Chemaly, R.F.; Moschopoulos, C.D.; Skevaki, C. How to interpret and use COVID-19 serology and immunology tests. Clin. Microbiol. Infect. 2021, 27, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Wang, Y.; Yuan, J.; Ye, H.; Wei, L.; Liao, X.; Wang, H.; Qian, S.; Wang, Z.; Liu, L.; et al. Early Viral Clearance and Antibody Kinetics of COVID-19 among Asymptomatic Carriers. Front. Med. 2021, 8, 595773. [Google Scholar] [CrossRef]
- Kerkhof, K.; Canier, L.; Kim, S.; Heng, S.; Sochantha, T.; Sovannaroth, S.; Vigan-Womas, I.; Coosemans, M.; Sluydts, V.; Ménard, D.; et al. Implementation and application of a multiplex assay to detect malaria-specific antibodies: A promising tool for assessing malaria transmission in Southeast Asian pre-elimination areas. Malar. J. 2015, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dobaño, C.; Vidal, M.; Santano, R.; Jiménez, A.; Chi, J.; Barrios, D.; Ruiz-Olalla, G.; Melero, N.R.; Carolis, C.; Parras, D.; et al. Highly Sensitive and Specific Multiplex Antibody Assays to Quantify Immunoglobulins M, A, and G against SARS-CoV-2 Antigens. J. Clin. Microbiol. 2021, 59, e01731-20. [Google Scholar] [CrossRef]
- Rosado, J.; Pelleau, S.; Cockram, C.; Merkling, S.H.; Nekkab, N.; Demeret, C.; Meola, A.; Kerneis, S.; Terrier, B.; Fafi-Kremer, S.; et al. Multiplex assays for the identification of serological signatures of SARS-CoV-2 infection: An antibody-based diagnostic and machine learning study. Lancet Microbe 2020, 2, e60–e69. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-N.; Huang, Y.; Wang, W.; Jing, Q.-L.; Zhang, C.-H.; Qin, P.-Z.; Guan, W.-J.; Gan, L.; Li, Y.-L.; Liu, W.-H.; et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: A test-negative case–control real-world study. Emerg. Microbes Infect. 2021, 10, 1751–1759. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, K.-L.; Jiang, X.-L.; Wang, X.-J.; Zhan, B.-D.; Gao, H.-X.; Geng, X.-Y.; Duan, L.-J.; Dai, E.-H.; Ma, M.-J. Omicron subvariants escape antibodies elicited by vaccination and BA.2.2 infection. Lancet Infect. Dis. 2022, 22, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2021, 22, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Ciabattini, A.; Sicuranza, A.; Pastore, G.; Santoni, A.; Simoncelli, M.; Polvere, J.; Galimberti, S.; Baratè, C.; Sammartano, V.; et al. The third dose of mRNA SARS-CoV-2 vaccines enhances the spike-specific antibody and memory B cell response in myelofibrosis patients. Front. Immunol. 2022, 13, 1017863. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Pastore, G.; Fiorino, F.; Polvere, J.; Lucchesi, S.; Pettini, E.; Auddino, S.; Rancan, I.; Durante, M.; Miscia, M.; et al. Evidence of SARS-CoV-2-Specific Memory B Cells Six Months after Vaccination with the BNT162b2 mRNA Vaccine. Front. Immunol. 2021, 12, 740708. [Google Scholar] [CrossRef] [PubMed]
- Gruell, H.; Vanshylla, K.; Tober-Lau, P.; Hillus, D.; Schommers, P.; Lehmann, C.; Kurth, F.; Sander, L.E.; Klein, F. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat. Med. 2022, 28, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Hens, N.; Ghebretinsae, A.H.; Hardt, K.; Van Damme, P.; Van Herck, K. Model based estimates of long-term persistence of inactivated hepatitis A vaccine-induced antibodies in adults. Vaccine 2014, 32, 1507–1513. [Google Scholar] [CrossRef] [PubMed]



| CoronaVac (n = 55) | BBIBP-CorV (n = 32) | ZF2001 (n = 8) | Mixed Vaccination (n = 5) | All Participants (n = 100) | p Value | |
|---|---|---|---|---|---|---|
| Gender | 0.424 | |||||
| Male | 21 (38.18%) | 17 (53.13%) | 3 (37.50%) | 1 (20.00%) | 42 (42.00%) | |
| Female | 34 (61.82%) | 15 (46.88%) | 5 (62.50%) | 4 (80.00%) | 58 (58.00%) | |
| Blood Type | 0.751 | |||||
| A | 18 (32.73%) | 9 (28.13%) | 1 (12.50%) | 2 (20.00%) | 30 (30.00%) | |
| B | 13 (23.64%) | 8 (25.00%) | 1 (12.50%) | 2 (20.00%) | 24 (24.00%) | |
| AB | 1 (1.82%) | 1 (3.13%) | 0 (0.00%) | 0 (0.00%) | 2 (2.00%) | |
| O | 20 (36.36%) | 14 (43.75%) | 6 (75.00%) | 1 (20.00%) | 41 (41.00%) | |
| Age, median age (IQR) | 20 (19–23) | 19.5 (19–22.75) | 21 (20–24.5) | 19 (19–19) | 20 (19–23) | 0.257 |
| BMI (Kg/m2) (IQR) | 21.34 (19.53–23.72) | 20.96 (19.58–24.96) | 21.98 (20.96–25.79) | 20.96 (20.42–21.77) | 21.51 (19.31–24.61) | 0.678 |
| The median interval days between two vaccinations (IQR) | 30 (23–31) | 27 (23–34) | The first and second dose (IQR) | 27 (21–28) | 30 (23–33) | 0.735 |
| 32 (30.5–35.5) | ||||||
| The second and third dose (IQR) | ||||||
| 33.5 (30.5–42.5) | ||||||
| The median interval days between the last dose and the first blood collection (IQR) | 59 (14–103) | 82 (54–117) | 88.5 (61–104.5) | 80 (76–82) | 76 (30.5–109.25) | 0.298 |
| Efficient | Anti WT-RBD IgG | Anti WT-NP1 IgG | Anti WT-NP2 IgG | Anti WT-NP3 IgG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimates | CI (95%) | p Value | Estimates | CI (95%) | p Value | Estimates | CI (95%) | p Value | Estimates | CI (95%) | p Value | |
| Gender | −0.171 | −0.418 to 0.076 | 0.172 | −0.26 | −0.511 to −0.009 | 0.042 * | −0.05 | −0.293 to 0.192 | 0.680 | −0.086 | −0.450 to 0.279 | 0.638 |
| Blood Type | 0.069 | −0.015 to 0.153 | 0.106 | −0.003 | −0.087 to 0.082 | 0.956 | −0.031 | −0.116 to 0.053 | 0.466 | −0.086 | −0.193 to 0.056 | 0.276 |
| Age | −0.016 | −0.038 to 0.006 | 0.162 | −0.017 | −0.039 to 0.005 | 0.130 | −0.028 | −0.048 to −0.007 | 0.010 * | −0.009 | −0.047 to 0.028 | 0.61 |
| BMI(Kg/m2) | 0.004 | −0.036 to 0.045 | 0.834 | −0.047 | −0.088 to −0.007 | 0.023 * | −0.032 | −0.071 to 0.007 | 0.108 | −0.038 | −0.094 to 0.018 | 0.181 |
| Vaccination brand | ||||||||||||
| CoronaVac | ||||||||||||
| BBIBP-CorV | −0.513 | −0.747 to −0.278 | 0.000 * | −0.384 | −0.638 to −0.131 | 0.004 * | −0.272 | −0.479 to −0.064 | 0.011 * | −0.337 | −0.689 to 0.015 | 0.060 |
| ZF2001 | 0.440 | 0.017 to 0.863 | 0.042 * | −0.728 | −1.162 to −0.294 | 0.001 * | −1.298 | −1.673 to −0.924 | 0.000 * | −0.977 | −1.483 to −0.471 | 0.000 * |
| Mixed Injection | −0.228 | −0.808 to 0.352 | 0.436 | −0.022 | −0.740 to 0.696 | 0.951 | 0.143 | −0.371 to 0.656 | 0.582 | 0.552 | −0.504 to 1.608 | 0.298 |
| The interval days between two vaccinations (IQR) | 0.011 | −0.003 to 0.024 | 0.117 | 0.004 | −0.010 to 0.019 | 0.546 | −0.008 | −0.022 to 0.005 | 0.210 | −0.005 | −0.026 to 0.015 | 0.595 |
| The interval days between the last dose andthe first blood collection (IQR) | −0.003 | −0.006 to 0.000 | 0.047 * | −0.002 | −0.005 to 0.001 | 0.147 | −0.002 | −0.004 to 0.001 | 0.276 | −0.003 | −0.007 to 0.000 | 0.081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Luo, Q.; Wan, L.; Zhu, Q.; Liu, R.; Yin, X.; Lu, X.; Wei, L.; Xiang, Z.; Zou, Y. Analysis of Antibodies Induced after SARS-CoV-2 Vaccination Using Antigen Coded Bead Array Luminex Technology. Vaccines 2023, 11, 442. https://doi.org/10.3390/vaccines11020442
Song Z, Luo Q, Wan L, Zhu Q, Liu R, Yin X, Lu X, Wei L, Xiang Z, Zou Y. Analysis of Antibodies Induced after SARS-CoV-2 Vaccination Using Antigen Coded Bead Array Luminex Technology. Vaccines. 2023; 11(2):442. https://doi.org/10.3390/vaccines11020442
Chicago/Turabian StyleSong, Zixuan, Qizhi Luo, Ling Wan, Quan Zhu, Rongjiao Liu, Xiangli Yin, Xiaofang Lu, Leiyan Wei, Zhiqing Xiang, and Yizhou Zou. 2023. "Analysis of Antibodies Induced after SARS-CoV-2 Vaccination Using Antigen Coded Bead Array Luminex Technology" Vaccines 11, no. 2: 442. https://doi.org/10.3390/vaccines11020442
APA StyleSong, Z., Luo, Q., Wan, L., Zhu, Q., Liu, R., Yin, X., Lu, X., Wei, L., Xiang, Z., & Zou, Y. (2023). Analysis of Antibodies Induced after SARS-CoV-2 Vaccination Using Antigen Coded Bead Array Luminex Technology. Vaccines, 11(2), 442. https://doi.org/10.3390/vaccines11020442






